Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extraction of Essential Oil
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oil
2.3. Determination of Antioxidant Activity
2.4. In-Vitro Anticancer Activity Assay
2.5. In-Silico Modelling for Potential Anti-Cancer Constituents
2.5.1. Preparation of Receptors and Ligands
2.5.2. Docking Using AutoDock Vina
2.5.3. Analysis of Docking Result
2.6. Complementarity Assessment Using AlteQ Orbit-Free Quantum Chemical Method
2.7. ADME Analysis
3. Results and Discussion
3.1. GC–MS Analysis of Essential Oil
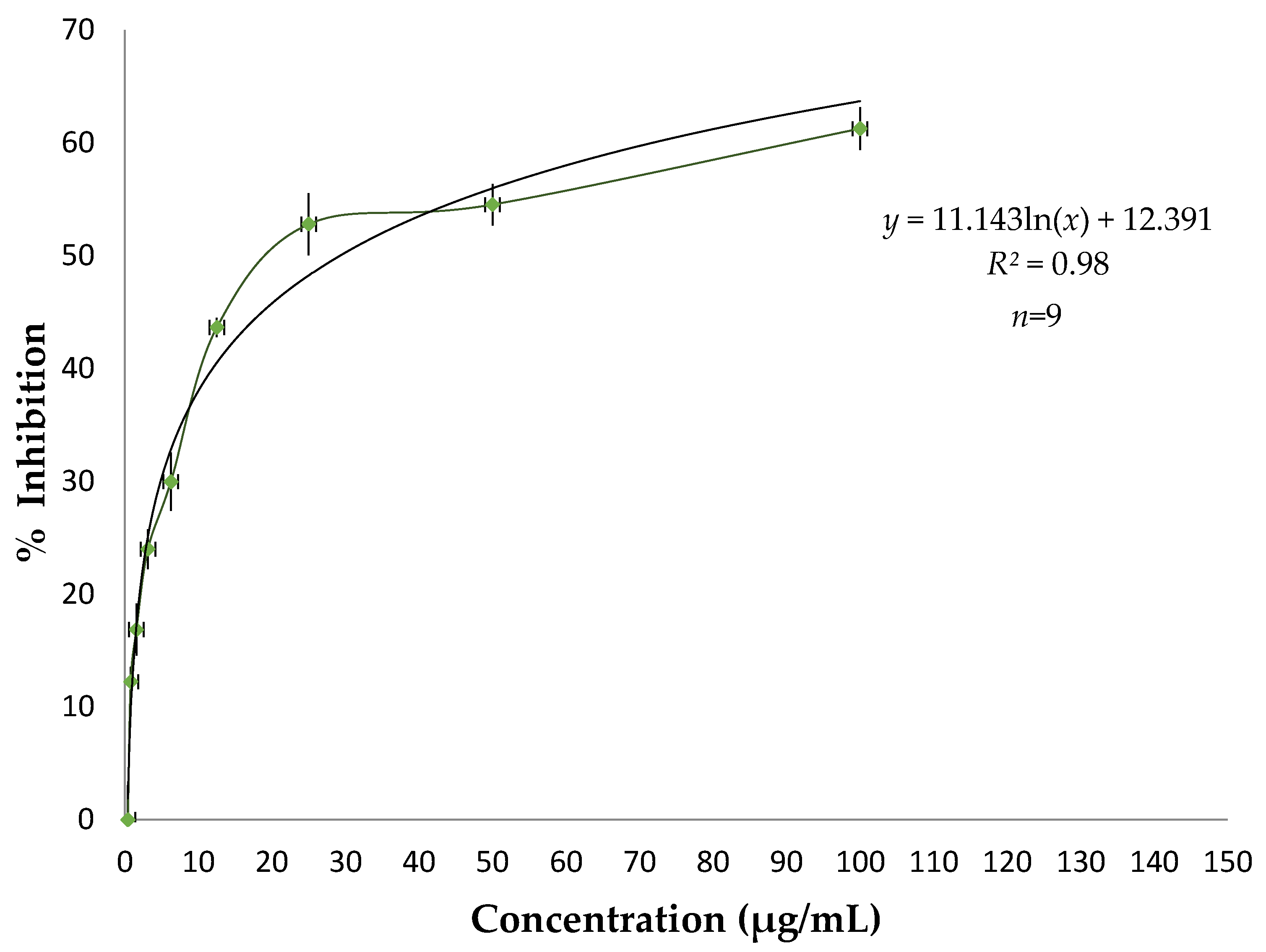
3.2. Antioxidant Activity of Essential Oil
3.3. In Vitro Anticancer Activity
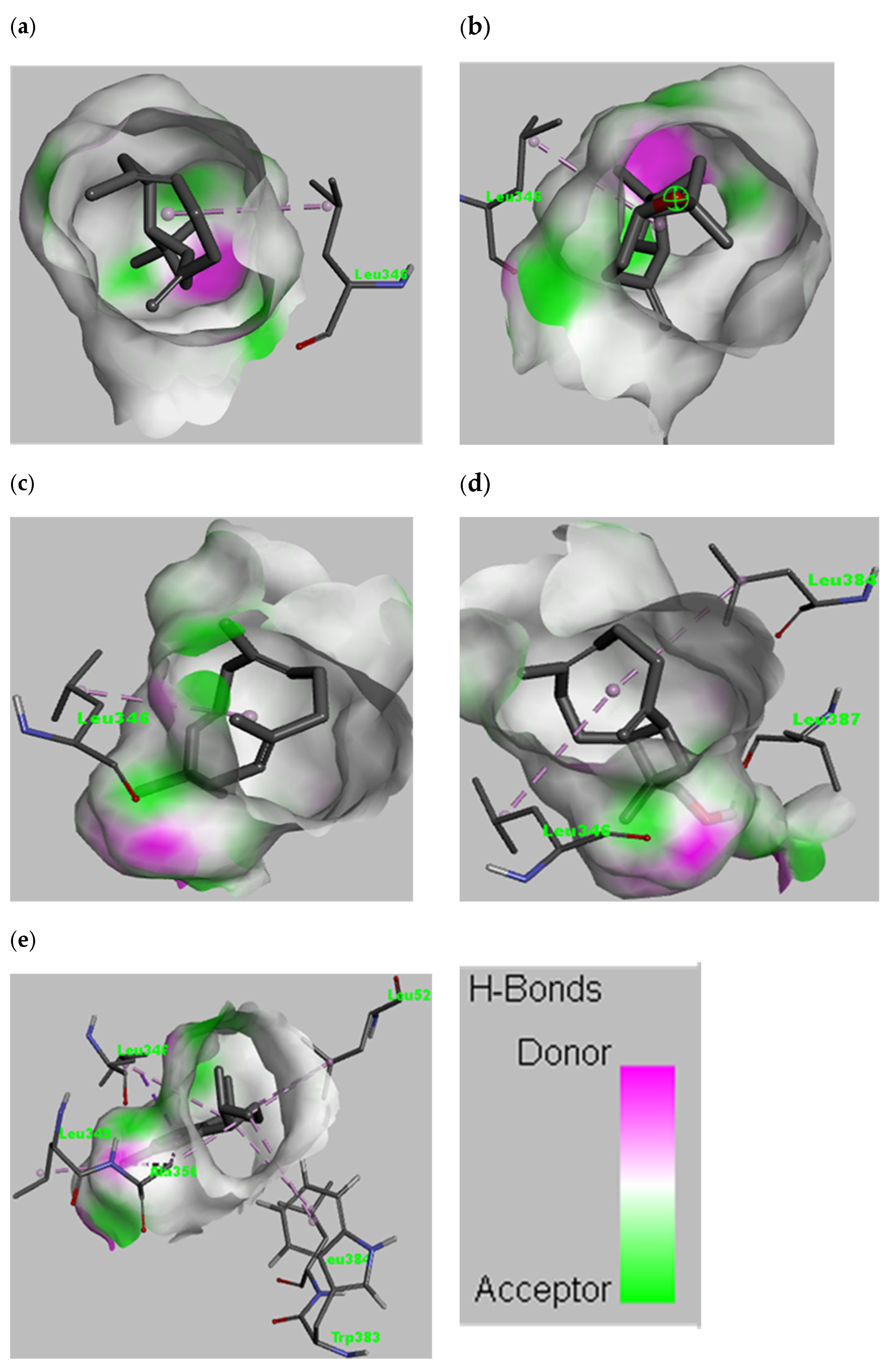
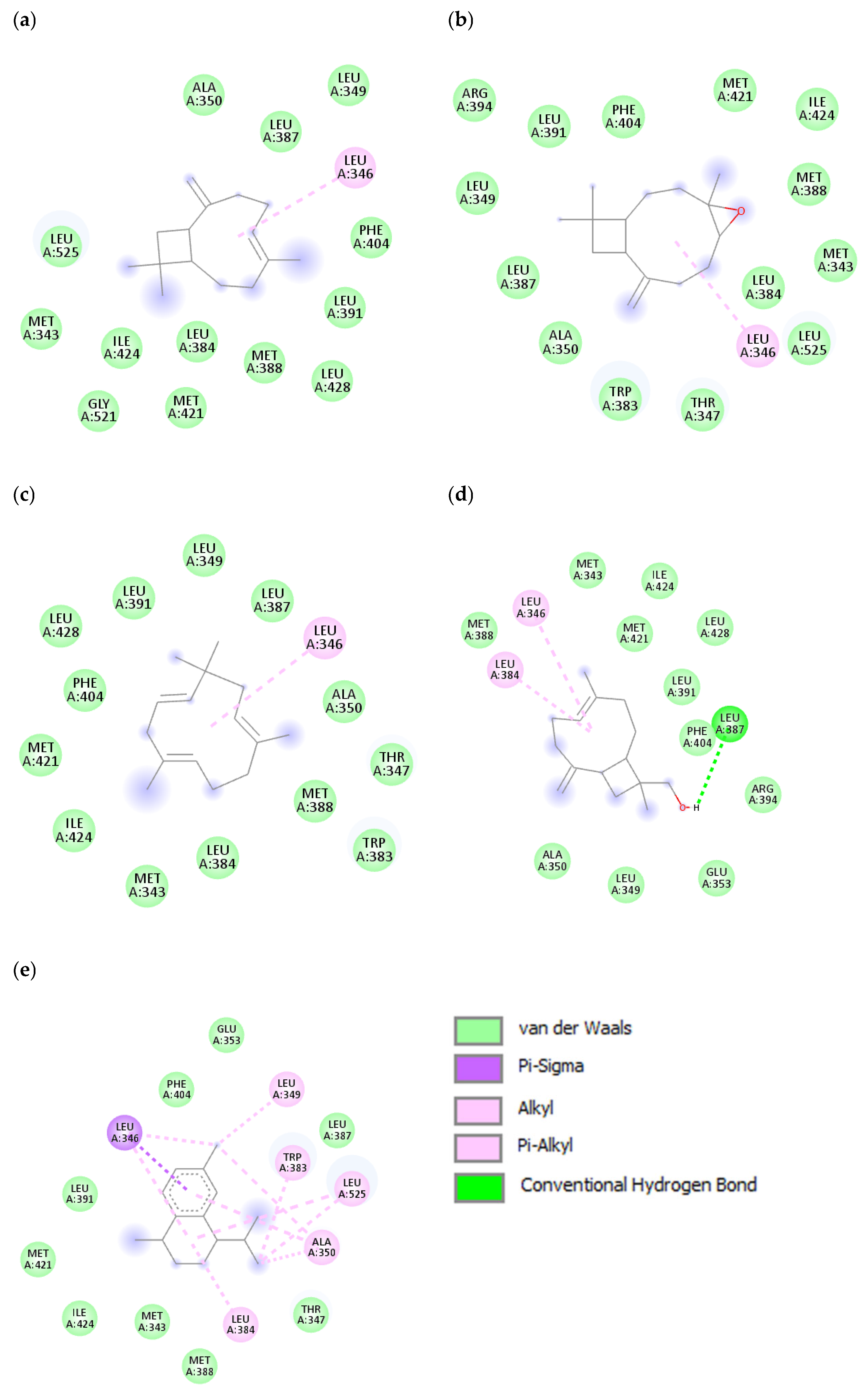
3.4. In Silico Modelling for Potential AntiCancer Constituents
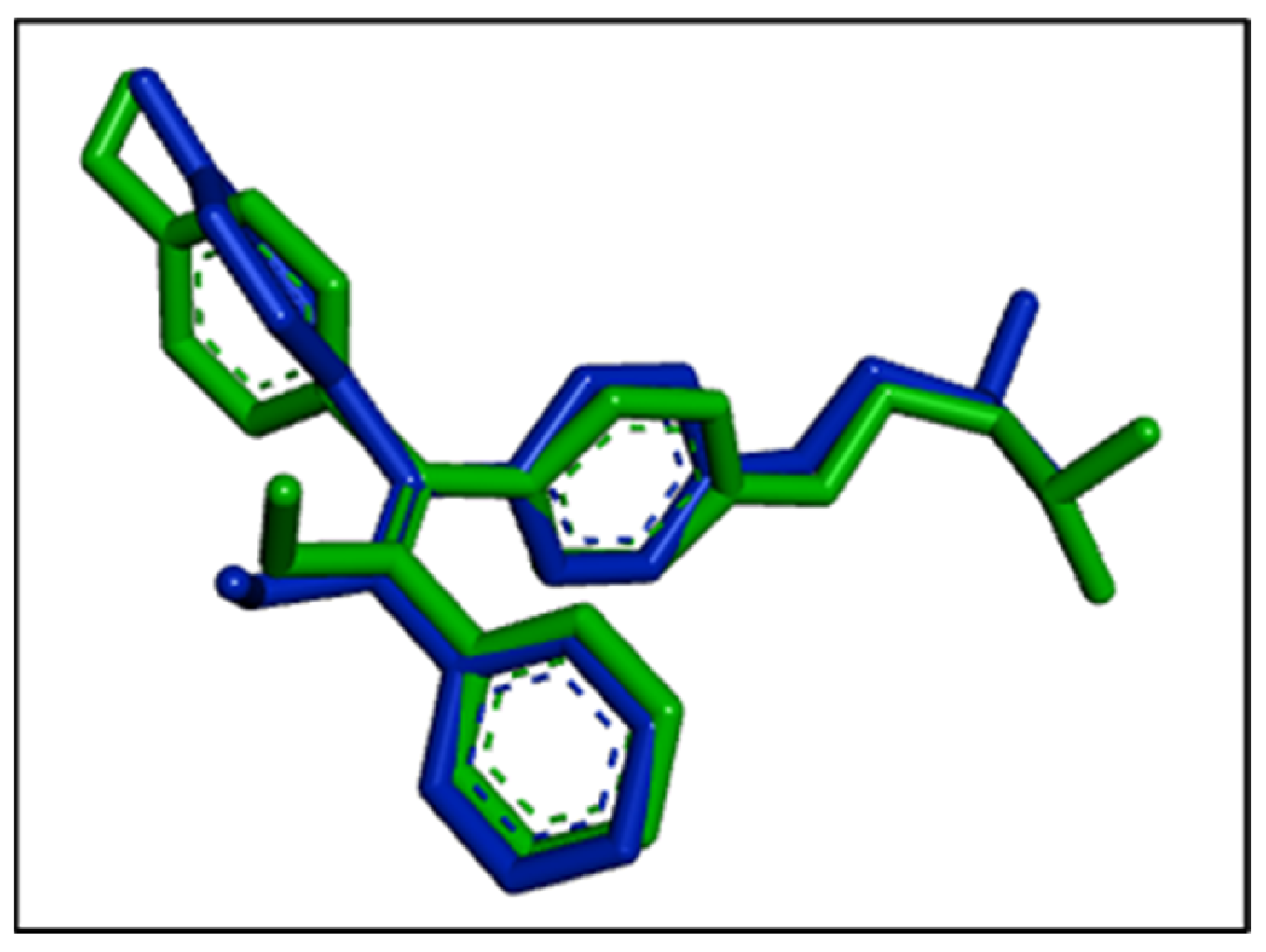
3.5. Complementarity Analysis of the Electronic Structures of Enzyme and Ligand
3.6. ADME Analysis
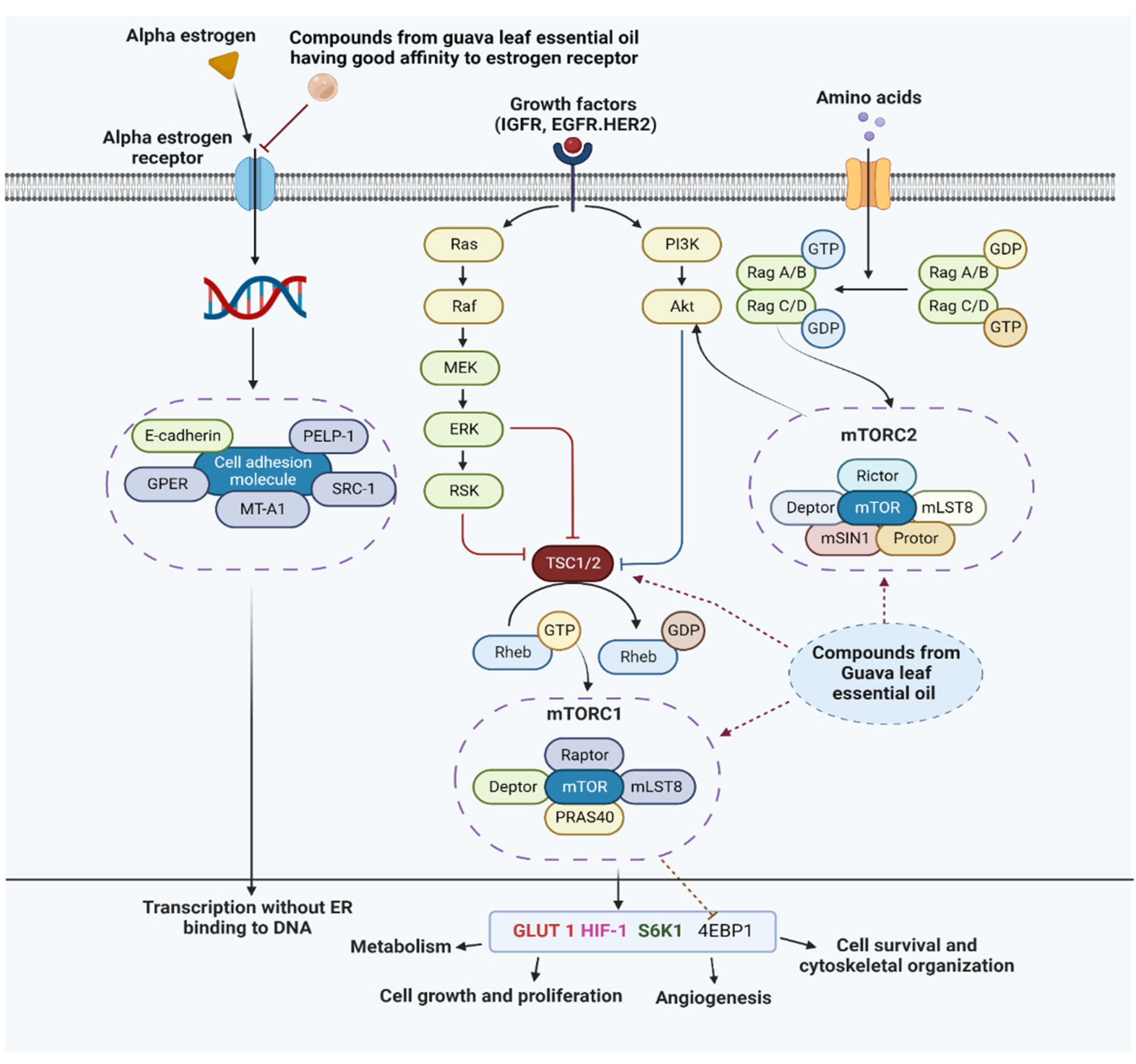
3.7. Probable Mechanism of Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weli, A.; Al-Kaabi, A.; Al-Sabahi, J.; Said, S.; Hossain, M.A.; Al-Riyami, S. Chemical composition and biological activities of the essential oils of Psidium guajava Leaf. J. King Saud Univ.-Sci. 2019, 31, 993–998. [Google Scholar] [CrossRef]
- Fischer, G.; Melgarejo, L.M. Ecophysiological apects of guava (Psidium guajava L.). A review. Rev. Colomb. Cienc. Hortícolas 2021, 15, e12355. [Google Scholar] [CrossRef]
- Shrestha, A.K. Critical appraisal of management practices in nepalese guava orchards. J. Inst. Agric. Anim. Sci. 2005, 26, 127–133. [Google Scholar] [CrossRef]
- Daswani, P.G.; Gholkar, M.S.; Birdi, T.J. Psidium guajava: A single plant for multiple health problems of rural indian population. Pharmacogn. Rev. 2017, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the west bank/palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef]
- WHO Western Pacific Region. Medicinal Plants in the South Pacific: Information on 102 Commonly Used Medicinal Plants in the South Pacific; WHO Regional Publications. Western Pacific Series, no.19; WHO Regional Office for the Western Pacific: Manila, Philippines, 1998. [Google Scholar]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N. Extraction of bioactive compounds from medicinal plants and herbs. In Pharmacognosy—Medicinal Plants [Working Title]; IntechOpen: London, UK, 2021. [Google Scholar]
- Sherazi, S.T.H.; Mahesar, S.A.; Arain, A. Sirajuddin guava (Psidium guajava) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 541–559. ISBN 978-3-030-12473-1. [Google Scholar]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2021, 35, 1393–1397. [Google Scholar] [CrossRef]
- Sul’ain, M.D.; Zazali, K.E.; Ahmad, N.S. Screening on anti-proliferative activity of Psidium guajava leaves extract towards selected cancer cell lines. J. US-China Med. Sci. 2012, 9, 30–37. [Google Scholar]
- Chen, K.-C.; Peng, C.-C.; Chiu, W.-T.; Cheng, Y.-T.; Huang, G.-T.; Hsieh, C.-L.; Peng, R.Y. Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr. Cancer 2010, 62, 260–270. [Google Scholar] [CrossRef]
- Fathilah, A.R.; Sujata, R.; Norhanom, A.W.; Adenan, M.I. Antiproliferative activity of aqueous extract of Piper betle L. and Psidium guajava L. on KB and HeLa cell lines. J. Med. Plants Res. 2010, 4, 987–990. [Google Scholar]
- Kawakami, Y.; Nakamura, T.; Hosokawa, T.; Suzuki-Yamamoto, T.; Yamashita, H.; Kimoto, M.; Tsuji, H.; Yoshida, H.; Hada, T.; Takahashi, Y. Antiproliferative activity of guava leaf extract via inhibition of prostaglandin endoperoxide H synthase isoforms. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 239–245. [Google Scholar] [CrossRef]
- Ryu, N.H.; Park, K.-R.; Kim, S.-M.; Yun, H.-M.; Nam, D.; Lee, S.-G.; Jang, H.-J.; Ahn, K.S.; Kim, S.-H.; Shim, B.S.; et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal P70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef]
- Hay, N. The Akt-MTOR tango and its relevance to cancer. Cancer Cell 2005, 8, 179–183. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Z.; Jiang, B.-H.; Shi, X. Role of PI3K/AKT/MTOR signaling in the cell cycle progression of human prostate cancer. Biochem. Biophys. Res. Commun. 2003, 310, 1124–1132. [Google Scholar] [CrossRef]
- Silva, F.L.N.; Carvalho, J.E.; Lammers, T.; Kiessling, F.; Foglio, M.A.; Rizzo, E.; Murakami, M.T.; Souza, T.A.C.B.; Eberlin, M.N.; Rizzo, L.Y.; et al. In vitro, in vivo and in silico analysis of the anticancer and estrogen-like activity of guava leaf extracts. Curr. Med. Chem. 2014, 21, 2322–2330. [Google Scholar]
- Skliris, G.P.; Leygue, E.; Watson, P.H.; Murphy, L.C. Estrogen receptor alpha negative breast cancer patients: Estrogen receptor beta as a therapeutic target. J. Steroid Biochem. Mol. Biol. 2008, 109, 1–10. [Google Scholar] [CrossRef]
- Bai, Z.; Gust, R. Breast cancer, estrogen receptor and ligands. Arch. Pharm. 2009, 342, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ellison, S.J.; Alarid, E.T.; Shapiro, D.J. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene 2007, 26, 4106–4114. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Swaby, R.F.; Sharma, C.G.N.; Jordan, V.C. SERMs for the treatment and prevention of breast cancer. Rev. Endocr. Metab. Disord. 2007, 8, 229–239. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995; ISBN 0-931710-42-1. [Google Scholar]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Naumovich, V.; Grishina, M.; Shukla, P.K.; Verma, A.; Potemkin, V. Quinazoline based 1,3,5-triazine derivatives as cancer inhibitors by impeding the phosphorylated RET tyrosine kinase pathway: Design, synthesis, docking, and QSAR study. Arch. Pharm. 2019, 352, 1900053. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Pathak, P.; Khalilullah, H.; Grishina, M.; Potemkin, V.; Kumar, V.; Majee, R.; Ramteke, P.W.; Abdellattif, M.H.; Shahbaaz, M.; et al. Green synthesis of silver nanoformulation of scindapsus officinalis as potent anticancer and predicted anticovid alternative: Exploration via experimental and computational methods. Biocatal. Agric. Biotechnol. 2021, 35, 102072. [Google Scholar] [CrossRef]
- Pathak, M.; Pathak, P.; Rimac, H.; Grishina, M.; Bagale, U.; Kumar, V.; Majee, R.; Potemkin, V.; Verma, A. Attenuation of hepatic and breast cancer cells by polygonatum verticillatum embedded silver nanoparticles. Biocatal. Agric. Biotechnol. 2020, 30, 101863. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- WL, D. The PyMOL molecular graphics system. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Rimac, H.; Grishina, M.A.; Potemkin, V.A. Electron density analysis of CDK complexes using the AlteQ method. Future Med. Chem. 2020, 12, 1387–1397. [Google Scholar] [CrossRef]
- Kandagalla, S.; Rimac, H.; Potemkin, V.A.; Grishina, M.A. Complementarity principle in terms of electron density for the study of EGFR complexes. Future Med. Chem. 2021, 13, 863–875. [Google Scholar] [CrossRef]
- Rimac, H.; Grishina, M.; Potemkin, V. Use of the complementarity principle in docking procedures: A new approach for evaluating the correctness of binding poses. J. Chem. Inf. Model. 2021, 61, 1801–1813. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2015, 3, 11–14. [Google Scholar]
- Sun, J. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Shah, B.B.; Mehta, A.A. In vitro evaluation of antioxidant activity of D-limonene. Asian J. Pharm. Pharmacol. 2018, 4, 883–887. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, H.; Wang, J.; Zhang, H. Chemical composition, antibacterial, antioxidant and enzyme inhibitory activities of the essential oil from leaves of Psidium guajava L. Chem. Biodivers. 2022, 19, e202100951. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Grosdidier, A.; Michielin, O. Docking, virtual high throughput screening and in silico fragment-based drug design. J. Cell. Mol. Med. 2009, 13, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J.-C. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by α-humulene as possible mechanism of action. Planta Med. 2003, 69, 402–407. [Google Scholar]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR signaling pathway in breast cancer: From molecular landscape to clinical aspects. Int. J. Mol. Sci. 2020, 26, 173. [Google Scholar] [CrossRef]
- Marina, K.H. The role of S6K1 in ER-positive breast cancer. Cell Cycle 2012, 11, 3159–3165. [Google Scholar] [CrossRef][Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Egeland, N.G.; Lunde, S.; Jonsdottir, K.; Lende, T.H.; Cronin-Fenton, D.; Gilje, B.; Janssen, E.A.; Søiland, H. The role of MicroRNAs as predictors of response to tamoxifen treatment in breast cancer patients. Int. J. Mol. Sci. 2015, 16, 24243–24275. [Google Scholar] [CrossRef]

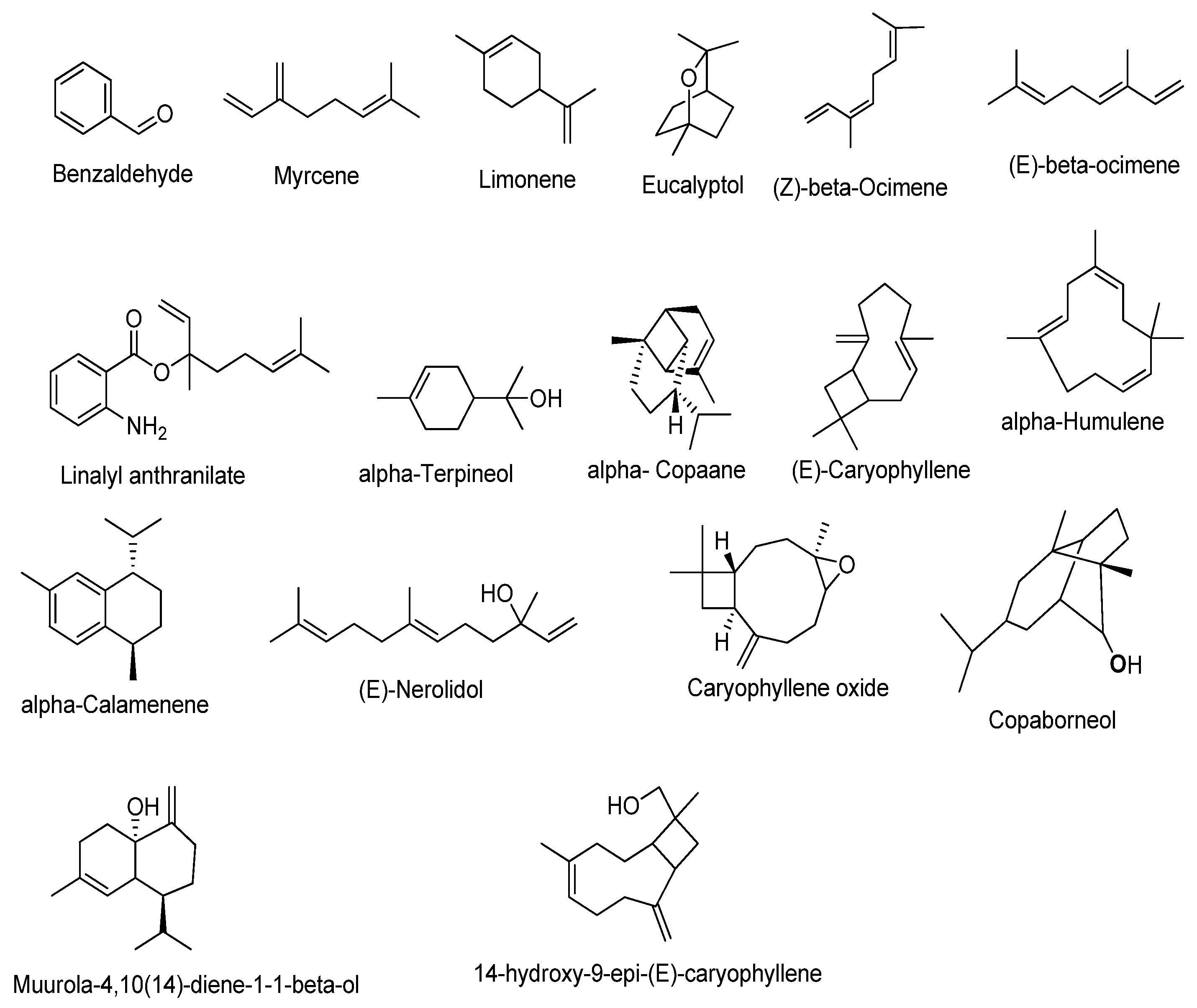
| Peak Number | Retention Time (min) | Compound | Area | Area Percentage |
|---|---|---|---|---|
| 1 | 14.5 | Benzaldehyde | 52,096 | 0.5 |
| 2 | 15.7 | Myrcene | 63,902 | 0.6 |
| 3 | 17.6 | Limonene | 5,248,363 | 51.3 |
| 4 | 17.8 | Eucalyptol | 2,177,857 | 21.3 |
| 5 | 17.9 | Ocimene <(Z)-, beta-> | 135,011 | 1.3 |
| 6 | 18.4 | Ocimene <(E)-, beta-> | 61,578 | 0.6 |
| 7 | 20.9 | Linalyl anthranilate | 83,586 | 0.8 |
| 8 | 25.4 | Terpineol <alpha-> | 129,922 | 1.3 |
| 9 | 34.0 | Copaane <alpha-> | 110,063 | 1.1 |
| 10 | 36.0 | Caryophyllene <(E)-> | 572,209 | 5.6 |
| 11 | 37.4 | Humulene <alpha-> | 72,787 | 0.7 |
| 12 | 40.2 | Calamenene <alpha-> | 88,207 | 0.9 |
| 13 | 41.5 | Nerolidol <(E)-> | 460,010 | 4.5 |
| 14 | 42.8 | Caryophyllene oxide | 637,587 | 6.2 |
| 15 | 43.6 | Copaborneol | 90,736 | 0.9 |
| 16 | 44.4 | Muurola-4,10(14)-diene-1-1-beta-ol | 119,809 | 1.2 |
| 17 | 44.8 | Caryophyllene <14-hydroxy-9-epi-(E)-> | 131,421 | 1.3 |
| Source | Concentration (µg/mL) | Percentage Viability | |||||
|---|---|---|---|---|---|---|---|
| Cancer Cell Lines | Normal Cell Line | ||||||
| HepG-2 | MCF-7 | MCF-12A | |||||
| Control | - | 0 | 0 | 0 | |||
| Oil extracted from P. guajava leaves | 1 | 98.3 ± 0.3 | 98.5 ± 0.4 | 99.5 ± 0.2 | |||
| 2 | 97.2 ± 0.4 | 96.2 ± 0.3 | 97.1 ± 0.3 | ||||
| 10 | 89.3 ± 0.5 | 89.5 ± 0.3 | 93.2 ± 0.5 | ||||
| 25 | 82.3 ± 0.4 | 84.3 ± 0.3 | 91.2 ± 0.5 | ||||
| 50 | 78.3 ± 0.2 | 78.7 ± 0.3 | 90.1 ± 0.3 | ||||
| 75 | 72.1 ± 0.2 | 72.4 ± 0.2 | 89.2 ± 0.5 | ||||
| 100 | 68.3 ± 0.5 | 67.3 ± 0.4 | 89.1 ± 0.4 | ||||
| 200 | 62.2 ± 0.5 | 58.3 ± 0.4 | 88.3 ± 0.2 | ||||
| 250 | 54.7 ± 0.3 | 52.6 ± 0.3 | 87.8 ± 0.3 | ||||
| S.N. | Name of the Ligand | Affinity (kcal/mol) |
|---|---|---|
| 1 | Hydroxytamoxifen | −9.7 |
| 2 | Caryophyllene | −8.4 |
| 3 | Caryophyllene oxide | −8.4 |
| 4 | Humulene | −8.3 |
| 5 | 14-Hydroxy-9-epi-(E)-caryophyllene | −8.2 |
| 6 | Calamenene | −8.0 |
| 7 | Muurola-4,10(14)-dien-8beta-ol | −7.9 |
| 8 | Nerolidol | −7.9 |
| 9 | Copaborneol | −7.8 |
| 10 | Copaane | −7.7 |
| 11 | Linalyl anthranilate | −7.2 |
| 12 | Eucalyptol | −6.3 |
| 13 | Terpineol | −6.2 |
| 14 | Limonenel | −6.1 |
| 15 | (E)-beta-ocimene | −5.4 |
| 16 | (Z)-beta-ocimene | −5.3 |
| 17 | Myrcene | −5.1 |
| S.N. | Name of Ligands | Amino Acids Responsible for Interaction | Types of Interaction |
|---|---|---|---|
| 1 | Caryophyllene | Leu346 | van der Waals, Alkyl |
| 2 | Caryophyllene oxide | Leu346 | van der Waals, Alkyl |
| 3 | Humulene | Leu346 | van der Waals, Alkyl |
| 4 | 14-Hydroxy-9-epi-(E)-caryophyllene | Leu346, Leu384, Leu387 | van der Waals, Alkyl, Conventional hydrogen bond |
| 5 | Calamenene | Leu346, Leu384, Leu349, Ala350, Trp383, Leu525 | van der Waals, Pi-sigma, Pi-alkyl, alkyl |
| Ligand | -Coefficient | -Coefficient | Rcor2 | Sigma | Npoints | MIN (SUMRLRE) | MAX (CF1) |
|---|---|---|---|---|---|---|---|
| calamenene (model 18) | 6.26 | −4.003 | 0.840 | 0.24 | 835 | 3.304 | −6.776 |
| Muurola-4,10(14)-diene-1-1-beta-ol (model 3) | 6.396 | −4.031 | 0.918 | 0.34 | 5650 | 2.742 | −4.184 |
| 3ERT | 9.041 | −4.820 | 0.972 | 0.29 | 5582 | 2.420 | −2.570 |
| Name of the Molecule | Molecular Weight (gm/mol) | Lipophilicity (MLOGP) | H-Bond Acceptor | H-Bond Donor | Molar Refractivity | Drug Likeness |
|---|---|---|---|---|---|---|
| Caryophyllene | 204.35 | 4.63 | 0 | 0 | 68.78 | Yes; 1 violation: MLOGP > 4.15 |
| Caryophyllene oxide | 220.35 | 3.67 | 1 | 0 | 68.27 | Yes; 0 violation |
| Humulene | 204.35 | 4.53 | 0 | 0 | 70.42 | Yes; 1 violation: MLOGP > 4.15 |
| 14-Hydroxy-9-epi-(E)-caryophyllene | 220.35 | 3.56 | 1 | 1 | 69.94 | Yes; 0 violation |
| Calamenene | 202.34 | 5.45 | 0 | 0 | 68.07 | Yes; 1 violation: MLOGP > 4.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.K.; Paudel, S.; Pandey, A.; Yadav, P.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; Khalilullah, H.; Verma, A. Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study. Antioxidants 2022, 11, 2204. https://doi.org/10.3390/antiox11112204
Mandal AK, Paudel S, Pandey A, Yadav P, Pathak P, Grishina M, Jaremko M, Emwas A-H, Khalilullah H, Verma A. Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study. Antioxidants. 2022; 11(11):2204. https://doi.org/10.3390/antiox11112204
Chicago/Turabian StyleMandal, Ashok Kumar, Samrat Paudel, Anisha Pandey, Parasmani Yadav, Prateek Pathak, Maria Grishina, Mariusz Jaremko, Abdul-Hamid Emwas, Habibullah Khalilullah, and Amita Verma. 2022. "Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study" Antioxidants 11, no. 11: 2204. https://doi.org/10.3390/antiox11112204
APA StyleMandal, A. K., Paudel, S., Pandey, A., Yadav, P., Pathak, P., Grishina, M., Jaremko, M., Emwas, A.-H., Khalilullah, H., & Verma, A. (2022). Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study. Antioxidants, 11(11), 2204. https://doi.org/10.3390/antiox11112204







