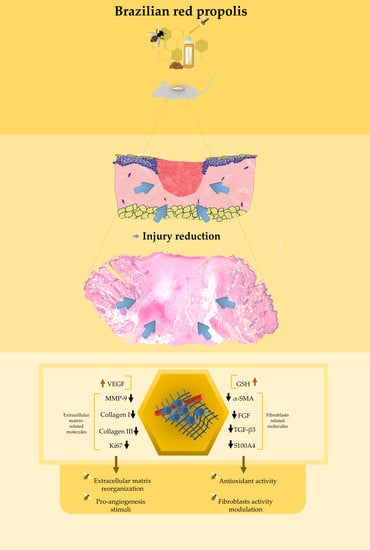
Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Red Propolis Extraction
2.3. Formulation of 1% Red Propolis Hydroalcoholic Extract and Paste
2.4. Animals
- Group 1 (WWT/negative control): Wounded animal without treatment;
- Group 2 (BRP-HS): Wounded animals treated with Brazilian red propolis hydroalcoholic solution at 1%; and
- Group 3 (BRP-Paste): Wounded animals treated with paste containing Brazilian red propolis at 1%.
2.5. Experimental Protocol of Wound Excision
2.6. Macroscopic Analysis
2.6.1. Wound Contraction Analysis
2.6.2. Clinical Parameters
2.7. Microscopic Analysis
2.7.1. Histological Parameters
2.7.2. Immunohistochemistry
2.8. Antioxidant Enzyme and Inflammatory Mediator Analysis
2.9. Statistical Analysis
3. Results
3.1. Macroscopic Analysis
3.1.1. Wound Contraction Analysis
3.1.2. Clinical Parameters
3.2. Microscopic Analysis
3.2.1. Histological Parameters
3.2.2. Immunohistochemistry
- α-SMA
- Collagen I
- Collagen III
- FGF
- Ki67
- MMP-9
- S100A4
- TGF-β3
- VEGF
3.3. Antioxidant Molecule and Inflammatory Mediator Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef] [Green Version]
- Pasterfield, M.; Clarke, S.A.; Thompson, A.R. The development of a self-help intervention to build social confidence in people living with visible skin conditions or scars: A think-aloud study. Scars Burn Heal. 2019, 5, 2059513118822954. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [Green Version]
- Rosa, D.F.; Sarandy, M.M.; Novaes, R.D.; Freitas, M.B.; do Carmo Gouveia Pelúzio, M.; Gonçalves, R.V. High-Fat Diet and Alcohol Intake Promotes Inflammation and Impairs Skin Wound Healing in Wistar Rats. Mediat. Inflamm. 2018, 2018, 4658583. [Google Scholar] [CrossRef]
- Qiao, Q.; Chen, L.; Li, X.; Lu, X.; Xu, Q. Roles of Dietary Bioactive Peptides in Redox Balance and Metabolic Disorders. Oxid. Med. Cell. Longev. 2021, 2021, 5582245. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the Biological Effects of Lyophilized Hydrophilic Extract of Rhus coriaria on Myeloperoxidase (MPO) Activity, Wound Healing, and Microbial Infections of Skin Wound Tissues. Evid. Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: For-mation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Nosenko, M.A.; Ambaryan, S.G.; Drutskaya, M.S. Proinflammatory cytokines and skin wound healing in mice. Mol. Biol. 2019, 53, 653–664. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Marsy, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic. Food Chem. Toxicol. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; De Souza, M.C.; Arruda, C.; Pereira, R.A.S.; Bastos, J.K. The Role of Baccharis dracunculifolia and its Chemical Profile on Green Propolis Production by Apis mellifera. J. Chem. Ecol. 2020, 46, 150–162. [Google Scholar] [CrossRef]
- Rufatto, L.C.; Luchtenberg, P.; Garcia, C.; Thomassigny, C.; Bouttier, S.; Henriques, J.; Roesch-Ely, M.; Dumas, F.; Moura, S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. [Google Scholar] [CrossRef]
- Afonso, A.M.; Gonçalves, J.; Luís, A.; Gallardo, E.; Duarte, A.P. Evaluation of the in vitro wound-healing activity and phytochemical characterization of propolis and honey. Appl. Sci. 2020, 10, 1845. [Google Scholar] [CrossRef]
- Curti, V.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and In Vivo Antioxidant Activity of a Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boufadi, Y.M.; Van Antwerpen, P.; Chikh Alard, I.; Nève, J.; Djennas, N.; Riazi, A.; Soubhye, J. Antioxidant effects and bioavailability evaluation of propolis extract and its content of pure polyphenols. J. Food Biochem. 2017, 42, e12434. [Google Scholar] [CrossRef]
- Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Squarisi, I.S.; Nascimento, S.; Tanimoto, M.H.; Ribeiro, V.P.; Arruda, C.; Nicolella, H.; Esperandim, T.; Ribeiro, A.B.; et al. Nonclinical toxicological studies of Brazilian red propolis and Its primary botanical source Dalbergia ecastaphyllum. Chem. Res. Toxicol. 2021, 34, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.; Ambrósio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.; Vaz, M.M.; Marchetti, J.M. Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–12. [Google Scholar] [CrossRef]
- Gushiken, L.; Hussni, C.A.; Bastos, J.K.; Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Padovani, C.R.; Lemos, M.; Polizello Junior, M.; da Silva, J.; et al. Skin Wound Healing Potential and Mechanisms of the Hydroalcoholic Extract of Leaves and Oleoresin of Copaifera langsdorffii Desf. Kuntze in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 6589270. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; de Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K.; et al. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- De Oliveira, M.L.; Bezerra, B.M.; Leite, L.O.; Girão, V.C.; Nunes-Pinheiro, D.C. Topical continuous use of Lippia sidoides Cham. essential oil induces cutaneous inflammatory response, but does not delay wound healing process. J. Ethnopharmacol. 2014, 153, 283–289. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. Anal. Free Radicals Biol. Syst. 1995, 237–248. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods in Enzimology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Orlando, FL, USA, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera Lf: The botanical sources of isoflavonoids and benzophenones in Brazilian red propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Boeing, T.; Mejía, J.A.A.; Ccana-Ccapatinta, G.V.; Mariott, M.; da Silva, R.C.M.V.A.F.; de Souza, P.; Mariano, L.N.B.; Oliveira, G.R.; da Rocha, I.M.; da Costa, G.A.; et al. The gastroprotective effect of red propolis extract from Northeastern Brazil and the role of its isolated compounds. J. Ethnopharmacol. 2021, 267, 113623. [Google Scholar] [CrossRef]
- Lucas, C.I.S.; Ferreira, A.F.; Costa, M.A.P.D.C.; Silva, F.L.; Estevinho, L.M.; Carvalho, C.A.L. Phytochemical study and antioxidant activity of Dalbergia ecastaphyllum. Rodriguésia 2020, 71, e00492019. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Gimeno-LLuch, I.; Benito-Jardón, M.; Guerrero-Barberà, G.; Burday, N.; Costell, M. The Role of the Fibronectin Synergy Site for Skin Wound Healing. Cells 2022, 11, 2100. [Google Scholar] [CrossRef]
- Thangavel, P.; Vilvanathan, S.P.; Kuttalam, I.; Lonchin, S. Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int. J. Biol. Macromol. 2020, 149, 395–403. [Google Scholar] [CrossRef]
- Abu-Seida, A.M. Effect of Propolis on Experimental Cutaneous Wound Healing in Dogs. Vet. Med. Int. 2015, 2015, 672643. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Surber, C.; Smith, E.W. The mystical effects of dermatological vehicles. Dermatology 2005, 210, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, D.V.; Lippert, C.N.; Crisp, J.L.; Savariar, E.N.; Hasselmann, J.; Kuo, C.; Nguyen, Q.T.; Tsien, R.Y.; Whitney, M.A.; Ellies, L.G. Impact of MMP-2 and MMP-9 enzyme activity on wound healing, tumor growth and RACPP cleavage. PLoS ONE 2018, 13, e0198464. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis Modifies Collagen Types I and III Accumulation in the Matrix of Burnt Tissue. Evid. Based Complement. Altern. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef] [Green Version]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-beta/Smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- Russo, B.; Brembilla, N.C.; Chizzolini, C. Interplay Between Keratino-cytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front Immunol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Sun, C.; Tian, X.; Jia, Y.; Yang, M.; Li, Y.; Fernig, D.G. Functions of exogenous FGF signals in regu-lation of fibroblast to myofibroblast differentiation and extracellular matrix protein ex-pression. Open Biol. 2022, 12, 210356. [Google Scholar] [CrossRef]
- Qu, Y.; Cao, C.; Wu, Q.; Huang, A.; Song, Y.; Li, H.; Zuo, Y.; Chu, C.; Li, J.; Man, Y. The dual delivery of KGF and bFGF by collagen membrane to promote skin wound healing. J. Tissue Eng. Regen Med. 2018, 12, 1508–1518. [Google Scholar] [CrossRef]
- Takaya, K.; Aramaki-Hattori, N.; Sakai, S.; Okabe, K.; Asou, T.; Kishi, K. Fibroblast Growth Factor 7 Suppresses Fibrosis and Promotes Epithelialization during Wound Healing in Mouse Fe-tuses. Int. J. Mol. Sci. 2022, 23, 7087. [Google Scholar] [CrossRef]
- Su, W.; Wang, P.; Dong, Q.; Li, S.; Hu, S. S100A8 accelerates wound healing by promoting adipose stem cell proliferation and suppressing inflammation. Regen Ther. 2022, 21, 166–174. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, S.; Qin, Z. Extracellular S100A4 as a key player in fibrotic diseases. J. Cell. Mol. Med. 2020, 24, 5973–5983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales Gil, R.; Vagnarelli, P. Ki-67: More Hidden behind a ‘Classic Proliferation Marker’. Trends Biochem. Sci. 2018, 43, 747–748. [Google Scholar] [CrossRef]
- Li-Tsang, C.W.; Feng, B.; Huang, L.; Liu, X.; Shu, B.; Chan, Y.T.; Cheung, K.K. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns 2015, 41, 1008–1016. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Limandjaja, G.C.; Belien, J.M.; Scheper, R.J.; Niessen, F.B.; Gibbs, S. Hypertrophic and keloid scars fail to progress from the CD34-/α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression. Br. J. Dermatol. 2020, 182, 974–986. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Peng, Y.; Gao, D.; Feng, C.; Yuan, X.; Li, H.; Wang, Y.; Yang, L.; Huang, S.; Fu, X. Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int. J. Low Extrem. Wound. 2015, 14, 50–62. [Google Scholar] [CrossRef]
- Samadikuchaksaraei, A.; Mehdipour, A.; Habibi Roudkenar, M.; Verdi, J.; Joghataei, M.T.; As’adi, K.; Amiri, F.; Dehghan Harati, M.; Gholipourmalekabadi, M.; Karkuki Osguei, N. A Dermal Equivalent Engineered with TGF-β3 Expressing Bone Marrow Stromal Cells and Amniotic Membrane: Cosmetic Healing of Full-Thickness Skin Wounds in Rats. Artif. Organs 2016, 40, E266–E279. [Google Scholar] [CrossRef]
- Chang, Z.; Kishimoto, Y.; Hasan, A.; Welham, N.V. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Model Mech. 2014, 7, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Brunner, L.M.; Rebling, J.; Ben-Yehuda Greenwald, M.; Werner, S.; Detmar, M.; Razansky, D. Non-invasive longitudinal imaging of VEGF-induced microvascular alterations in skin wounds. Theranostics 2022, 12, 558–573. [Google Scholar] [CrossRef]
- Orgill, D.; Demling, R.H. Current concepts and approaches to wound healing. Crit. Care Med. 1988, 16, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ishida, Y.; Kimura, A.; Takayasu, T.; Eisenmenger, W.; Kondo, T. Forensic application of VEGF expression to skin wound age determination. Int. J. Legal Med. 2004, 118, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hotta, S.; Uchiyama, S.; Ichihara, K. Brazilian red propolis extract enhances expression of antioxidant enzyme genes in vitro and in vivo. Biosci. Biotechnol. Biochem. 2020, 84, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Vieira de Morais, D.; Rosalen, P.L.; Ikegaki, M.; de Souza Silva, A.P.; Massarioli, A.P.; de Alencar, S.M. Active Antioxidant Phenolics from Brazilian Red Propolis: An Optimization Study for Their Recovery and Identification by LC-ESI-QTOF-MS/MS. Antioxidants 2021, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.; Ribeiro, A.; Lima, A.K.; Bezerra, G.B.; Pinheiro, M.S.; Albuquerque-Júnior, R.; Gomes, M.Z.; Padilha, F.F.; Thomazzi, S.M.; Novellino, E.; et al. Red propolis and its dyslipidemic regulator formononetin: Evaluation of antioxidant activity and gastroprotective effects in rat model of gastric ulcer. Nutrients 2020, 12, 2951. [Google Scholar] [CrossRef]
- De Mendonça, I.C.; Porto, I.C.; do Nascimento, T.G.; de Souza, N.S.; Oliveira, J.M.; Arruda, R.E.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Mujica, V.; Orrego, R.; Fuentealba, R.; Leiva, E.; Zúñiga-Hernández, J. Propolis as an Adjuvant in the Healing of Human Diabetic Foot Wounds Receiving Care in the Diagnostic and Treatment Centre from the Regional Hospital of Talca. J. Diabetes Res. 2019, 2019, 2507578. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound. J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genomics. 2016, 48, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.J.; Kim, B.; Lee, Y.S.; Kim, T.Y. Role of superoxide dismutase 3 in skin inflammation. J. Dermatol. Sci. 2012, 67, 81–87. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods. 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Iio, A.; Ohguchi, K.; Inoue, H.; Maruyama, H.; Araki, Y.; Nozawa, Y.; Ito, M. Ethanolic extracts of Brazilian red propolis promote adipocyte differentiation through PPARγ activation. Phytomedicine 2010, 17, 974–979. [Google Scholar] [CrossRef]
- Carvalho, C.; Fernandes, W.H.C.; Mouttinho, T.B.F.; Souza, D.M.; Marcucci, M.C.; D’Alpino, P.H.P. Evidence-Based Studies and Perspectives of the Use of Brazilian Green and Red Propolis in Dentistry. Eur. J. Dent. 2019, 13, 459–465. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, M.; Gushiken, L.F.S.; Aldana-Mejía, J.A.; Tanimoto, M.H.; Ferreira, M.V.d.S.; Alves, A.C.M.; Miyashita, M.N.; Bastos, J.K.; Beserra, F.P.; Pellizzon, C.H. Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis. Antioxidants 2022, 11, 2188. https://doi.org/10.3390/antiox11112188
Conceição M, Gushiken LFS, Aldana-Mejía JA, Tanimoto MH, Ferreira MVdS, Alves ACM, Miyashita MN, Bastos JK, Beserra FP, Pellizzon CH. Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis. Antioxidants. 2022; 11(11):2188. https://doi.org/10.3390/antiox11112188
Chicago/Turabian StyleConceição, Mariana, Lucas Fernando Sérgio Gushiken, Jennyfer Andrea Aldana-Mejía, Matheus Hikaru Tanimoto, Marcos Vital de Sá Ferreira, Andreia Cristina Miranda Alves, Marina Naomi Miyashita, Jairo Kenupp Bastos, Fernando Pereira Beserra, and Cláudia Helena Pellizzon. 2022. "Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis" Antioxidants 11, no. 11: 2188. https://doi.org/10.3390/antiox11112188
APA StyleConceição, M., Gushiken, L. F. S., Aldana-Mejía, J. A., Tanimoto, M. H., Ferreira, M. V. d. S., Alves, A. C. M., Miyashita, M. N., Bastos, J. K., Beserra, F. P., & Pellizzon, C. H. (2022). Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis. Antioxidants, 11(11), 2188. https://doi.org/10.3390/antiox11112188