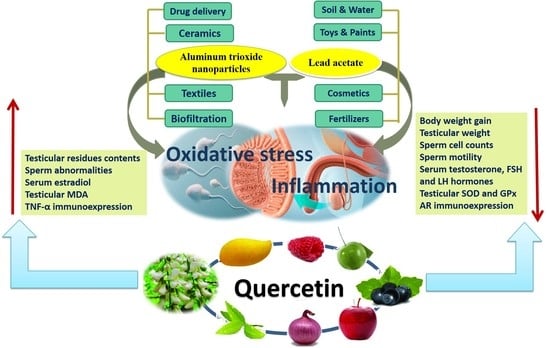
Quercetin Abates Aluminum Trioxide Nanoparticles and Lead Acetate Induced Altered Sperm Quality, Testicular Oxidative Damage, and Sexual Hormones Disruption in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Design
2.3. PbAc, Al2O3NPs, and QTN Dose Selection
2.4. Blood Sampling Collection
2.5. Testicular Tissues Weights and Sampling
2.6. Semen Assessment
2.7. Analysis of Al and Pb Residues
2.8. Hormones Measurements
2.9. Oxidative Stress Biomarkers Analysis
2.10. Histopathological Evaluation
2.11. Immunohistochemistry of AR and TNF-α
2.12. Statistical Analysis
3. Results
3.1. Effects on Body WEIGHT Changes and Testicular Weight
3.2. Effects on Semen Quality
3.3. Effects on Reproductive Hormones
3.4. Effects on Testicular Oxidative Stress Biomarkers
3.5. Changes in Testicular Content of Al and Pb
3.6. Histopathological Findings
3.6.1. Testis
3.6.2. Prostate Glands
3.6.3. Seminal Vesicles
3.7. Immunohistochemistry Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd-Elhakim, Y.M.; Hashem, M.M.; Abo-El-Sooud, K.; Hassan, B.A.; Elbohi, K.M.; Al-Sagheer, A.A. Effects of Co-Exposure of Nanoparticles and Metals on Different Organisms: A Review. Toxics 2021, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Dantas, G.P.F.; Ferraz, F.S.; Andrade, L.M.; Costa, G.M.J. Male reproductive toxicity of inorganic nanoparticles in rodent models: A systematic review. Chem.-Biol. Interact. 2022, 363, 110023. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.Q.; Ma, D.D.; Hao, S.L.; Yang, W.X.; Kovacs, T.; Tan, F.Q. Titanium dioxide nanoparticles perturb the blood-testis barrier via disruption of actin-based cell adhesive function. Aging 2021, 13, 25440–25452. [Google Scholar] [CrossRef]
- Asare, N.; Instanes, C.; Sandberg, W.J.; Refsnes, M.; Schwarze, P.; Kruszewski, M.; Brunborg, G. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology 2012, 291, 65–72. [Google Scholar] [CrossRef]
- Hamdi, H. Testicular dysfunction induced by aluminum oxide nanoparticle administration in albino rats and the possible protective role of the pumpkin seed oil. J. Basic Appl. Zool. 2020, 81, 42. [Google Scholar] [CrossRef]
- Li, N.; Mruk, D.D.; Chen, H.; Wong, C.K.C.; Lee, W.M.; Cheng, C.Y. Rescue of perfluorooctanesulfonate (PFOS)-mediated Sertoli cell injury by overexpression of gap junction protein connexin 43. Sci. Rep. 2016, 6, 29667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood-testis barrier and spermatogenesis: Lessons from genetically-modified mice. Asian J. Androl. 2014, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Yang, W.-X. Nanoparticles and spermatogenesis: How do nanoparticles affect spermatogenesis and penetrate the blood–testis barrier. Nanomedicine 2012, 7, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Gupta, H.; Singh, D.; Mohanty, I.R.; Maheswari, U.; Vanage, G.; Joshi, D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (Chronic study) of repeated oral administration. J. Nanobiotechnol. 2014, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canli, E.G.; Ila, H.B.; Canli, M. Responses of biomarkers belonging to different metabolic systems of rats following oral administration of aluminium nanoparticle. Environ. Toxicol. Pharmacol. 2019, 69, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Mutar, T.F.; Kamel, M.A.E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019, 6, 336–346. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef]
- Asztemborska, M. Alumina nanoparticles and plants: Environmental transformation, bioaccumulation, and phytotoxicity. In Phytotoxicity of Nanoparticles; Springer: Cham, Switzerland, 2018; pp. 335–345. [Google Scholar]
- Kim, Y.S.; Chung, Y.H.; Seo, D.S.; Choi, H.S.; Lim, C.H. Twenty-Eight-Day Repeated Inhalation Toxicity Study of Aluminum Oxide Nanoparticles in Male Sprague-Dawley Rats. Toxicol. Res. 2018, 34, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Elkhadrawy, B.; Abouzeid, S.; El Borai, N.; El-Sabbagh, H.; El-bialy, B. Potential Toxic Effects of Aluminum Nanoparticles: An overview. J. Curr. Vet. Res. 2021, 3, 94–106. [Google Scholar] [CrossRef]
- Yousef, M.; Al-Hamadani, M.; Kamel, M. Reproductive toxicity of aluminum oxide nanoparticles and zinc oxide nanoparticles in male rats. Nanoparticle 2019, 1, 3. [Google Scholar] [CrossRef]
- De, A.; Ghosh, S.; Chakrabarti, M.; Ghosh, I.; Banerjee, R.; Mukherjee, A. Effect of low-dose exposure of aluminium oxide nanoparticles in Swiss albino mice: Histopathological changes and oxidative damage. Toxicol. Ind. Health 2020, 36, 567–579. [Google Scholar] [CrossRef]
- Bas, H.; Kalender, S. Antioxidant status, lipid peroxidation and testis-histoarchitecture induced by lead nitrate and mercury chloride in male rats. Braz. Arch. Biol. Technol. 2016, 59, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, B.; Cheng, Q.; Li, X.; Li, Z. Removal of Toxic Heavy Metal Ions (Pb, Cr, Cu, Ni, Zn, Co, Hg, and Cd) from Waste Batteries or Lithium Cells Using Nanosized Metal Oxides: A Review. J. Nanosci. Nanotechnol. 2020, 20, 7231–7254. [Google Scholar] [CrossRef]
- DeWitt, R.D. Pediatric lead exposure and the water crisis in Flint, Michigan. J. Am. Acad. PAs 2017, 30, 43–46. [Google Scholar] [CrossRef]
- Halder, D.; Saha, J.K.; Biswas, A. Accumulation of essential and non-essential trace elements in rice grain: Possible health impacts on rice consumers in West Bengal, India. Sci. Total Environ. 2020, 706, 135944. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Meharg, A.A. Cadmium and lead in vegetable and fruit produce selected from specific regional areas of the UK. Sci. Total Environ. 2015, 533, 520–527. [Google Scholar] [CrossRef] [Green Version]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Flora, S.J.; Flora, G.; Saxena, G. Environmental occurrence, health effects and management of lead poisoning. In Lead; Elsevier: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Kelainy, E.G.; Ibrahim Laila, I.M.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 31675–31684. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Abd-Elhakim, Y.M.; Farouk, S.M. Protective effects of ethanolic extract of rosemary against lead-induced hepato-renal damage in rabbits. Exp. Toxicol. Pathol. 2016, 68, 451–461. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; El Bohi, K.M.; El Sharkawy, N.I.; Ghali, M.A.; Haseeb, S. The impacts of individual and combined exposure to cadmium and lead on intraocular pressure, electroretinography, and residual changes in the rabbit eyes. Environ. Sci. Pollut. Res. 2019, 26, 33321–33328. [Google Scholar] [CrossRef]
- Dolati, P.; Zamiri, M.J.; Akhlaghi, A.; Khodabandeh, Z.; Mehrabani, D.; Atashi, H.; Jamhiri, I. Reproductive and embryological toxicity of lead acetate in male mice and their offspring and mitigation effects of quercetin. J. Trace Elem. Med. Biol. 2021, 67, 126793. [Google Scholar] [CrossRef]
- Udi, O.A.; Oyem, J.C.; Ebeye, O.A.; Chris-Ozoko, L.E.; Igbigbi, P.S.; Olannye, D.U. The effects of aqueous extract of ocimum gratissimum on the cerebellum of male wistar rats challenged by lead acetate. Clin. Nutr. Open Sci. 2022, 44, 28–41. [Google Scholar] [CrossRef]
- Elgawish, R.A.R.; Abdelrazek, H.M.A. Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol. Rep. 2014, 1, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Ileriturk, M.; Benzer, F.; Aksu, E.H.; Yildirim, S.; Kandemir, F.M.; Dogan, T.; Dortbudak, M.B.; Genc, A. Chrysin protects against testicular toxicity caused by lead acetate in rats with its antioxidant, anti-inflammatory, and antiapoptotic properties. J. Food Biochem. 2021, 45, e13593. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Dong, W.; Li, Y.; Zheng, X.; Piao, F.; Li, S. Subchronic exposure to lead acetate inhibits spermatogenesis and downregulates the expression of Ddx3y in testis of mice. Reprod. Toxicol. 2013, 42, 242–250. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Yadegari, P.; Imani, A.; Taghdir, M. Vitamin D3 protects against lead-induced testicular toxicity by modulating Nrf2 and NF-κB genes expression in rat. Reprod. Toxicol. 2021, 103, 36–45. [Google Scholar] [CrossRef]
- Mesa-Garcia, M.D.; Plaza-Diaz, J.; Gomez-Llorente, C. Chapter 3—Molecular Basis of Oxidative Stress and Inflammation. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 41–62. [Google Scholar]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Zhang, P.; Sun, F.; Chen, Z.; Ma, W.; Meng, F.; Hao, H.; Shang, X. Silica nanoparticles cause spermatogenesis dysfunction in mice via inducing cell cycle arrest and apoptosis. Ecotoxicol. Environ. Saf. 2022, 231, 113210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Mo, Y.; Tollerud, D.J.; Gu, A.; Zhang, Q. Sublethal effects of zinc oxide nanoparticles on male reproductive cells. Toxicol. In Vitro 2016, 35, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Basalamah, M.; Abdelghany, A.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018, 8, 4853. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Rajamohan, T. The Role of Coconut Water on Nicotine-Induced Reproductive Dysfunction in Experimental Male Rat Model. Food Nutr. Sci. 2014, 5, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Holdcraft, R.W.; Shima, J.E.; Griswold, M.D.; Braun, R.E. Androgens regulate the permeability of the blood–testis barrier. Proc. Natl. Acad. Sci. USA 2005, 102, 16696–16700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, L.; Smith, L.B. Androgen receptor roles in spermatogenesis and infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Wahab, O.A.; Princely, A.C.; Oluwadamilare, A.A.; Oore-oluwapo, D.O.; Blessing, A.O.; Alfred, E.F. Clomiphene citrate ameliorated lead acetate-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2019, 23, 336. [Google Scholar]
- Abd-Elhakim, Y.M.; Ghoneim, M.H.; Ebraheim, L.L.; Imam, T.S. Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rats via modulating oxidative stress and inflammation. Life Sci. 2020, 254, 117782. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Hussein, M.M.; Saber, T.; Abd-Elhakim, Y.M. Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats. Int. J. Environ. Res. Public Health 2022, 19, 8171. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Simple, G.; Onuoha, C.S. Morphometric Evaluation of the Seminiferous Tubules and the Antioxidant Protective Effects of Gallic Acid and Quercetin in the Testis and Liver of Butyl Phthalate Treated Rats. Indian J. Clin. Biochem. 2020, 35, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saber, T.M.; Abd El-Aziz, R.M.; Ali, H.A. Quercetin mitigates fenitrothion-induced testicular toxicity in rats. Andrologia 2016, 48, 491–500. [Google Scholar] [CrossRef]
- Kanter, M.; Aktoz, T.; Aktas, C.; Ozen, F.; Yarali, O.; Kanter, B. Role of Quercetin in Cadmium-Induced Oxidative Stress, Testicular Damage, and Apoptosis in Rats. Anal. Quant. Cytopathol. Histpathol. 2016, 38, 45–51. [Google Scholar]
- Bharti, S.; Misro, M.M.; Rai, U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 2014, 383, 10–20. [Google Scholar] [CrossRef]
- Zaidi, R.; Ullah Khan, S.; Farooqi, I.H.; Azam, A. Rapid adsorption of Pb (II) and Cr (VI) from aqueous solution by Aluminum hydroxide nanoparticles: Equilibrium and kinetic evaluation. Mater. Today Proc. 2021, 47, 1430–1437. [Google Scholar] [CrossRef]
- Siahpoosh, S.M.; Salahi, E.; Hessari, F.A.; MOBASHERPOUR, I. Facile Preparation of Mesoporous γ-Alumina Nanoparticles with High-Surface-Area via Sol-Gel Method and their Efficiency for the Removal of Lead from Aqueous Solution. Bulletin de la Société Royale des Sciences de Liège 2016, 85, 890–911. [Google Scholar] [CrossRef]
- Saadi, Z.; Saadi, R.; Fazaeli, R. Fixed-bed adsorption dynamics of Pb (II) adsorption from aqueous solution using nanostructured γ-alumina. J. Nanostruct. Chem. 2013, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Mushak, P. Chapter 3—Lead in the Human Environment: Production, Uses, Trends. In Trace Metals and other Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2011; Volume 10, pp. 41–72. [Google Scholar]
- Stanley, J.K.; Coleman, J.G.; Weiss Jr, C.A.; Steevens, J.A. Sediment toxicity and bioaccumulation of nano and micron-sized aluminum oxide. Environ. Toxicol. Chem. 2010, 29, 422–429. [Google Scholar] [CrossRef]
- Załęska-Radziwiłł, M.; Doskocz, N.; Affek, K.; Muszyński, A. Effect of aluminum oxide nanoparticles on aquatic organisms–A microcosm study. Desalin. Water Treat. 2020, 195, 286–296. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, P.; Liu, T. Literature analysis on present status of occupational lead hazards in lead-acid battery manufacture industry. J. Environ. Occup. Med. 2010, 27, 641–644. [Google Scholar]
- Shumakova, A.A.; Trushina, E.N.; Mustafina, O.K.; Soto, S.; Gmoshinsky, I.V.; Khotimchenko, S.A. Lead toxicity in its joint administration with the aluminium oxide nanoparticles to rats. Vopr. Pitan. 2015, 84, 40–49. [Google Scholar] [PubMed]
- Güleş, Ö.; Kum, Ş.; Yıldız, M. Protective effect of coenzyme Q10 against bisphenol-A-induced toxicity in the rat testes. Toxicol. Ind. Health 2019, 35, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Usman, U.Z.; Ofutet, E.O.; Owu, D.U. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride—induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem. Toxicol. 2017, 102, 143–155. [Google Scholar] [CrossRef]
- Adler, I.-D. Comparison of the duration of spermatogenesis between male rodents and humans. Mutat. Res./Fundam. Mol. Mech. Mutagenes. 1996, 352, 169–172. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Abubakar, K.; Chiroma, S.M.; Danmaigoro, A.; Rahim, E.B.A.; Mohd Moklas, M.A.; Zakaria, Z.A.B. Curcumin-loaded cockle shell-derived calcium carbonate nanoparticles: A novel strategy for the treatment of lead-induced hepato-renal toxicity in rats. Saudi J. Biol. Sci. 2020, 27, 1538–1552. [Google Scholar] [CrossRef]
- Ayuba, Y.; Ekanem, A.G.S.H. Effect of Oral Administration of Lead Acetate Exposure on the Histology of the Testis and Testicular Sperm Concentration in Wistar Albino Rats. Sch. J. Appl. Med. Sci. 2017, 1, 2337–2344. [Google Scholar]
- Sudjarwo, S.A.; Sudjarwo, G.W. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017, 12, 381. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kulkarni, V.H.; Habbu, P.V.; Chakraborty, M. Detrimental effects of lead on human health and protective effect by natural polyphenols: A review. Int. Res. J. Pharm. 2018, 9, 4–13. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Husain, S. NeurohistologicalEffects of Lead on Cerebellum of Adult Albimo Rat Dr SM Dawar Husain Medical Science. Int. J. Sci. Res. 2015, 4, 2277–8179. [Google Scholar]
- Kim, J.; Lee, Y.; Yang, M. Environmental exposure to lead (Pb) and variations in its susceptibility. J. Environ. Sci. Health Part C 2014, 32, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; Yabe, J.; Makumba, J.; Schutzmeier, P.; Ericson, B.; Caravanos, J. Lead intoxicated children in Kabwe, Zambia. Environ. Res. 2018, 165, 420–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalili, P.; Huet, S.; Lanceleur, R.; Jarry, G.; Hegarat, L.L.; Nesslany, F.; Hogeveen, K.; Fessard, V. Genotoxicity of aluminum and aluminum oxide nanomaterials in rats following oral exposure. Nanomaterials 2020, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- El-Borai, N.B.; Elkhadrawey, B.A.; AbuBakr, H.O.; Anis, A.; El-Bialy, B.E.; Elsabbagh, H.S.; Abou-Zeid, S.M. Sesamol protects against aluminum oxide nanoparticles-induced hepatorenal toxicity in rats via modulation of oxidative stress, inflammation, apoptosis, and DNA damage. Environ. Toxicol. 2022, 37, 1914–1924. [Google Scholar] [CrossRef]
- Jo, E.; Seo, G.-B.; Kim, H.; Choi, K.; Kwon, J.-T.; Kim, P.; Eom, I. Toxic effects of alumina nanoparticles in rat cerebrums and kidneys. J. Environ. Health Sci. 2016, 42, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Oyewopo, A.O.; Adeleke, O.; Johnson, O.; Akingbade, A.; Olaniyi, K.S.; Areola, E.D.; Tokunbo, O. Regulatory effects of quercetin on testicular histopathology induced by cyanide in Wistar rats. Heliyon 2021, 7, e07662. [Google Scholar] [CrossRef]
- Osawe, S.O.; Farombi, E.O. Quercetin and rutin ameliorates sulphasalazine-induced spermiotoxicity, alterations in reproductive hormones and steroidogenic enzyme imbalance in rats. Andrologia 2018, 50, e12981. [Google Scholar] [CrossRef]
- Alanbaki, A.A.; AL-Mayali, H.M.; AL-Mayali, H.K. Ameliorative effect of quercetin and hesperidin on antioxidant and histological changes in the testis of etoposide-induced adult male rats. Res. J. Pharm. Technol. 2018, 11, 564–574. [Google Scholar] [CrossRef]
- Kempinas, W.G.; Lamano-Carvalho, T.L. A method for estimating the concentration of spermatozoa in the rat cauda epididymidis. Lab. Anim. 1988, 22, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Slott, V.L.; Suarez, J.D.; Perreault, S.D. Rat sperm motility analysis: Methodologic considerations. Reprod. Toxicol. 1991, 5, 449–458. [Google Scholar] [CrossRef]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filler, R. Methods for evaluation of rat epididymal sperm morphology. Methods Toxicol. 1993, 3, 334–343. [Google Scholar]
- Zirkin, B.R.; Chen, H. Regulation of Leydig cell steroidogenic function during aging. Biol. Reprod. 2000, 63, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Banchroft, J.; Stevens, A.; Turner, D. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Hsu, S.-M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Guckland, A.; Murfitt, R.; Ebeling, M.; Sprenger, D.; Foudoulakis, M.; Koutsaftis, A. Relationship between magnitude of body weight effects and exposure duration in mammalian toxicology studies and implications for ecotoxicological risk assessment. Environ. Sci. Eur. 2019, 31, 38. [Google Scholar] [CrossRef]
- Ali, S.; Al-Derawi, K.H.; Almansour, N.; Monsour, A. Testicular toxic effect of lead acetate on adult male rats and the potential protective role of alcoholic extract of ginseng (histological, histomorphometrical and physiological). Sci. J. Med. Res. 2018, 2, 87–92. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Adewumi, I. Biochemical Evaluation of Silver Nanoparticles in Wistar Rats. Int. Sch. Res. Not. 2014, 2014, 196091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafabadi, R.E.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Quercetin Prevents Body Weight Loss Due to the Using of Superparamagnetic Iron Oxide Nanoparticles in Rat. Adv. Biomed. Res. 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.-P.; Bernard, J.R.; Hsu, H.-C.; Hsu, C.-L.; Liao, S.-F.; Cheng, I.-S. Short-Term Oral Quercetin Supplementation Improves Post-exercise Insulin Sensitivity, Antioxidant Capacity and Enhances Subsequent Cycling Time to Exhaustion in Healthy Adults: A Pilot Study. Front. Nutr. 2022, 9, 872. [Google Scholar] [CrossRef]
- Santos, M.R.; Mira, L. Protection by flavonoids against the peroxynitrite-mediated oxidation of dihydrorhodamine. Free. Radic. Res. 2004, 38, 1011–1018. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Mitochondria in exercise-induced oxidative stress. Neurosignals 2001, 10, 125–140. [Google Scholar] [CrossRef]
- Reichmann, D.; Voth, W.; Jakob, U. Maintaining a Healthy Proteome during Oxidative Stress. Mol. Cell 2018, 69, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Hasanein, P.; Fazeli, F.; Parviz, M.; Roghani, M. Ferulic acid prevents lead-induced testicular oxidative stress and suppressed spermatogenesis in rats. Andrologia 2018, 50, e12798. [Google Scholar] [CrossRef]
- Ibrahim, N.M.; Eweis, E.A.; El-Beltagi, H.S.; Abdel-Mobdy, Y.E. Retraction note to: The effect of lead acetate toxicity on experimental male albino rat. Biol. Trace Elem. Res. 2013, 151, 156. [Google Scholar] [CrossRef] [Green Version]
- Khorsandi, L.; Orazizadeh, M.; Moradi-Gharibvand, N.; Hemadi, M.; Mansouri, E. Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environ. Sci. Pollut. Res. 2017, 24, 5595–5606. [Google Scholar] [CrossRef] [PubMed]
- Nateghian¹, Z.; Aliabadi, E. Aspects of environmental pollutants on male fertility and sperm parameters. J. Environ. Treat. Tech. 2020, 8, 299–309. [Google Scholar]
- Algefare, A.; Sedky, A.M.; Alfwuaires, M. Apigenin Ameliorates Lead Acetate induced Hyperlipidemia, Hypothyroidism and Hypogonadism in Male Rats. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offor, S.J.; Mbagwu, H.O.; Orisakwe, O.E. Improvement of Lead Acetate-Induced Testicular Injury and Sperm Quality Deterioration by Solanum Anomalum Thonn. Ex. Schumach Fruit Extracts in Albino Rats. J. Fam. Reprod. Health 2019, 13, 98–108. [Google Scholar]
- Dhurvey, V.; Gotmare, B.; Karim, F. Lead Acetate induced Histological alterations in Seminal-vesicle and Prostate Gland of Wistar rats. J. Indian Soc. Toxicol. 2018, 14, 26–29. [Google Scholar] [CrossRef]
- Ricardo, L.H.J. Male accessory glands and sperm function. In Spermatozoa: Facts and Perspectives; IntechOpen: Cambridge, UK, 2018; pp. 101–116. [Google Scholar]
- Oyeyemi, W.A.; Akinola, A.O.; Daramola, O.-O.O.; Aikpitanyi, I.; Durotoluwa, O.T.; Alele, P.-G.O.; Ogieriakhi, I.O.; Okoro, T.D. Vitamin E and quercetin attenuated the reproductive toxicity mediated by lead acetate in male Wistar. Bull. Natl. Res. Cent. 2022, 46, 22. [Google Scholar] [CrossRef]
- Yelumalai, S.; Giribabu, N.; Karim, K.; Omar, S.Z.; Salleh, N.B. In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Arch. Med. Sci. 2019, 15, 240–249. [Google Scholar] [CrossRef]
- Taepongsorat, L.; Tangpraprutgul, P.; Kitana, N.; Malaivijitnond, S. Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J. Androl. 2008, 10, 249–258. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, F.M.; Mahgoub, H.A.; Ateya, A.I. Ameliorative effect of curcumin against lead acetate-induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 10950–10965. [Google Scholar] [CrossRef] [PubMed]
- Cavallin, M.D.; Wilk, R.; Oliveira, I.M.; Cardoso, N.C.S.; Khalil, N.M.; Oliveira, C.A.; Romano, M.A.; Romano, R.M. The hypothalamic–pituitary–testicular axis and the testicular function are modulated after silver nanoparticle exposure. Toxicol. Res. 2017, 7, 102–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdieh, Y.; Shariat, S.; Mahmoudreza, G.; Mahsa, S.; Negin, H.; Andishe, K.; Modaresi, M. The effects of titanium dioxide nanoparticles on pituitary-gonad axis in male mice. J. Chem. Pharm. Res. 2015, 7, 720–723. [Google Scholar]
- Lamia, H.; Mounir, T.; Adel, C.; Abdelhamid, K.; Najjar, M.; Rachid, S. Effects of oral intoxication by lead acetate on pituitary-testicular axis in the pubertal rat. Ital. J. Public Health 2008, 5, 297–303. [Google Scholar] [CrossRef]
- Sokol, R.Z.; Wang, S.; Wan, Y.J.; Stanczyk, F.Z.; Gentzschein, E.; Chapin, R.E. Long-term, low-dose lead exposure alters the gonadotropin-releasing hormone system in the male rat. Environ. Health Perspect. 2002, 110, 871–874. [Google Scholar] [CrossRef] [Green Version]
- Bin-Jaliah, I. Quercetin Inhibits ROS-p53-Bax-caspase-3 Axis of Apoptosis and Augments Gonadotropin and Testicular Hormones in Chronic Unpredictable Stress-Induced Testis Injury. Int. J. Morphol. 2021, 39, 839–847. [Google Scholar] [CrossRef]
- Sharma, P.; Aslam Khan, I.; Singh, R. Curcumin and Quercetin Ameliorated Cypermethrin and Deltamethrin-Induced Reproductive System Impairment in Male Wistar Rats by Upregulating The Activity of Pituitary-Gonadal Hormones and Steroidogenic Enzymes. Int. J. Fertil. Steril. 2018, 12, 72–80. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006, 11, 114–127. [Google Scholar]
- Alshatwi, A.A.; Subbarayan, P.V.; Ramesh, E.; Al-Hazzani, A.A.; Alsaif, M.A.; Alwarthan, A.A. Aluminium oxide nanoparticles induce mitochondrial-mediated oxidative stress and alter the expression of antioxidant enzymes in human mesenchymal stem cells. Food Addit. Contam. Part A 2013, 30, 1–10. [Google Scholar] [CrossRef]
- Srikanth, K.; Mahajan, A.; Pereira, E.; Duarte, A.C.; Venkateswara Rao, J. Aluminium oxide nanoparticles induced morphological changes, cytotoxicity and oxidative stress in Chinook salmon (CHSE-214) cells. J. Appl. Toxicol. 2015, 35, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid.-Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Libová, Ľ.; Lukáč, N. Protective effects of quercetin on selected oxidative biomarkers in bovine spermatozoa subjected to ferrous ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- H.M., O.; Hassan, K.A.; S.Kh, A.-E.; E.A., A. Aluminium toxicity in rats: The role of tannic acid as antioxidant. Ass. Univ. Bull. Environ. Res. 2003, 6, 1–14. [Google Scholar]
- Yokel, R.A.; Sjögren, B. Chapter 1—Aluminum. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–22. [Google Scholar]
- Sudjarwo, S.A.; Eraiko, K.; Sudjarwo, G.W.; Koerniasari. Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2017, 20, 1227–1231. [Google Scholar] [CrossRef]
- Wills, M.R.; Savory, J. Aluminum and chronic renal failure: Sources, absorption, transport, and toxicity. Crit. Rev. Clin. Lab. Sci. 1989, 27, 59–107. [Google Scholar] [CrossRef]
- Nesse, A.; Garbossa, G.; Stripeikis, J.; Gálvez, G.; Castro, M.E.; Rizzo, N.; Lauricella, A.M.; Gutnisky, A. Aluminium accumulation in chronic renal failure affects erythropoiesis. Nephrology 1997, 3, 347–351. [Google Scholar] [CrossRef]
- Loughran, D.; Calello, D.; Nelson, L. Treatment of acute aluminum toxicity due to alum bladder irrigation in a hemodialysis patient: A case report. Toxicol. Commun. 2022, 6, 35–38. [Google Scholar] [CrossRef]
- Di Virgilio, A.L.; Reigosa, M.; Arnal, P.M.; Fernández Lorenzo de Mele, M. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminium oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. J. Hazard Mater. 2010, 177, 711–718. [Google Scholar] [CrossRef]
- Matos, B.; Publicover, S.J.; Castro, L.F.C.; Esteves, P.J.; Fardilha, M. Brain and testis: More alike than previously thought? Open Biol. 2021, 11, 200322. [Google Scholar] [CrossRef] [PubMed]
- Arden, R.; Gottfredson, L.S.; Miller, G.; Pierce, A. Intelligence and semen quality are positively correlated. Intelligence 2009, 37, 277–282. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef]
- Apostoli, P.; Porru, S.; Bisanti, L. Critical aspects of male fertility in the assessment of exposure to lead. Scand. J. Work. Environ. Health 1999, 25 (Suppl. 1), 40–43. [Google Scholar]
- Dolati, P.; Khodabandeh, Z.; Zamiri, M.J.; Jamhiri, I.; Mehrabani, D. The effect of lead acetate and quercetin on the tight and gap junctions in the mouse testis. Biol. Trace Elem. Res. 2020, 198, 535–543. [Google Scholar] [CrossRef]
- Anjum, M.R.; Reddy, P.S. Recovery of lead-induced suppressed reproduction in male rats by testosterone. Andrologia 2015, 47, 560–567. [Google Scholar] [CrossRef]
- Cornard, J.; Dangleterre, L.; Lapouge, C. Computational and spectroscopic characterization of the molecular and electronic structure of the Pb (II)− Quercetin complex. J. Phys. Chem. A 2005, 109, 10044–10051. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, M.; Jiang, Y.; Zhang, M.; Wang, C.; Wang, X.; Yu, G.; Tang, Z. Beneficial Effects of Quercetin on Microcystin-LR Induced Tight Junction Defects. Front. Pharmacol. 2021, 12, 733993. [Google Scholar] [CrossRef]
- Guo, C. Chapter 189—Testis: Anatomy and histology. In Uropathology, 2nd ed.; Zhou, M., Netto, G.J., Epstein, J.I., Eds.; Elsevier: St. Louis, MO, USA, 2023; pp. 427–429. [Google Scholar]
- Noda, T.; Ikawa, M. Physiological function of seminal vesicle secretions on male fecundity. Reprod. Med. Biol. 2019, 18, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.L.; Majumder, P.K. Prostate gland: Structure, functions and regulation. Int. Urol. Nephrol. 1995, 27, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Abdrabou, M.I.; Elleithy, E.M.M.; Yasin, N.A.E.; Shaheen, Y.M.; Galal, M. Ameliorative effects of Spirulina maxima and Allium sativum on lead acetate-induced testicular injury in male albino rats with respect to caspase-3 gene expression. Acta Histochem. 2019, 121, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Aldaddou, W.A.; Aljohani, A.S.; Ahmed, I.A.; Al-Wabel, N.A.; El-Ashmawy, I.M. Ameliorative effect of methanolic extract of Tribulus terrestris L. on nicotine and lead-induced degeneration of sperm quality in male rats. J. Ethnopharmacol. 2022, 295, 115337. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.M.; Abd El-Hamid, M.I.; Noshy, P.A. Reproductive toxicity investigation of titanium dioxide nanoparticles in male albino rats. World J. Pharm. Pharmaceut. Sci. 2015, 4, 34–49. [Google Scholar]
- Shahed, A.R.; Shoskes, D.A. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: Correlation with gram positive bacterial growth and treatment response. J. Androl. 2000, 21, 669–675. [Google Scholar] [PubMed]
- Shahed, A.R.; Shoskes, D.A. Correlation of beta-endorphin and prostaglandin E2 levels in prostatic fluid of patients with chronic prostatitis with diagnosis and treatment response. J. Urol. 2001, 166, 1738–1741. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Meng, L.Q.; Yang, F.Y.; Wang, M.S.; Shi, B.K.; Chen, D.X.; Chen, D.; Zhou, Q.; He, Q.B.; Ma, L.X.; Cheng, W.L.; et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate 2018, 78, 790–800. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Men, Q.-L.; Meng, X.-G.; Fang, X.-X.; Tao, M.-Z. Chronic Toxic Effect of Lead on Male Testis Tissue in Adult Pelophylax nigromaculata. Nat. Environ. Pollut. Technol. 2017, 16, 213–218. [Google Scholar]
- Ibrahim, I.A.; Shalaby, A.A.; Abd Elaziz, R.T.; Bahr, H.I. Chlorella vulgaris or Spirulina platensis mitigate lead acetate-induced testicular oxidative stress and apoptosis with regard to androgen receptor expression in rats. Environ. Sci. Pollut. Res. 2021, 28, 39126–39138. [Google Scholar] [CrossRef] [PubMed]
- Doumouchtsis, K.K.; Doumouchtsis, S.K.; Doumouchtsis, E.K.; Perrea, D.N. The effect of lead intoxication on endocrine functions. J. Endocrinol. Investig. 2009, 32, 175–183. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Pant, A.B.; Farombi, E.O. Effects of quercetin on mRNA expression of steroidogenesis genes in primary cultures of Leydig cells treated with atrazine. Toxicol. In Vitro 2013, 27, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Nagpal, M.L.; Stocco, D.M.; Lin, T. Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. J. Endocrinol. 2007, 192, 527–537. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, D.M.; Ezzat, S.M.; Salama, M.M.; Mahrous, E.A.; Attia, Y.M.; Ahmed, M.S.; Elmazar, M.M. Anti-estrogenic and anti-aromatase activities of citrus peels major compounds in breast cancer. Sci. Rep. 2021, 11, 7121. [Google Scholar] [CrossRef]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of Inflammation on Male Reproductive Tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar]
- El-Khadragy, M.; Al-Megrin, W.A.; AlSadhan, N.A.; Metwally, D.M.; El-Hennamy, R.E.; Salem, F.E.H.; Kassab, R.B.; Abdel Moneim, A.E. Impact of Coenzyme Q10 Administration on Lead Acetate-Induced Testicular Damage in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 4981386. [Google Scholar] [CrossRef]
- Khan, H.A.; Abdelhalim, M.A.K.; Alhomida, A.S.; Al-Ayed, M.S. Effects of Naked Gold Nanoparticles on Proinflammatory Cytokines mRNA Expression in Rat Liver and Kidney. BioMed Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Shi, T.; Duffin, R.; Albrecht, C.; van Berlo, D.; Höhr, D.; Fubini, B.; Martra, G.; Fenoglio, I.; Borm, P.J. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007, 222, 141–151. [Google Scholar] [CrossRef]
- Val, S.; Hussain, S.; Boland, S.; Hamel, R.; Baeza-Squiban, A.; Marano, F. Carbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial epithelial cells: Need for multiparametric evaluation due to adsorption artifacts. Inhal. Toxicol. 2009, 21, 115–122. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef] [PubMed]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [PubMed]






| Estimated Parameters | Experimental Groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | CO | QTN | Al2O3NPs | Pb Ac | Al2O3NPs + Pb Ac | Al2O3NPs + Pb Ac + QTN | |
| Initial Body weight (g) | 182.00 ± 0.71 | 181.33 ± 3.79 | 182.00 ± 2.55 | 186.67 ± 3.70 | 185.33 ± 1.89 | 188.33 ± 3.01 | 189.33 ± 2.05 |
| Final body weight (g) | 250.67 b ± 10.02 | 245.00 b ± 14.71 | 273.33 a ± 15.74 | 231.67 bc ± 1.65 | 202.33 c ± 7.10 | 208.00 c ± 8.64 | 270.67 a ± 14.27 |
| Body weight change (g) | 68.67 ab ± 10.73 | 63.00 ab ± 12.90 | 86.67 a ± 16.41 | 45.00 bc ± 2.94 | 17.00 c ± 6.75 | 19.67 c ± 7.55 | 81.33 a ± 14.06 |
| Testes weight (g) | 2.03 a ± 0.08 | 1.92 a ± 0.07 | 2.19 a ± 0.02 | 1.40 b ± 0.12 | 1.20 b ± 0.11 | 1.10 b ± 0.11 | 2.15 a ± 0.20 |
| Gonadosomatic index (%) | 0.82 a ± 0.05 | 0.80 a ± 0.08 | 0.81 a ± 0.05 | 0.61 b ± 0.06 | 0.60 b ± 0.07 | 0.52 b ± 0.03 | 0.80 a ± 0.07 |
| Sperm count (sp.cc/mL × 125 × 104) | 75.00 ± 3.46 bc | 76.00 b ± 1.53 | 82.00 a ± 2.08 | 61.67 d ± 1.20 | 54.33 e ± 0.88 | 40.3 f ± 0.88 | 69.67 c ± 1.76 |
| Sperm abnormalities (%) | 16.66 d ± 1.09 | 17.50 d ± 0.58 | 13.16 d ± 1.59 | 40.50 b ± 2.08 | 43.33 ab ± 2.40 | 50.00 a ± 5.20 | 32.50 c ± 1.60 |
| Sperm motility (%) | 88.67 b ± 0.88 | 85.33 b ± 0.33 | 94.33 a ± 1.20 | 50.00 d ± 2.89 | 33.33 e ± 1.67 | 16.67 f ± 0.88 | 63.33 c ± 1.67 |
| Estimated Parameters | Experimental Groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | CO | QTN | Al2O3NPs | Pb Ac | Al2O3NPs + Pb Ac | Al2O3NPs + Pb Ac + QTN | |
| Testosterone (pg/mL) | 0.16 bc ± 0.02 | 0.18 b ± 0.00 | 0.27 a ± 0.06 | 0.10 bc ± 0.00 | 0.09 c ± 0.00 | 0.01 d ± 0.00 | 0.15 bc ± 0.02 |
| Estradiol (mIU/mL) | 30.90 c ± 1.09 | 31.50 c ± 0.67 | 19.13 d ± 2.02 | 38.43 b ± 1.13 | 40.63 b ± 3.28 | 46.67 a ± 1.64 | 26.97 c ± 2.27 |
| LH (mIU/mL) | 2.50 ab ± 0.11 | 2.53 ab ± 0.10 | 3.23 a ± 0.66 | 1.87 bc ± 0.09 | 1.33 c ± 0.13 | 1.20 c ± 0.11 | 2.30 b ± 0.11 |
| FSH (mIU/mL) | 4.03 bc ± 0.47 | 4.20 b ± 0.66 | 5.37 a ± 0.42 | 2.93 cd ± 0.28 | 2.93 cd ± 0.06 | 2.00 d ± 0.07 | 3.23 bc ± 0.06 |
| SOD (IU/g. protein) | 44.99 b ± 1.31 | 42.42 b ± 2.33 | 74.58 a ± 3.84 | 31.63 c ± 1.31 | 17.46 d ± 0.48 | 15.71 d ± 0.87 | 42.67 b ± 1.28 |
| GPx-like activity (IU/g. protein) | 66.25 b ± 1.31 | 64.37 b ± 2.64 | 94.26 a ± 2.13 | 53.47 c ± 2.06 | 52.69 c ± 2.25 | 36.65 d ± 1.60 | 62.59 b ± 3.60 |
| MDA (nmol/g. protein) | 113.74 d ± 0.98 | 115.08 cd ± 2.31 | 91.58 e ± 0.88 | 128.08 b ± 0.91 | 131.08 ab ± 2.08 | 136.08 a ± 3.35 | 120.09 c ± 1.49 |
| Pb residues (ppm) | ND | ND | ND | 0.08 d ± 0.00 | 60.75 b ± 0.01 | 0.93 a ± 0.0 | 0.57 c ± 0.01 |
| Al residues (ppm) | 8.13 e ± 0.76 | 6.90 e ± 0.56 | 5.0 e ± 0.13 | 64.05 b ± 1.11 | 25.90 c ± 0.33 | 138.50 a ± 2.29 | 19.60 d ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behairy, A.; Hashem, M.M.; Abo-El-Sooud, K.; El-Metwally, A.E.; Hassan, B.A.; Abd-Elhakim, Y.M. Quercetin Abates Aluminum Trioxide Nanoparticles and Lead Acetate Induced Altered Sperm Quality, Testicular Oxidative Damage, and Sexual Hormones Disruption in Male Rats. Antioxidants 2022, 11, 2133. https://doi.org/10.3390/antiox11112133
Behairy A, Hashem MM, Abo-El-Sooud K, El-Metwally AE, Hassan BA, Abd-Elhakim YM. Quercetin Abates Aluminum Trioxide Nanoparticles and Lead Acetate Induced Altered Sperm Quality, Testicular Oxidative Damage, and Sexual Hormones Disruption in Male Rats. Antioxidants. 2022; 11(11):2133. https://doi.org/10.3390/antiox11112133
Chicago/Turabian StyleBehairy, Amany, Mohamed M. Hashem, Khaled Abo-El-Sooud, Abeer E. El-Metwally, Bayan A. Hassan, and Yasmina M. Abd-Elhakim. 2022. "Quercetin Abates Aluminum Trioxide Nanoparticles and Lead Acetate Induced Altered Sperm Quality, Testicular Oxidative Damage, and Sexual Hormones Disruption in Male Rats" Antioxidants 11, no. 11: 2133. https://doi.org/10.3390/antiox11112133
APA StyleBehairy, A., Hashem, M. M., Abo-El-Sooud, K., El-Metwally, A. E., Hassan, B. A., & Abd-Elhakim, Y. M. (2022). Quercetin Abates Aluminum Trioxide Nanoparticles and Lead Acetate Induced Altered Sperm Quality, Testicular Oxidative Damage, and Sexual Hormones Disruption in Male Rats. Antioxidants, 11(11), 2133. https://doi.org/10.3390/antiox11112133