Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin
Abstract
:1. Introduction
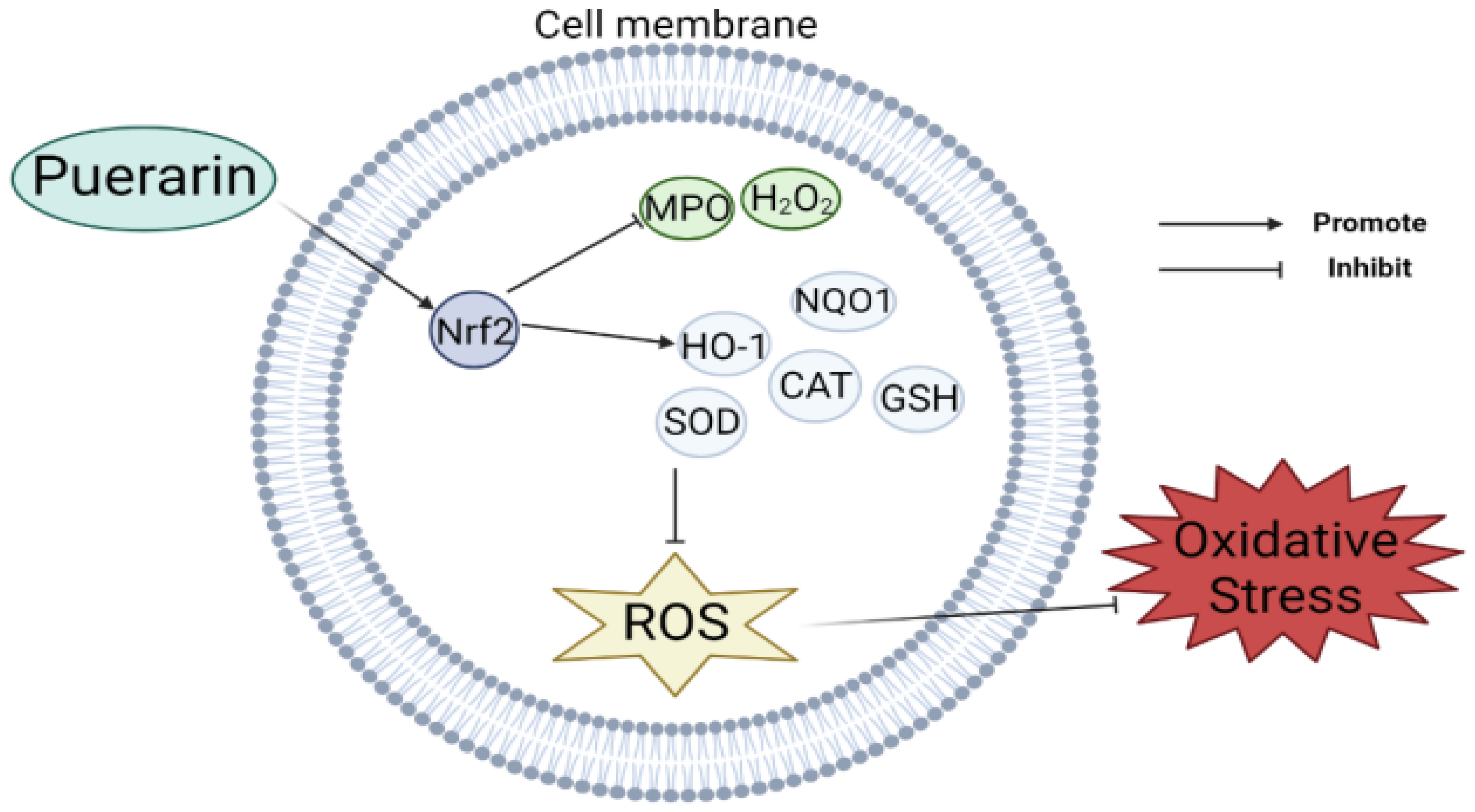
2. Antioxidant
3. The Role of Puerarin in Inflammatory Diseases
3.1. Liver Disease
3.1.1. Hepatitis
3.1.2. Liver Fibrosis
3.1.3. Non-Alcoholic Fatty Liver Disease
3.2. Joint Disease
3.2.1. Osteoarthritis (OA)
3.2.2. Total Joint Arthroplasty
3.2.3. Osteoporosis
3.3. Respiratory Diseases
3.3.1. Lung Injury
3.3.2. Chronic Obstructive Pulmonary Disease
3.3.3. Viral Pneumonia
3.4. Nervous System Diseases
3.4.1. Alzheimer’s Disease
3.4.2. Depression
3.4.3. Stroke
3.4.4. Intracerebral Hemorrhage
3.4.5. Neuropathic Pain
3.4.6. Vascular Dementia
3.5. Cardiovascular Diseases
3.5.1. Ischemic Heart Disease (IHD)
3.5.2. Heart Failure
3.5.3. Atherosclerosis
3.5.4. Hypertension
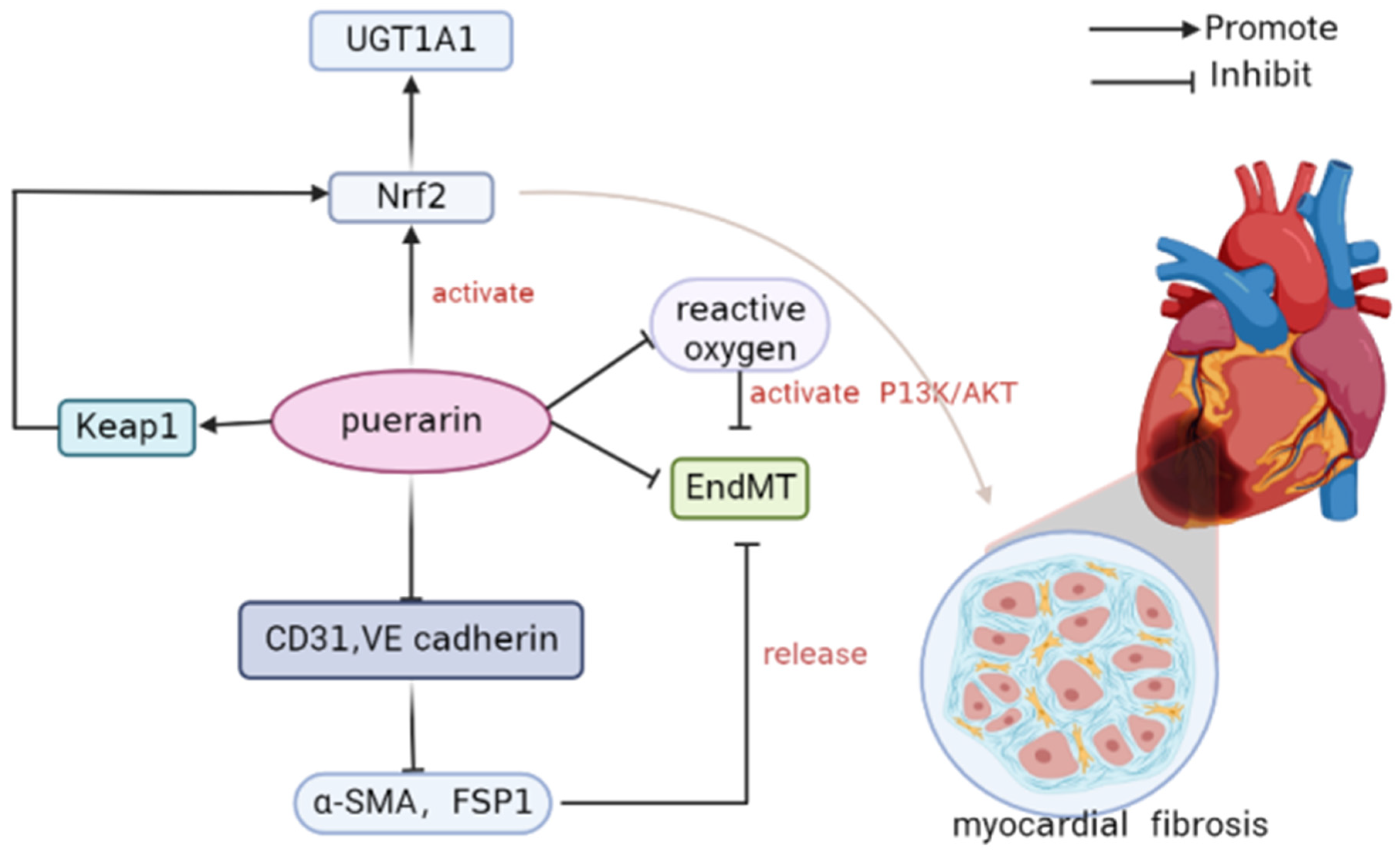
3.5.5. Myocardial Fibrosis
3.5.6. Radiation-Induced Cardiovascular Disease
3.5.7. Diabetic Cardiomyopathy
3.5.8. Cardiotoxicity
3.6. Kidney Diseases
3.6.1. Chronic Kidney Disease
3.6.2. Renal Fibrosis
3.7. Inflammatory Gastrointestinal Diseases
3.8. Diabetes
3.9. Other Inflammatory Diseases
3.10. Regulation of Puerarin on Immune Cells
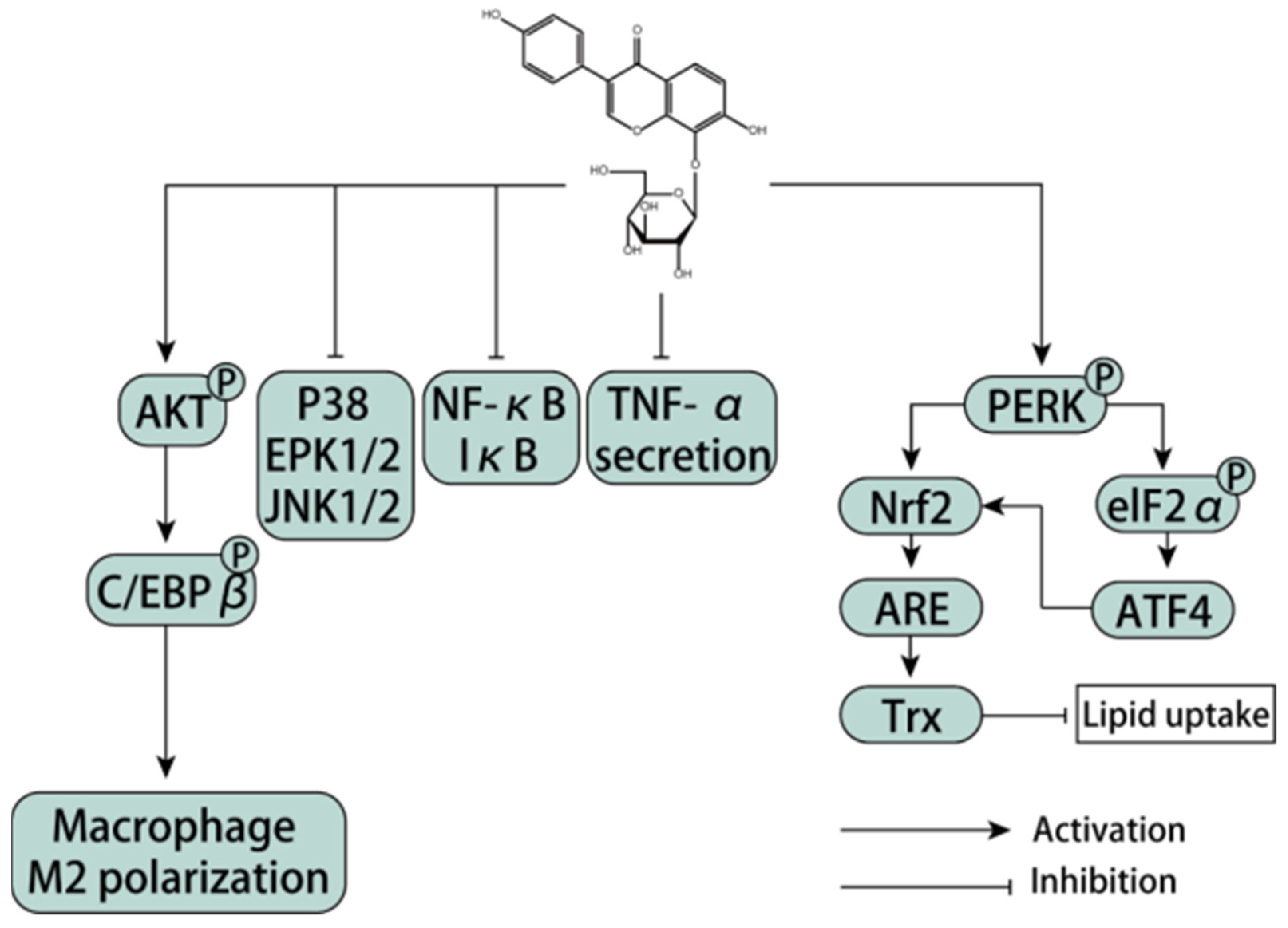
3.10.1. Regulation of Macrophage Polarization
3.10.2. Regulation of Lymphocytes
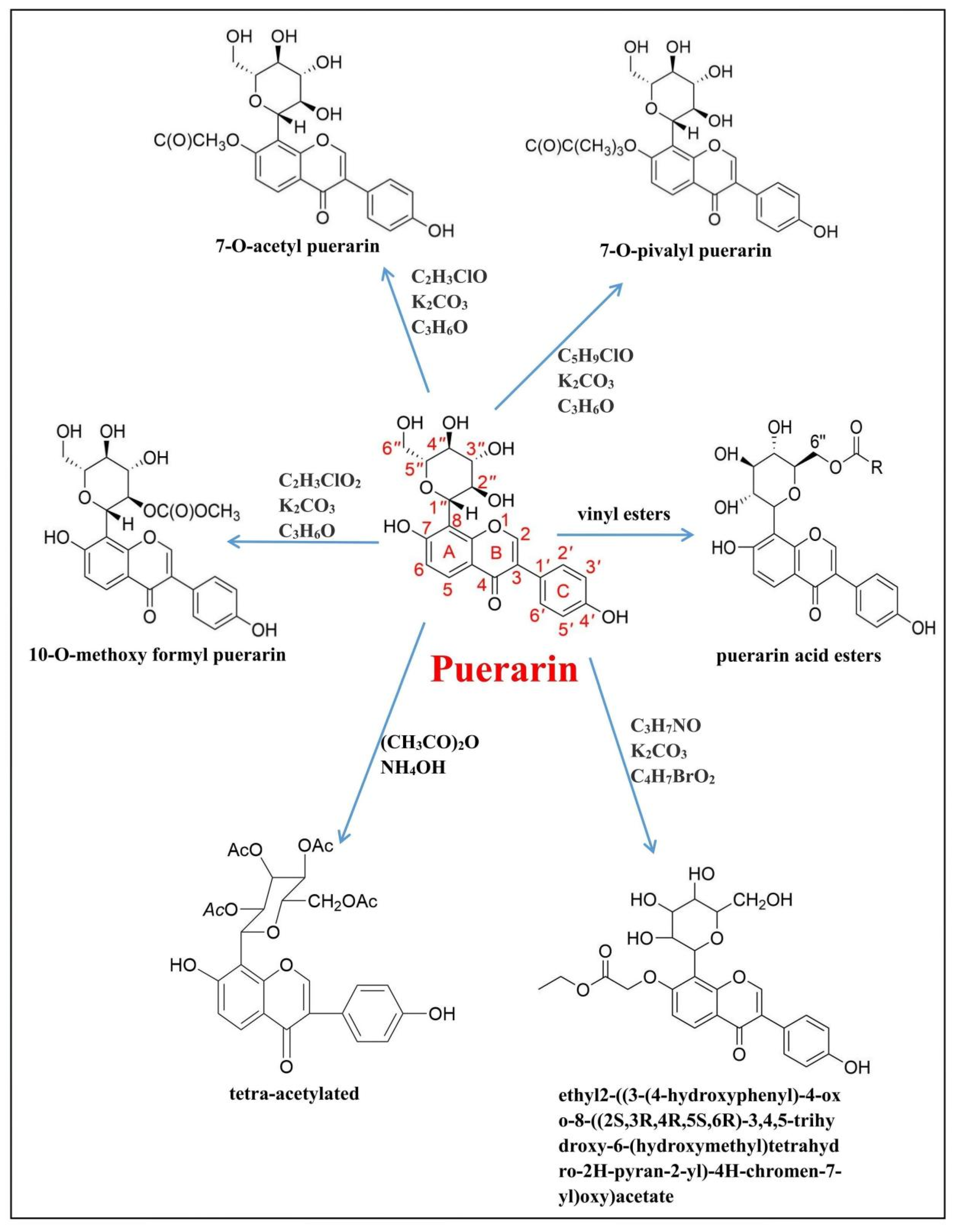
4. Puerarin and Its Derivatives
5. Pharmacokinetics of Puerarin
6. A New Drug Delivery System for Puerarin
7. Clinical Trial of Puerarin
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Ge, S.; Wang, Y.; Liu, Y.; Qiu, L.; Li, J.; Huang, X.; Sun, L. Puerarin Alleviates UUO-Induced Inflammation and Fibrosis by Regulating the NF-κB P65/STAT3 and TGFβ1/Smads Signaling Pathways. Drug Des. Devel. Ther. 2021, 15, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, X.; Yao, Y.; Meng, Q.; Wu, X.; Wang, H.; Xue, J. Puerarin: A Potential Therapeutic for Colon Adenocarcinoma (COAD) Patients Suffering From SARS-CoV-2 Infection. Front. Pharmacol. 2022, 13, 921517. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Lee, D.S.; Lee, J.K.; Kim, Y.C.; Cho, H.K.; Kim, S.Y. Protective activity of kudzu (Pueraria thunbergiana) vine on chemically-induced hepatotoxicity: In vitro and in vivo studies. BMC Complement. Altern. Med. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Gao, F.; Yang, L.; Xu, L.; Wang, Z.; Ye, H. Biotransformation of puerarin into puerarin-6″-O-phosphate by Bacillus cereus. J. Ind. Microbiol. Biotechnol. 2012, 39, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.Z.; Zhang, P.H.; Fu, Y.; Yu, W.F.; Ju, M.T. Hepatoprotective activity of puerarin against carbon tetrachloride-induced injuries in rats: A randomized controlled trial. Food Chem. Toxicol. 2013, 59, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, Y.D.; Lee, Y.M.; Kim, D.K. The suppressive effect of puerarin on atopic dermatitis-like skin lesions through regulation of inflammatory mediators in vitro and in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 707–714. [Google Scholar] [CrossRef]
- Yang, J.; Wu, M.; Fang, H.; Su, Y.; Zhang, L.; Zhou, H. Puerarin Prevents Acute Liver Injury via Inhibiting Inflammatory Responses and ZEB2 Expression. Front. Pharmacol. 2021, 12, 727916. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Shang, Y.; Chen, K. Radical Scavenging Activity of Puerarin: A Theoretical Study. Antioxidants 2019, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Lee, O.H.; Seo, D.H.; Park, C.S.; Kim, Y.C. Puerarin enhances adipocyte differentiation, adiponectin expression, and antioxidant response in 3T3-L1 cells. Biofactors 2010, 36, 459–467. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, X.; Dong, M. Puerarin suppresses MPP+/MPTP-induced oxidative stress through an Nrf2-dependent mechanism. Food Chem. Toxicol. 2020, 144, 111644. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, B.; Hu, Y.; Chen, J.; Zhang, S.; Chen, D.; Wang, J. Puerarin Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis in Mice by Inhibiting JAKs. J. Pharmacol. Exp. Ther. 2021, 379, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yi, D.; Zhang, Q.; Wu, T.; Yu, K.; Peng, M.; Wang, L.; Zhao, D.; Hou, Y.; Wu, G. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus. Sci. Rep. 2021, 11, 6552. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.Y.; Zhao, Y.R.; Ma, P.; Xu, C.Y.; He, P.; Yang, X.Y.; Zhang, L.; Qiang, G.F.; DU, G.H. Hypoglycemic activity of puerarin through modulation of oxidative stress and mitochondrial function via AMPK. Chin. J. Nat. Med. 2020, 18, 818–826. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Liu, D.; Meng, Q.; Yan, P.; Luo, D.; Wang, X.; Zhou, X. Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control. Oxid. Med. Cell. Longev. 2021, 2021, 6659240. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Hu, Y.; Zhou, C.; Wu, J.; Wu, Y.; Wang, H.; Lenahan, C.; Huang, L.; Nie, S.; et al. Puerarin Attenuates Oxidative Stress and Ferroptosis via AMPK/PGC1α/Nrf2 Pathway after Subarachnoid Hemorrhage in Rats. Antioxidants 2022, 11, 1259. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Lin, A.Q.; Chen, X.F.; Wu, Y.Z.; Zhang, B.; Zhang, Y.; Min, H.Y.; Wen, Y.T.; Song, S.Y.; et al. Puerarin Increases Survival and Protects Against Organ Injury by Suppressing NF-kappa B/JNK Signaling in Experimental Sepsis. Front. Pharmacol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Zheng, J.A.; Huang, Q.; Fang, J.J. Puerarin mitigates acute liver injury in septic rats by regulating proinflammatory factors and oxidative stress levels. Trop. J. Pharm. Res. 2021, 20, 2305–2310. [Google Scholar] [CrossRef]
- Li, L.; Yin, H.Y.; Zhao, Y.; Zhang, X.F.; Duan, C.L.; Liu, J.; Huang, C.X.; Liu, S.H.; Yang, S.Y.; Li, X.J. Protective role of puerarin on LPS/D-Gal induced acute liver injury via restoring autophagy. Am. J. Transl. Res. 2018, 10, 957–965. [Google Scholar]
- Zhou, B.G.; Zhao, H.M.; Lu, X.Y.; Zhou, W.; Liu, F.C.; Liu, X.K.; Liu, D.Y. Effect of Puerarin Regulated mTOR Signaling Pathway in Experimental Liver Injury. Front. Pharmacol. 2018, 9, 1165. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.G.; Fan, F.C.; Zheng, S.H.; Tong, Q.Y. Puerarin Exerts the Hepatoprotection from Chronic Alcohol-Induced Liver Injury via Inhibiting the Cyclooxygenase-2 and the 5-Lipoxygenase Pathway in Rats. Complement. Med. Res. 2021, 28, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Zhu, H.L.; Zhang, C.; Wang, H.W.; Yang, Z.J.; Liu, Z.P. Puerarin protects rat liver and kidney against cadmium-induced oxidative stress. Indian J. Anim. Sci. 2019, 89, 927–931. [Google Scholar]
- Wan, X.M.; Chen, J.; Wang, M.; Zheng, C.; Zhou, X.L. Puerarin attenuates cadmium-induced hepatic lipid metabolism disorder by inhibiting oxidative stress and inflammation in mice. J. Inorg. Biochem. 2021, 222, 111521. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.M.; Wan, X.M.; Xiao, M.; Zheng, C.; Zhou, X.L. Puerarin induces Nrf2 as a cytoprotective mechanism to prevent cadmium-induced autophagy inhibition and NLRP3 inflammasome activation in AML12 hepatic cells. J. Inorg. Biochem. 2021, 217, 111389. [Google Scholar] [CrossRef]
- Wang, L.Y.; Fan, R.F.; Yang, D.B.; Zhang, D.; Wang, L. Puerarin reverses cadmium-induced lysosomal dysfunction in primary rat proximal tubular cells via inhibiting Nrf2 pathway. Biochem. Pharmacol. 2019, 162, 132–141. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Zhang, Y.; Sun, J.; Xie, Y.L.; Yuan, Y.; Gu, J.H.; Bian, J.C.; Liu, Z.P.; Zou, H. Puerarin Restores Autophagosome-Lysosome Fusion to Alleviate Cadmium-Induced Autophagy Blockade via Restoring the Expression of Rab7 in Hepatocytes. Front. Pharmacol. 2021, 12, 632825. [Google Scholar] [CrossRef]
- Wen, S.Q.; Wang, L.; Zou, H.; Gu, J.H.; Song, R.L.; Bian, J.C.; Yuan, Y.; Liu, Z.P. Puerarin Attenuates Cadmium-Induced Neuronal Injury via Stimulating Cadmium Excretion, Inhibiting Oxidative Stress and Apoptosis. Biomolecules 2021, 11, 978. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, H.; Pan, L.J.; Zou, H.O.; Miao, X.N.; Cheng, J.; Wu, Y.S. Puerarin alleviates liver fibrosis via inhibition of the ERK1/2 signaling pathway in thioacetamide-induced hepatic fibrosis in rats. Exp. Ther. Med. 2019, 18, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.R.; Wei, S.J.; Huang, Y.Q.; Xing, W.; Wang, L.Y.; Liang, L.L. Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/beta-catenin signalling pathway. World. J. Gastroenterol. 2018, 24, 4178–4185. [Google Scholar] [CrossRef]
- Gong, M.J.; Zhu, C.Y.; Zou, Z.J.; Han, B.; Huang, P. Therapeutic potential of puerarin against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis determined by combination of H-1 NMR spectroscopy-based metabonomics and 16S rRNA gene sequencing. J. Pharm. Biomed. Anal. 2021, 197, 113964. [Google Scholar] [CrossRef]
- Wang, S.; Yang, F.J.; Shang, L.C.; Zhang, Y.H.; Zhou, Y.; Shi, X.L. Puerarin protects against high-fat high-sucrose diet-induced non-alcoholic fatty liver disease by modulating PARP-1/PI3K/AKT signaling pathway and facilitating mitochondrial homeostasis. Phytother. Res. 2019, 33, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, K.X.; Zhou, J. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.Y.; Zhao, Y.R.; Qiang, G.F.; Yang, X.Y.; Xu, C.Y.; Chen, X.; Liu, C.G.; Wang, X.B.; Zhang, L.; Du, G.H. Puerarin Mitigates Diabetic Hepatic Steatosis and Fibrosis by Inhibiting TGF-beta Signaling Pathway Activation in Tune 2 Diabetic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 4545321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.M.; Huang, C.A.; Sun, H.Q.; Hong, H.F.; Jin, J.; Bei, C.Y.; Lu, Z.Q.; Zhang, X.L. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021, 12, 2075–2089. [Google Scholar] [CrossRef]
- Li, G.S.; Rao, H.M.; Xu, W.H. Puerarin plays a protective role in chondrocytes by activating Beclin1-dependent autophagy. Biosci. Biotechnol. Biochem. 2021, 85, 621–625. [Google Scholar] [CrossRef]
- Wang, L.Y.; Shan, H.J.; Wang, B.; Wang, N.; Zhou, Z.B.; Pan, C.H.; Wang, F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-gamma Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology 2018, 102, 117–125. [Google Scholar] [CrossRef]
- Peng, L.B.; Xie, Z.K.; Pei, J.; Wang, B.; Gao, Y.; Qu, Y.X. Puerarin alters the function of monocytes/macrophages and exhibits chondroprotection in mice. Mol. Med. Rep. 2019, 19, 2876–2882. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Luo, Y.; Liu, T.; Zhong, X.L.; Yan, J.; Huang, Q.; Tao, J.; He, Q.J.; Guo, M.Y.; Hu, Y.H. The Effect of Puerarin on Carotid Intima-media Thickness in Patients With Active Rheumatoid Arthritis: A Randomized Controlled Trial. Clin. Ther. 2018, 40, 1752–1764. [Google Scholar] [CrossRef]
- Ma, T.W.; Wen, Y.J.; Song, X.P.; Hu, H.L.; Li, Y.; Bai, H.; Zhao, M.C.; Gao, L. Puerarin inhibits the development of osteoarthritis through antiinflammatory and antimatrix-degrading pathways in osteoarthritis-induced rat model. Phytother. Res. 2020, 35, 2579–2593. [Google Scholar] [CrossRef]
- Tang, W.K.; Xiao, L.; Ge, G.R.; Zhong, M.D.; Zhu, J.; Qin, J.L.; Feng, C.C.; Zhang, W.H.; Bai, J.X.; Zhu, X.S.; et al. Puerarin inhibits titanium particle-induced osteolysis and RANKL-induced osteoclastogenesis via suppression of the NF-kappa B signaling pathway. J. Cell. Mol. Med. 2020, 24, 11972–11983. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.H.; Zhu, K.C.; Yuan, X.W.; Cheng, T.; Qian, Y.B.; Zhang, X.L. Puerarin Exerts Protective Effects on Wear Particle-Induced Inflammatory Osteolysis. Front. Pharmacol. 2019, 10, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Liu, M.Y.; Wang, Y.; Gong, S.Q.; Yao, W.F.; Li, W.S.; Gao, H.; Wei, M.J. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal. Biomed. Pharmacother. 2020, 132, 110923. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhong, M.; Huang, Y.; Zhu, J.; Tang, W.; Li, D.; Shi, J.; Lu, A.; Yang, H.; Geng, D.; et al. Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-κB signaling pathways. Aging 2020, 12, 21706–21729. [Google Scholar] [CrossRef]
- Guo, C.J.; Xie, J.J.; Hong, R.H.; Pan, H.S.; Zhang, F.G.; Liang, Y.M. Puerarin alleviates streptozotocin (STZ)-induced osteoporosis in rats through suppressing inflammation and apoptosis via HDAC1/HDAC3 signaling. Biomed. Pharmacother. 2019, 115, 108570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Liu, P.P.; Di, P.; Li, M.Y.; Wang, C.B. Puerarin exhibits antiinflammatory properties in gunpowder smog-induced acute lung injury in rats via regulation of the renin-angiotensin system and the NFκB signaling pathway. Exp. Ther. Med. 2021, 22, 809. [Google Scholar] [CrossRef]
- Hu, X.M.; Huang, X.L. Alleviation of Inflammatory Response of Pulmonary Fibrosis in Acute Respiratory Distress Syndrome by Puerarin via Transforming Growth Factor (TGF-beta 1). Med. Sci. Monit. 2019, 25, 6523–6531. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yan, J.J.; Xu, X.H.; Duan, C.Y.; Xie, Z.; Su, Z.Q.; Ma, H.X.; Ma, H.; Wei, X.; Du, X.C. Puerarin prevents LPS-induced acute lung injury via inhibiting inflammatory response. Microb. Pathog. 2018, 118, 170–176. [Google Scholar] [CrossRef]
- Xu, B.Y.; Wang, H.D.; Chen, Z. Puerarin Inhibits Ferroptosis and Inflammation of Lung Injury Caused by Sepsis in LPS Induced Lung Epithelial Cells. Front. Pediatr. 2021, 9, 706327. [Google Scholar] [CrossRef]
- Deng, H.F.; Wang, S.; Wang, X.L.; Li, L.; Xie, F.; Zeng, Z.W.; Zhang, W.X. Puerarin Protects Against LPS-Induced Vascular Endothelial Cell Hyperpermeability via Preventing Downregulation of Endothelial Cadherin. Inflammation 2019, 42, 1504–1510. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, W.Z.; Wang, J.; Xie, Y.Y.; Wang, W.S. Puerarin inhibits FUNDC1-mediated mitochondrial autophagy and CSE-induced apoptosis of human bronchial epithelial cells by activating the PI3K/AKT/mTOR signaling pathway. Aging 2022, 14, 1253–1264. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhao, J.; Gu, X.Y. The effects of genistein and puerarin on the activation of nuclear factor-kappaB and the production of tumor necrosis factor-alpha in asthma patients. Pharmazie 2010, 65, 127–131. [Google Scholar]
- Zhang, P.; Zhang, Y.; Wang, L.; Wang, X.; Xu, S.; Zhai, Z.; Wang, C.; Cai, H. Reversal of NADPH Oxidase-Dependent Early Oxidative and Inflammatory Responses in Chronic Obstructive Pulmonary Disease by Puerarin. Oxid. Med. Cell. Longev. 2022, 2022, 5595781. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pathak, Y.; Kumar, A.; Mishra, S.K.; Tripathi, V. Natural compounds as potential inhibitors of SARS-CoV-2 main protease: An in-silico study. Asian. Pac. J. Trop. Biomed. 2021, 11, 155–163. [Google Scholar]
- Tung, B.T.; Nhung, N.H.; Hang, T.T.T.; Linh, V.K.; Ngoc, L. Evaluating natural product compound inhibitors of SARS-CoV-2 main protease and spike protein target by molecular docking approach. Vietnam. J. Chem. 2021, 59, 846–861. [Google Scholar]
- Qin, X.Y.; Huang, C.; Wu, K.; Li, Y.; Liang, X.; Su, M.; Li, R. Anti-coronavirus disease 2019 (COVID-19) targets and mechanisms of puerarin. J. Cell. Mol. Med. 2021, 25, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.Y.; Fang, S.B.; Zhang, J.; Pan, Y.; Liu, H.; Wang, Y.; Li, M.; Liu, L.R. Chinese herbal compounds against SARS-CoV-2: Puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor. Comput. Struct. Biotechnol. J. 2020, 18, 3518–3527. [Google Scholar] [CrossRef]
- Wang, H.X.; Zeng, M.S.; Ye, Y.; Liu, J.Y.; Xu, P.P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2021, 35, 324–336. [Google Scholar] [CrossRef]
- Zeng, M.S.; Yu, W.D.; Wang, H.X.; Xu, P.P.; Liu, J.Y. Puerarin reduces impairment of intestinal and adipose immune responses to influenza virus infection in mice. Arch. Virol. 2021, 166, 2387–2397. [Google Scholar] [CrossRef]
- Liu, S.; Cao, X.L.; Liu, G.Q.; Zhou, T.; Yang, X.L.; Ma, B.X. The in silico and in vivo evaluation of puerarin against Alzheimer’s disease. Food Funct. 2019, 10, 799–813. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, W.N.; Chai, X.Q. Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice. Neural. Regen. Res. 2018, 13, 731–736. [Google Scholar]
- Yu, W.J.; An, S.J.; Shao, T.M.; Xu, H.J.; Chen, H.X.; Ning, J.D.; Zhou, Y.J.; Chai, X.Q. Active compounds of herbs ameliorate impaired cognition in APP/PS1 mouse model of Alzheimer’s disease. Aging 2019, 11, 11186–11201. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Liu, M.J.; Liang, J.T.; Li, N.N.; Yang, D.D.; Cai, J.J.; Zhang, Y.; He, Y.; Chen, Z.G.; Ma, T. Ferroptosis as a New Mechanism in Parkinson’s Disease Therapy Using Traditional Chinese Medicine. Front. Pharmacol. 2021, 12, 659584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, G.H.; Shao, N.; Qin, Y.P.; Chen, Q.; Wang, Y.; Zhou, P.; Cai, B. Thioredoxin-interacting protein (TXNIP) as a target for Alzheimer’s disease: Flavonoids and phenols. Inflammopharmacology 2021, 29, 1317–1329. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, Y.Z.; Zhao, W.; Chen, H.X.; Zhang, X.Y.; Ngo, F.Y.; Luo, D.; Song, Y.Q.; Lao, L.X.; Rong, J.H. Botanical Drug Puerarin Ameliorates Liposaccharide-Induced Depressive Behaviors in Mice via Inhibiting RagA/mTOR/p70S6K Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 7716201. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.N.; Yan, M.C.; Zhou, L.R.; Wang, J.A.; Sai, C.M.; Fu, Y.J.; Liu, Y.; Ding, L. Puerarin Alleviates Depression-Like Behavior Induced by High-Fat Diet Combined With Chronic Unpredictable Mild Stress via Repairing TLR4-Induced Inflammatory Damages and Phospholipid Metabolism Disorders. Front. Pharmacol. 2021, 12, 767333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, M.; Zhu, J.X.; Li, C.F.; Zhang, Q.P.; Geng, D.; Liu, Q.; Yi, L.T. FGF-2 signaling activation in the hippocampus contributes to the behavioral and cellular responses to puerarin. Biochem. Pharmacol. 2019, 168, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tsai, M.H.; Wu, Y.C.; Chen, K.T.; Chuang, H.W.; Chen, Y.; Tseng, G.W.; Fu, P.I.; Wei, I.H. Activity Dependent Mammalian Target of Rapamycin Pathway and Brain Derived Neurotrophic Factor Release is Required for the Rapid Antidepressant Effects of Puerarin. Am. J. Chin. Med. 2018, 46, 1519–1534. [Google Scholar] [CrossRef]
- Song, X.J.; Wang, W.H.; Ding, S.S.; Liu, X.Y.; Wang, Y.; Ma, H. Puerarin ameliorates depression-like behaviors of with chronic unpredictable mild stress mice by remodeling their gut microbiota. J. Affect. Disord. 2021, 290, 353–363. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shen, C.Y.; Jiang, J.G. Antidepressant active ingredients from herbs and nutraceuticals used in TCM: Pharmacological mechanisms and prospects for drug discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef]
- Tantipongpiradet, A.; Monthakantirat, O.; Vipatpakpaiboon, O.; Khampukdee, C.; Umehara, K.; Noguchi, H.; Fujiwara, H.; Matsumoto, K.; Sekeroglu, N.; Kijjoa, A.; et al. Effects of Puerarin on the Ovariectomy-Induced Depressive-Like Behavior in ICR Mice and Its Possible Mechanism of Action. Molecules 2019, 24, 4569. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, Y.B.; Wang, Z.; Li, J.F.; Liang, Y.; Li, M. Puerarin mitigates symptoms of depression in ovariectomized female rats by regulating hippocampal cAMP-CREB-BDNF signaling pathway. Trop. J. Pharm. Res. 2021, 20, 1403–1409. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Qiao, H.; Li, Y.; Xia, M.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Xie, Y.; et al. Research progress of natural products for the treatment of ischemic stroke. J. Integr. Neurosci. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 2021, 225, 107848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zheng, S.Z.; Chen, Y.Z.; Qu, Y.M.; Xie, J.Y.; Hong, E.H.; Lv, H.Z.; Ding, R.; Feng, L.; Xie, Z.C. Puerarin attenuates intracerebral hemorrhage-induced early brain injury possibly by PI3K/Akt signal activation-mediated suppression of NF-κB pathway. J. Cell. Mol. Med. 2021, 25, 7809–7824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.W.; Xu, X.Z.; Kong, J.L.; Rong, C.X.; She, J.H.; Guo, W.L.; Shi, L.J.; Zhao, D.A.F. Effect of Puerarin on EBI after SAH. Food Sci. Tech. 2022, 42, 45021. [Google Scholar]
- Zhou, Y.Q.; Mei, W.; Tian, X.B.; Tian, Y.K.; Liu, D.Q.; Ye, D.W. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 2021, 225, 107846. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Chen, Y.Y.; Du, K.R.; Wu, W.; Feng, X.B. Puerarin alleviates vincristine-induced neuropathic pain and neuroinflammation via inhibition of nuclear factor-kappa B and activation of the TGF-beta/Smad pathway in rats. Int. Immunopharmacol. 2020, 89 Pt B, 107060. [Google Scholar] [CrossRef]
- Du, K.R.; Wu, W.; Feng, X.B.; Ke, J.J.; Xie, H.T.; Chen, Y.Y. Puerarin Attenuates Complete Freund’s Adjuvant-Induced Trigeminal Neuralgia and Inflammation in a Mouse Model via Sirt1-Mediated TGF-beta 1/Smad3 Inhibition. J. Pain Res. 2021, 14, 2469–2479. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, Y.L.; Hu, Y.M.; Zhu, L.R.; Zhao, Y.S. Puerarin alleviate radicular pain from lumbar disc herniation by inhibiting ERK-dependent spinal microglia activation. Neuropeptides 2018, 72, 30–37. [Google Scholar] [CrossRef]
- Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khalid, S.; Naveed, M.; Ali, H.; Kim, Y.S.; Khan, S. Attenuation of inflammatory pain by puerarin in animal model of inflammation through inhibition of pro-inflammatory mediators. Int. Immunopharmacol. 2018, 61, 306–316. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhu, M.L.; Qiu, Y.; Wu, Z.Q.; Huang, N.; Wan, G.R.; Xu, J.; Song, P.; Wang, S.X.; Yin, Y.L.; et al. Puerarin Alleviates Vascular Cognitive Impairment in Vascular Dementia Rats. Front. Behav. Neurosci. 2021, 15, 717008. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Yuan, T.Y.; Chen, D.; Chen, Y.C.; Sun, S.C.; Wang, D.S.; Fang, L.H.; Lu, Y.; Du, G.H. Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-gamma/NF-kappa B Pathway. Molecules 2018, 23, 3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Wang, T.; Chen, B.W.; Lu, F.M.; Xu, J. Puerarin inhibits apoptosis and inflammation in myocardial cells via PPAR alpha expression in rats with chronic heart failure. Exp. Ther. Med. 2019, 18, 3347–3356. [Google Scholar] [PubMed] [Green Version]
- Chen, Z.Q.; Zhou, Y.; Huang, J.W.; Chen, F.; Zheng, J.; Li, H.L.; Li, T.; Li, L. Puerarin pretreatment attenuates cardiomyocyte apoptosis induced by coronary microembolization in rats by activating the PI3K/Akt/GSK-3β signaling pathway. Korean J. Physiol. Pharmacol. 2021, 25, 147–157. [Google Scholar] [CrossRef]
- Ni, S.Y.; Zhong, X.L.; Li, Z.H.; Huang, D.J.; Xu, W.T.; Zhou, Y.; Ou, C.W.; Chen, M.S. Puerarin Alleviates Lipopolysaccharide-Induced Myocardial Fibrosis by Inhibiting PARP-1 to Prevent HMGB1-Mediated TLR4-NF-κB Signaling Pathway. Cardiovasc. Toxicol. 2020, 20, 482–491. [Google Scholar] [CrossRef]
- Wang, Z.K.; Chen, R.R.; Li, J.H.; Chen, J.Y.; Li, W.; Niu, X.L.; Wang, F.F.; Wang, J.; Yang, J.X. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-κB pathway. Int. Immunopharmacol. 2020, 89 Pt B, 107086. [Google Scholar] [CrossRef]
- Shi, Z.G.; Wu, Q.Y.; Shi, H.Y.; Ying, S.T.; Tao, L. Puerarin inactivates NLRP3-mediated pyroptotic cell death to alleviate cerebral ischemia/reperfusion (I/R) injury through modulating the LncRNA DUXAP8/miR-223-3p axis. Biocell 2022, 46, 979–988. [Google Scholar] [CrossRef]
- Zhao, L.P.; Wang, L.; Zhang, D.M.; Chen, Y.Q.; Jin, F.L. Puerarin alleviates coronary heart disease via suppressing inflammation in a rat model. Gene 2021, 771, 145354. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, C.X.; Li, H.K.; Chen, X.Q.; Ding, Y.; Xu, S.D. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 2018, 497, 233–240. [Google Scholar] [CrossRef]
- Fu, C.; Chen, B.X.; Jin, X.L.; Liu, X.M.; Wang, F.L.; Guo, R.J.; Chen, Z.G.; Zheng, H.; Wang, L.; Zhang, Y.L. Puerarin protects endothelial progenitor cells from damage of angiotensin II via activation of ERK1/2-Nrf2 signaling pathway. Mol. Med. Rep. 2018, 17, 3877–3883. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Lei, T.W.; Li, H.M.; Mo, X.C.; Wang, Z.T.; Ou, H.L. ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.S.; Wang, M.Y. Effects of puerarin on chronic inflammation: Focus on the heart, brain, and arteries. Aging Med. 2021, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Z.Y.; Yang, Y.P. Puerarin inhibits vascular smooth muscle cells proliferation induced by fine particulate matter via suppressing of the p38 MAPK signaling pathway. BMC Complement. Altern. Med. 2018, 18, 146. [Google Scholar]
- Hu, Y.W.; Li, H.T.; Li, R.L.; Wu, Z.J.; Yang, W.X.; Qu, W. Puerarin protects vascular smooth muscle cells from oxidized low-density lipoprotein-induced reductions in viability via inhibition of the p38 MAPK and JNK signaling pathways. Exp. Ther. Med. 2020, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.L.; Yuan, R.; Chen, X.; Xin, Q.Q.; Wang, Y.; Shang, X.H.; Cong, W.H.; Chen, K.J. Puerarin reduces blood pressure in spontaneously hypertensive rats by targeting eNOS. Am. J. Chin. Med. 2019, 47, 19–38. [Google Scholar] [CrossRef]
- Zhou, T.T.; Wang, Z.W.; Guo, M.T.; Zhang, K.; Geng, L.; Mao, A.Q.; Yang, Y.J.; Yu, F. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food Funct. 2020, 11, 10137–10148. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.D.; Dai, X.L.; Wang, X.; Lu, J.; Zhang, X.M.; Li, X.F. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.F.; Yuan, T.Y.; Sun, S.C.; Wang, R.R.; Wang, S.B.; Fang, L.H.; Lyu, Y.; Du, G.H. Puerarin-V prevents the progression of hypoxia- and monocrotaline-induced pulmonary hypertension in rodent models. Acta. Pharmacol. Sin. 2022, 43, 2325–2339. [Google Scholar] [CrossRef]
- Cai, S.A.; Hou, N.; Zhao, G.J.; Liu, X.W.; He, Y.Y.; Liu, H.L.; Hua, Y.Q.; Li, L.R.; Huang, Y.; Ou, C.W.; et al. Nrf2 Is a Key Regulator on Puerarin Preventing Cardiac Fibrosis and Upregulating Metabolic Enzymes UGT1A1 in Rats. Front. Pharmacol. 2018, 9, 540. [Google Scholar] [CrossRef]
- Li, X.G.; Sun, S.C.; Chen, D.; Yuan, T.Y.; Chen, Y.C.; Wang, D.S.; Fang, L.H.; Lu, Y.; Du, G.H. Puerarin attenuates the endothelial-mesenchymal transition induced by oxidative stress in human coronary artery endothelial cells through PI3K/AKT pathway. Eur. J. Pharmacol. 2020, 886, 173472. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Xu, X.T.; Zhang, L.; Cui, F.M.; Chen, Q.; Li, H.X.; Sang, R.; Li, G.; He, Y.M. Puerarin Reduces Radiation-Induced Vascular Endothelial Cell Damage Via miR-34a/Placental Growth Factor. Dose-Response 2022, 20, 15593258211068649. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.W.; Liu, J.Y.; Han, R.F.; Jin, J.Q.; Zhu, L.; Zhang, Y.H.; Huang, Y.; Wang, X.; Xian, S.X.; Chen, Y. Kakonein restores diabetes-induced endothelial junction dysfunction via promoting autophagy-mediated NLRP3 inflammasome degradation. J. Cell. Mol. Med. 2021, 25, 7169–7180. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.W.; Yuan, H.Q.; Yin, X.Z.; Wu, Y.J.; He, R.R.; Huang, Y.; Chen, Y. Puerarin inhibits hyperglycemia-induced inter-endothelial junction through suppressing endothelial Nlrp3 inflammasome activation via ROS-dependent oxidative pathway. Phytomedicine 2019, 55, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.S.; Zhang, Y.C.; Xu, S.H.; Liu, J.J.; Sun, X.H.; Liang, C.; Wang, Y.; Li, J.; Wang, F.W.; Wang, Q.L.; et al. Puerarin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of inflammation. J. Asian. Nat. Prod. Res. 2019, 21, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yan, H.L.; Wang, L.X.; Xu, J.F.; Peng, C.; Ao, H.; Tan, Y.Z. Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef]
- Sun, X.H.; Ding, J.P.; Li, H.; Pan, N.; Gan, L.; Yang, X.L.; Xu, H.B. Activation of large-conductance calcium-activated potassium channels by puerarin: The underlying mechanism of Puerarin-mediated vasodilation. J. Pharmacol. Exp. Ther. 2007, 323, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Targeting PPARs Signaling Pathways in Cardiotoxicity by Natural Compounds. Cardiovasc. Toxicol. 2022, 22, 281–291. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Zhang, Z.; He, X.; Fan, Q.; Cheng, X.; Qiao, Y.; Huang, H.; Lai, S.; Wan, Q.; et al. Puerarin activates adaptive autophagy and protects the myocardium against doxorubicin-induced cardiotoxicity via the 14-3-3γ/PKCε pathway. Biomed. Pharmacother. 2022, 153, 113403. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhang, X.L.; Zhong, X.L.; Li, Z.H.; Cai, S.A.; Yang, P.Z.; Ou, C.W.; Chen, M.S. Puerarin inhibits vascular calcification of uremic rats. Eur. J. Pharmacol. 2019, 855, 235–243. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.F.; Li, Q.; Li, J.; Lu, X. Puerarin alleviates cisplatin-induced acute renal damage and upregulates microRNA-31-related signaling. Exp. Ther. Med. 2020, 20, 3122–3129. [Google Scholar] [CrossRef]
- Dey, P. Targeting gut barrier dysfunction with phytotherapies: Effective strategy against chronic diseases. Pharmacol. Res. 2020, 161, 105135. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Yamamoto, T.; Kadowaki, M. Ginger Increases ALDH1A1 Expression and Enhances Retinoic Acid Signaling in a Human Colonic Epithelial Cell Line. J. Nutr. Sci. Vitaminol. 2020, 66, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.F.; Ruan, Z.; Li, J.J.; Zhang, L.; Lu, H.; Xu, Z.J. Puerarin Rebuilding the Mucus Layer and Regulating Mucin-Utilizing Bacteria to Relieve Ulcerative Colitis. J. Agric. Food Chem. 2020, 68, 11402–11411. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Matsunami, E.; Komori, K.; Hayashi, S.; Kadowaki, M. The isoflavone puerarin induces Foxp3(+) regulatory T cells by augmenting retinoic acid production, thereby inducing mucosal immune tolerance in a murine food allergy model. Biochem. Biophys. Res. Commun. 2019, 516, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lin, Y.J.; Cao, Z.Q.; Xue, Y.; Wang, W.; Wang, X.Y. Network pharmacology analysis and experimental study strategy reveals the potential mechanism of puerarin against rotavirus. Ann. Transl. Med. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.T.; Liu, H. Puerarin attenuates LPS-induced inflammatory injury in gastric epithelial cells by repressing NLRP3 inflammasome-mediated apoptosis. Toxicol. In Vitro 2022, 81, 105350. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.B.; Duan, T.T.; Wu, Z.; Tang, C.F.; Cao, Z.H. Puerarin Inhibits the PERK-eIF2 alpha-ATF4-CHOP Pathway through Inactivating JAK2/STAT3 Signal in Pancreatic beta-Cells. Am. J. Chin. Med. 2021, 49, 1723–1738. [Google Scholar] [CrossRef]
- Noh, J.W.; Yang, H.K.; Jun, M.S.; Lee, B.C. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines 2022, 10, 175. [Google Scholar] [CrossRef]
- Xu, W.T.; Tang, M.Y.; Wang, J.H.; Wang, L.H. Anti-inflammatory activities of puerarin in high-fat diet-fed rats with streptozotocin-induced gestational diabetes mellitus. Mol. Biol. Rep. 2020, 47, 7537–7546. [Google Scholar] [CrossRef]
- Guo, J.J.; Bian, W.Q.; Jiang, H.D. Puerarin attenuates preeclampsia-induced trophoblast mobility loss and inflammation by modulating miR-181b-5p/RBAK axis. Am. J. Reprod. Immunol. 2022, 87, e13510. [Google Scholar] [CrossRef]
- Liang, X.P.; Liu, Y.J.; Chen, L.; Chen, S.Y. The natural compound puerarin alleviates inflammation and apoptosis in experimental cell and rat preeclampsia models. Int. Immunopharmacol. 2021, 99, 108001. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.P.; Zeng, J.H.; Lin, X.; Ni, Y.H.; Jiang, C.S.; Li, D.Z.; He, X.J.; Wang, R.; Wang, W. Puerarin Ameliorates Caerulein-Induced Chronic Pancreatitis via Inhibition of MAPK Signaling Pathway. Front. Pharmacol. 2021, 12, 686992. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Y.; Zhang, Y.J.; Zhao, S.Z. Puerarin regulates neovascular glaucoma through pigment epithelium-derived growth factor-induced NF-κ B signaling pathway. Mol. Med. Rep. 2018, 17, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Li, C.; Zhang, Y.; Gong, J.; Wang, G.; Tian, P.; Shen, N. Puerarin ameliorates retinal ganglion cell damage induced by retinal ischemia/reperfusion through inhibiting the activation of TLR4/NLRP3 inflammasome. Life Sci. 2020, 256, 117935. [Google Scholar] [CrossRef]
- Liu, J.L.; Liu, J.Y.; Bai, M.M.; Wang, H. Protective effect of puerarin against burn-induced heart injury in rats. Exp. Ther. Med. 2020, 20, 275–282. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Ahn, S.H.; Choi, M.J.; Yang, I.J.; Shin, H.M. Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration. Appl. Sci. 2021, 11, 9343. [Google Scholar] [CrossRef]
- Deng, H.F.; Wang, S.; Li, L.; Zhou, Q.; Guo, W.B.; Wang, X.L.; Liu, M.D.; Liu, K.; Xiao, X.Z. Puerarin prevents vascular endothelial injury through suppression of NF-kappa B activation in LPS-challenged human umbilical vein endothelial cells. Biomed. Pharmacother. 2018, 104, 261–267. [Google Scholar] [CrossRef]
- Nguyen Ngo Le, M.A.; Wen, Y.T.; Ho, Y.C.; Kapupara, K.; Tsai, R.K. Therapeutic Effects of Puerarin Against Anterior Ischemic Optic Neuropathy Through Antiapoptotic and Anti-Inflammatory Actions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3481–3491. [Google Scholar] [CrossRef]
- Wang, C.; Yan, M.Y.; Jiang, H.; Wang, Q.; He, S.; Chen, J.W.; Wang, C.B. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 2018, 193, 270–281. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Wang, H.; Lv, L.; Zhu, Z.Y. Metabolic responses of BV-2 cells to puerarin on its polarization using ultra-performance liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2020, 34, e4796. [Google Scholar] [CrossRef]
- Li, W.C.; Xu, X.T.; Dong, D.D.; Lei, T.W.; Ou, H.L. Up-regulation of thioredoxin system by puerarin inhibits lipid uptake in macrophages. Free Radic. Biol. Med. 2021, 162, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Guo, A.L.; Jing, Y.L.; Lin, J.J.; Sun, Y.Y.; Kong, L.L.; Zheng, H.H.; Deng, Y. Immunomodulatory activity of puerarin in RAW264.7 macrophages and cyclophosphamide-induced immunosuppression mice. Immunopharmacol. Immunotoxicol. 2021, 43, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Ma, S.; Fu, L.Y.; Wu, Y.; Geng, J. Puerarin plays an anti-inflammatory role by down-regulating the phosphorylation of MAPKS signal and up-regulating the level of O-GlCNAc glycosylated protein. Acta Medica 2020, 36, 2221–2226. [Google Scholar]
- Tong, J.; Hu, X.J.; Cai, W.Q.; Dai, X.; Wang, L. Puerarin alleviates delayed-type hypersensitivity via cytokine inhibition by modulating Th1/Th2 balance. Exp. Ther. Med. 2018, 15, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez-López, G.; Herrera-González, A.; Hernández, L.; Amaya-Delgado, L.; Sandoval, G.; Gschaedler, A.; Arrizon, J.; Remaud-Simeon, M.; Morel, S. Fructosylation of phenolic compounds by levansucrase from Gluconacetobacter diazotrophicus. Enzyme Microb. Technol. 2019, 122, 19–25. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Wang, Y.Y.; Guo, Z.R.; Chu, F.M.; Sun, P.Y. The synthesis of puerarin derivatives and their protective effect on the myocardial ischemia and reperfusion injury. J. Asian Nat. Prod. Res. 2010, 12, 843–850. [Google Scholar] [CrossRef]
- Xing, Z.H.; Ma, Y.C.; Li, X.P.; Zhang, B.; Zhang, M.D. Research progress of puerarin and its derivatives on anti-inflammatory and anti-gout activities. Zhongguo Zhong Yao Za Zhi 2017, 42, 3703–3708. [Google Scholar] [PubMed]
- Jiang, P.; Ji, Y.; Demetrescu, I.; Oh, K.; Kaushik, N.K.; Butu, A.; Othman, F. Effects of Puerarin Derivative P on Learning, Memory and MPO Activity in Vascular Dementia Model Mice. MATEC Web Conf. 2016, 60, 03003. [Google Scholar] [CrossRef]
- Han, R.M.; Tian, Y.; Peng, W.; Xiang, J.F.; Ai, X.C.; Zhang, J.P. Synthesis and characterization of puerarin derivatives and the mechanism of derivation reaction. Chem. J. Chin. Univ.-Chin. Ed. 2006, 27, 1716–1720. [Google Scholar]
- Ji, Y.; Jiang, P.; Yan, X. Anticerebral Ischemia-Reperfusion Injury Activity of Synthesized Puerarin Derivatives. BioMed Res. Int. 2016, 2016, 9821767. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhao, G.L.; Li, X.F.; Xiao, X.L.; He, N.; Ma, J.J.; Yu, Y.G. Evaluation of the digestion and transport profiles and potential immunocompetence of puerarin and its acylated derivatives. Food Funct. 2021, 12, 5949–5958. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Qu, L.B.; Yuan, J.W.; Zhao, Y.F. Synthesis of a novel type of phosphates of puerarin. J. Chin. Chem. Soc. 2007, 54, 583–585. [Google Scholar] [CrossRef]
- He, H.; Peng, S.; Song, X.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Protective effect of isoflavones and triterpenoid saponins from pueraria lobata on liver diseases: A review. Food Sci. Nutr. 2021, 10, 272–285. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, X.; Qu, P.; Shang, C.; Xiang, M.; Wang, J. Roles and mechanisms of puerarin on cardiovascular disease: A review. Biomed. Pharmacother. 2022, 147, 112655. [Google Scholar] [CrossRef]
- Zhang, M.F.; Liu, Y.X.; Jiang, K.Y.; Niu, H.M.; Jiang, J.L.; Dong, S.T.; Wang, X.; Wang, D.F.; Meng, S.N. Alteration of UDP-glucuronosyltransferase 1a1, 1a7 and P-glycoprotein expression in hepatic fibrosis rats and the impact on pharmacokinetics of puerarin. Phytomedicine 2019, 52, 264–271. [Google Scholar] [CrossRef]
- Anukunwithaya, T.; Poo, P.; Hunsakunachai, N.; Rodsiri, R.; Malaivijitnond, S.; Khemawoot, P. Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Chen, R.; Peng, W.; Cai, Z.; Tang, L.; Ye, L.; Hou, C.; Na, Y.; Zhao, J. The combination of Puerariae Lobatae Radix and Chuanxiong Rhizoma enhanced the absorption and pharmacokinetics of puerarin by modulating the intestinal barrier and influenced gut microbiota. J. Funct. Foods 2018, 47, 72–82. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, X.; Dong, G. Effects of verapamil on the pharmacokinetics of puerarin in rats. Xenobiotica 2019, 49, 1178–1182. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Zhang, Y. Effects of quercetin on the pharmacokinetics of puerarin in rats. Lat. Am. J. Pharm. 2018, 37, 1870–1876. [Google Scholar]
- Wu, J.Y.; Li, Y.J.; Han, M.; Hu, X.B.; Yang, L.; Wang, J.M.; Xiang, D.X. A microemulsion of puerarin-phospholipid complex for improving bioavailability: Preparation, in vitro and in vivo evaluations. Drug Dev. Ind. Pharm. 2018, 44, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yuan, F.; Liu, J.; Liu, W.; Feng, J.; Jin, Y.; Tu, L. Fabrication of Fine Puerarin Nanocrystals by Box-Behnken Design to Enhance Intestinal Absorption. AAPS PharmSciTech 2020, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, W.; Li, D.; Chen, X.; Liu, F.; Yuan, D.; Pan, H.; Wang, Q.; Fang, S.; Chen, T. Oral Delivery of Puerarin Nanocrystals To Improve Brain Accumulation and Anti-Parkinsonian Efficacy. Mol. Pharm. 2019, 16, 1444–1455. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, S.; Yi, T. Development of an Oral Compound Pickering Emulsion Composed of Nanocrystals of Poorly Soluble Ingredient and Volatile Oils from Traditional Chinese Medicine. Pharmaceutics 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, Z.; Huang, Y.; Zhu, Q.; Wu, W.; Yi, T.; Chen, Z.; Lu, Y. Utility of Pickering emulsions in improved oral drug delivery. Drug Discov. Today 2020, 25, 2038–2045. [Google Scholar] [CrossRef]
- Inam, M.; Liu, L.; Wang, J.W.; Yu, K.X.; Phan, C.U.; Shen, J.; Zhang, W.H.; Tang, G.; Hu, X. Enhancing the Physiochemical Properties of Puerarin via L-Proline Co-Crystallization: Synthesis, Characterization, and Dissolution Studies of Two Phases of Pharmaceutical Co-Crystals. Int. J. Mol. Sci. 2021, 22, 928. [Google Scholar] [CrossRef]
- Tu, L.; Cheng, M.; Sun, Y.; Fang, Y.; Liu, J.; Liu, W.; Feng, J.; Jin, Y. Fabrication of ultra-small nanocrystals by formation of hydrogen bonds: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 573, 118730. [Google Scholar] [CrossRef]
- Yan, J.; Guan, Z.Y.; Zhu, W.F.; Zhong, L.Y.; Qiu, Z.Q.; Yue, P.F.; Wu, W.T.; Liu, J.; Huang, X. Preparation of Puerarin Chitosan Oral Nanoparticles by Ionic Gelation Method and Its Related Kinetics. Pharmaceutics 2020, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, S.; Sun, W.; Cui, M.; Wen, H.; Li, Q.; Pan, W.; Yang, X. Flexibility of 3D Extruded Printing for a Novel Controlled-Release Puerarin Gastric Floating Tablet: Design of Internal Structure. AAPS PharmSciTech 2019, 20, 236. [Google Scholar] [CrossRef]
- Ye, Q.; Li, J.; Li, T.; Ruan, J.; Wang, H.; Wang, F.; Zhang, X. Development and evaluation of puerarin-loaded controlled release nanostructured lipid carries by central composite design. Drug Dev. Ind. Pharm. 2021, 47, 113–125. [Google Scholar] [CrossRef]
- Barro, L.; Hsiao, J.T.; Chen, C.Y.; Chang, Y.L.; Hsieh, M.F. Cytoprotective Effect of Liposomal Puerarin on High Glucose-Induced Injury in Rat Mesangial Cells. Antioxidants 2021, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xing, X.; Lv, N.; Zhao, J.; Liu, Y.; Gong, H.; Du, Y.; Lu, Q.; Dong, Z. Therapy for myocardial infarction: In vitro and in vivo evaluation of puerarin-prodrug and tanshinone co-loaded lipid nanoparticulate system. Biomed. Pharmacother. 2019, 120, 109480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, B.N.; Zhang, Y.T.; Xie, J.X.; Li, W.S.; Zhang, H.T.; Liu, Y.; Feng, N.P. Exploring the Potential of Mesoporous Silica as a Carrier for Puerarin: Characterization, Physical Stability, and In Vivo Pharmacokinetics. AAPS PharmSciTech 2019, 20, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Wu, Y.; Liu, J.; Ye, T.; Wang, S. Succinylated whey protein isolate as a sustained-release excipient of puerarin derivative oral tablets: Preparation, optimization and pharmacokinetics. Asian J. Pharm. Sci. 2018, 13, 383–394. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Qin, J.; Wa, W. Interactions between puerarin/daidzein and micellar casein. J. Food Biochem. 2022, 46, e14048. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zhang, J.; Wang, J.; Xiang, D.; Luo, S.; Li, J.; Liu, X. Puerarin-loaded PEG-PE micelles with enhanced anti-apoptotic effect and better pharmacokinetic profile. Drug Deliv. 2018, 25, 827–837. [Google Scholar] [CrossRef]
- Gao, J.M.; Wang, Z.P.; Meng, X.P.; Chen, T.S.; Wang, Y.F. Anti-hepatocarcinoma effects of puerarin-nanoethosomes against human HepG2 cells. In Nanophotonics and Micro/Nano Optics IV; SPIE: Bellingham, WA, USA, 2018. [Google Scholar]
- Chen, H.; Pang, Z.; Qiao, Q.; Xia, Y.; Wei, Y.; Gao, Y.; Zhang, J.; Qian, S. Puerarin-Na Chelate Hydrate Simultaneously Improves Dissolution and Mechanical Behavior. Mol. Pharm. 2021, 18, 2507–2520. [Google Scholar] [CrossRef]
- Han, Q.; Chen, K.; Su, C.; Liu, X.; Luo, X. Puerarin Loaded PLGA Nanoparticles: Optimization Processes of Preparation and Anti-alcohol Intoxication Effects in Mice. AAPS PharmSciTech 2021, 22, 217. [Google Scholar] [CrossRef]
- Liu, W.; Lu, H.W.; Rao, X.Y.; Li, X.; Lu, H.D.; Li, F.F.; He, Y.; Yu, R.Y.; Zhong, R.S.; Zhang, Y.; et al. Enhanced treatment for cerebral ischemia-reperfusion injury of puerarin loading liposomes through neutrophils-mediated targeted delivery. Nano Res. 2021, 14, 4634–4643. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Yang, L.; Hu, Y.Y.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Borneol and -asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018, 25, 1858–1864. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Liu, W.; Xiong, S.; Li, D.; Fang, S.; Wu, Z.; Wang, Q.; Chen, X. Nanoparticles Mediating the Sustained Puerarin Release Facilitate Improved Brain Delivery to Treat Parkinson’s Disease. ACS. Appl. Mater. Interfaces 2019, 11, 45276–45289. [Google Scholar] [CrossRef] [PubMed]
- Hesari, M.; Mohammadi, P.; Khademi, F.; Shackebaei, D.; Momtaz, S.; Moasefi, N.; Farzaei, M.H.; Abdollahi, M. Current Advances in the Use of Nanophytomedicine Therapies for Human Cardiovascular Diseases. Int. J. Nanomed. 2021, 16, 3293–3315. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Wu, J.Y.; Xiang, D.X.; Luo, S.L.; Hu, X.B.; Tang, T.T.; Sun, T.L.; Liu, X.Y. Micelles Loaded With Puerarin And Modified With Triphenylphosphonium Cation Possess Mitochondrial Targeting And Demonstrate Enhanced Protective Effect Against Isoprenaline-Induced H9c2 Cells Apoptosis. Int. J. Nanomed. 2019, 14, 8345–8360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, S.; Di, L. Acute myocardial infarction therapy: In vitro and in vivo evaluation of atrial natriuretic peptide and triphenylphosphonium dual ligands modified, baicalin-loaded nanoparticulate system. Drug Deliv. 2021, 28, 2198–2204. [Google Scholar] [CrossRef]
- Xue, J.T.; Zhou, N.Q.; Yang, Y.P.; Jin, Y.; Qiu, Y.; Liu, Y.H.; Lu, J.X.; Tian, X.Q.; Yin, Y.L.; Li, P. Puerarin-loaded ultrasound microbubble contrast agent used as sonodynamic therapy for diabetic cardiomyopathy rats. Colloids. Surf. B. Biointerfaces 2020, 190, 110887. [Google Scholar]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.; Wang, Y.; Wu, J. Polydopamine/puerarin nanoparticle-incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Ou, Q.; Zhang, S.; Fu, C.; Yu, L.; Xin, P.; Gu, Z.; Cao, Z.; Wu, J.; Wang, Y. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 2021, 19, 237. [Google Scholar] [CrossRef]
- Zhou, X.; Saiding, Q.; Wang, X.; Wang, J.; Cui, W.; Chen, X. Regulated Exogenous/Endogenous Inflammation via “Inner-Outer” Medicated Electrospun Fibers for Promoting Tissue. Adv. Healthc. Mater. 2022, 11, e2102534. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, K.; Li, J.; Liao, J.; Ma, L. Engineering of hybrid anticancer drug-loaded polymeric nanoparticles delivery system for the treatment and care of lung cancer therapy. Drug Deliv. 2021, 28, 1539–1547. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Miao, C.; Liu, R. Improved Therapeutic Effect of Puerarin-Encapsulated PEG-PLGA Nanoparticle on an In Vitro Cerebral Infarction Model. Adv. Polym. Tech. 2020, 714, 7145738. [Google Scholar] [CrossRef]
- Xiong, S.; Luo, J.; Wang, Q.; Li, Z.; Li, J.; Liu, Q.; Gao, L.; Fang, S.; Li, Y.; Pan, H.; et al. Targeted graphene oxide for drug delivery as a therapeutic nanoplatform against Parkinson’s disease. Biomater. Sci. 2021, 9, 1705–1715. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Zhao, Z.; Wang, Z.; Li, S.; Chen, C.; Yu, S.; Qu, X.; Li, K.; Tian, Y.; et al. Preparation of a novel ginkgolide B niosomal composite drug. Open Chem. 2020, 18, 1064–1074. [Google Scholar] [CrossRef]
- Yi, T.; Huang, J.; Chen, X.; Xiong, H.; Kang, Y.; Wu, J. Synthesis, characterization, and formulation of poly-puerarin as a biodegradable and biosafe drug delivery platform for anti-cancer therapy. Biomater. Sci. 2019, 7, 2152–2164. [Google Scholar] [CrossRef]
- Li, W. Supramolecular nanofiber-reinforced Puerarin hydrogels as drug carriers with synergistic controlled release and antibacterial properties. J. Mater. Sci. 2020, 55, 1–9. [Google Scholar] [CrossRef]
- Feng, X.; Luo, Y.; Li, F.; Jian, X.; Liu, Y. Development of Natural-Drugs-Based Low-Molecular-Weight Supramolecular Gels. Gels 2021, 7, 105. [Google Scholar] [CrossRef]
- Cai, Y.B.; Zhang, J.W.; He, Y.Y.; Li, Z.H.; Hua, Y.Q.; Wu, Z.Y.; Gao, J.; Ou, C.W.; Chen, M.S. A Supramolecular Hydrogel of Puerarin. J. Biomed. Nanotechnol. 2018, 14, 257–266. [Google Scholar] [CrossRef]
- Pang, Z.; Wei, Y.; Wang, N.; Zhang, J.; Gao, Y.; Qian, S. Gel formation of puerarin and mechanistic study during its cooling process. Int. J. Pharm. 2018, 548, 625–635. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, J.; Dai, T.; Ye, J.; Wu, J.; Chen, T.; Liu, C. Fabrication of Oil-in-Water Emulsions with Whey Protein Isolate-Puerarin Composites: Environmental Stability and Interfacial Behavior. Foods 2021, 10, 705. [Google Scholar] [CrossRef]
- Yuan, H.; Li, W.; Song, C.; Huang, R. An injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties as potential carriers for drug delivery. Int. J. Biol. Macromol. 2022, 205, 563–573. [Google Scholar] [CrossRef]
- Yang, B.; Du, S.; Lu, Y.; Jia, S.; Zhao, M.; Bai, J.; Li, P.; Wu, H. Influence of paeoniflorin and menthol on puerarin transport across MDCK and MDCK-MDR1 cells as blood-brain barrier invitro model. J. Pharm. Pharmacol. 2018, 70, 349–360. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wu, T.; Liu, B.; Yang, J.; Liu, W. Chinese herb-crosslinked hydrogel bearing rBMSCs-laden polyzwitterion microgels: Self-adaptive manipulation of micromilieu and stemness maintenance for restoring infarcted myocardium. Nano Today 2021, 41, 101306. [Google Scholar] [CrossRef]
- Lin, X.X.; Zhang, H.T.; Qin, Y.Q.; Hu, X.H. Design and Fabrication of Photo-Responsive Hydrogel for the Application of Functional Contact Lens. Front. Mater. 2021, 8, 680359. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhang, H.B.; Wang, G.F.; Xu, D.; Zhang, W.Y.; Wang, Q.S.; Cui, Y.L. Colon-specific microspheres loaded with puerarin reduce tumorigenesis and metastasis in colitis-associated colorectal cancer. Int. J. Pharm. 2019, 570, 118644. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, Y.; Zhang, Y.; Jiang, Z.P.; Cui, Y.L.; Wang, Q.S. ROS-responsive thioketal-linked alginate/chitosan carriers for irritable bowel syndrome with diarrhea therapy. Int. J. Biol. Macromol. 2022, 209 Pt A, 70–82. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, J.; Yi, T. A Novel Solid Nanocrystals Self-Stabilized Pickering Emulsion Prepared by Spray-Drying with Hydroxypropyl-β-cyclodextrin as Carriers. Molecules 2021, 26, 1809. [Google Scholar] [CrossRef]
- Li, R.; Yuan, G.; Li, D.; Xu, C.; Du, M.; Tan, S.; Liu, Z.; He, Q.; Rong, L.; Li, J. Enhancing the bioaccessibility of puerarin through the collaboration of high internal phase Pickering emulsions with β-carotene. Food Funct. 2022, 13, 2534–2544. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Liu, L.; Gao, C.S.; Liu, N.N.; Fa, X.E. Puerarin pre-conditioning on the expression levels of CK-MB, cTnI and inflammatory factors in patients undergoing cardiac valve replacement. Exp. Ther. Med. 2019, 17, 2598–2602. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.Y.; Xiao, W.X.; Ding, X.M.; Jing, N.; Tang, M.; Yin, L.L.; Li, Y.; Zhong, T.; Guo, Z.T. The effect of puerarin, as add-on therapy, on renal outcomes, oxidative stress and inflammatory cytokines in type 2 diabetic patients with hypertension. Int. J. Clin. Exp. Med. 2019, 12, 1299. [Google Scholar]
- Chen, L.; Bi, X.Y.; Zhu, L.X.; Qiu, Y.Q.; Ding, S.J.; Deng, B.Q. Flavonoids of puerarin versus tanshinone II A for ischemic stroke: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1215–1220. [Google Scholar] [CrossRef]
- Wang, J.W.; Gao, J.M.; Huang, Y.J. Effects of puerarin on the vascular active factor related to cerebral vasospasm after aneurysm subarachnoid hemorrhage. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012, 32, 164–167. [Google Scholar] [PubMed]
- Luo, Z.K.; Liu, Y.; Li, H.M. A clinical efficacy and safety study on coronary heart disease and angina treatment with Puerarin Injection. Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 614–616. [Google Scholar] [PubMed]
- Li, L.; Nie, S.; Dong, Z.; Ning, H. Clinical efficacy and CT perfusion of puerarin combined with naloxone in the treatment of traumatic cerebral infarction. Pak. J. Pharm. Sci. 2020, 33, 423–428. [Google Scholar]


| Disease Type | Delivery Type | Major Findings | Reference |
|---|---|---|---|
| / | Microemulsion (ME) | Exploit a late-model ME on the basis of phospholipid complex technique to increase the oral bioavailability of puerarin. | [151] |
| / | Nanocrystals | The optimal nanocrystals were prepared by Box–Behnken design, which can strengthen the intestinal absorption of puerarin by increasing permeability and restraining P-gp efflux. | [152] |
| Parkinson’s disease (PD). | Nanocrystals | Puerarin nanocrystals could serve as a promising oral delivery system for PD, strengthening the ability of puerarin to incorporate into the brain by improving its bioavailability. | [153] |
| / | Nanocrystals self-stabilized Pickering emulsion (NSSPE) | Puerarin nanocrystals could fix Pickering emulsion of Ligusticum Chuanxiong essential oil and could boost the oral bioavailability of puerarin. | [154] |
| / | Solid nanocrystals | The solid nanocrystals self-stabilized Pickering emulsion (NSSPE) can preserve the particular microstructure and the excellent properties in vitro of the liquid NSSPE for weakly soluble drugs. | [197] |
| / | High internal phase Pickering emulsion (HIPPE) | Owing to the individualized formulation and the extraordinary structure of the HIPPE, which can slow down lipid digestion and restrained puerarin degradation, a synergistic interaction occurred between β-carotene and HIPPE to boost puerarin bioaccessibility. | [198] |
| / | Nanocrystals | The ultra-small nanocrystals were prepared which can increase bioavailability of poorly soluble drugs. | [157] |
| / | Chitosan nanoparticles | The prepared nanoparticles can be conducive to enlarging the absorption acreage and improving the oral absorption of puerarin. Meanwhile, this effectively encapsulates puerarin and prevents the intestinal first-pass elimination, which eminently increases puerarin absorption in the small intestine and the colon. | [158] |
| / | 3D-printed tablet | The puerarin gastric floating 3D-printed tablets could achieve good gastric residence time and controlled release; 3D-extrusion-based printing may be fit for the production of oral drug delivery systems. | [159] |
| Myocardial infarction (MI) | Solid lipid nanoparticles (SLN) | PUE (puerarin)-prodrug and TAN (tanshinone) co-loaded solid lipid nanoparticles (SLN), which proved that the double drugs co-loaded with SLN can be employed as a promising candidate delivery system for cardioprotective drugs in remedy of myocardial infarction. | [162] |
| Cerebral ischemia-reperfusion injury (CIRI) | Liposomes | Using neutrophils as supporters to penetrate the BBB for liposomes loaded with puerarin and enhance the consistence of puerarin in the brain parenchyma. Enhancing the neuroprotection effect at the ischemic penumbra. | [170] |
| Parkinson’s disease (PD) | 6-Armed star-shaped poly(lactide-co-glycolide) nanoparticles (6-s-PLGA NPs) | Puerarin-NPs can enhance puerarin oral absorption and improve its delivery to the brain wherein it can contribute to the remedy of PD. | [172] |
| myocardial infarction | Micelles | Puerarin (PUE) was carried by mitochondria-targeted micelles (PUE@TPP/PEG-PE) for accurately delivering PUE into mitochondria to counter myocardial infarction. | [166] |
| Colitis-associated colorectal cancer (CAC) | pH-responsive alginate microspheres | The microspheres loaded with puerarin demonstrated high retention time in the colon, low inflammatory response, and a promising therapeutic strategy for colitis-associated colorectal cancer. | [195] |
| Diabetic cardiomyopathy (DCM) | Ultrasound microbubble contrast agent | A puerarin-loaded ultrasound sulfur hexafluoride microbubble contrast agent could significantly improve the migration ability of human umbilical vein endothelial cells and improve targeted drug delivery and pharmacodynamic effects in diabetic cardiomyopathy (DCM) treatment. | [176] |
| Cerebral infarction | PEG-PLGA Nanoparticle | The PEG-PLGA/PUE nanoparticles prepared by the thin-film hydration method had uniform particle size, regular shape, and good stability and were not toxic to cells. Furthermore, they inhibited the expression of PDCD4 protein by lowering the expression level of miR-424 in cells, thereby reducing the hazard of cerebral infarction. | [182] |
| Lung cancer | Polymeric nanoparticles. | Developing compostable polymeric nanomaterials (NMs) for the delivery of puerarin (PRN) and 5-fluorouracil (5FU), which showed excellent biocompatibility and significant promise to improve the effectiveness of lung cancer cells. | [180] |
| Triple negative breast cancer | Nanoemulsion | A novel puerarin nanoemulsion (nanoPue) was developed to improve the solubility and bioavailability of puerarin. NanoPue significantly deactivated the stromal microenvironment and facilitated chemotherapy effect of nano-paclitaxel in the desmoplastic triple-negative breast cancer model. | [181] |
| Parkinson’s disease (PD) | Pue-loaded graphene oxide nanosheets (GO) | Using Pue-loaded graphene oxide nanosheets (GO), which had an excellent drug-loading ability, modifiable surface functional groups, and good biocompatibility, in vivo and in vitro results indicated that this multifunctional brain-targeted drug delivery system was an effective and safe therapy for PD. | [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. https://doi.org/10.3390/antiox11112121
Wang D, Bu T, Li Y, He Y, Yang F, Zou L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants. 2022; 11(11):2121. https://doi.org/10.3390/antiox11112121
Chicago/Turabian StyleWang, Di, Tong Bu, Yangqian Li, Yueyue He, Fan Yang, and Liang Zou. 2022. "Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin" Antioxidants 11, no. 11: 2121. https://doi.org/10.3390/antiox11112121
APA StyleWang, D., Bu, T., Li, Y., He, Y., Yang, F., & Zou, L. (2022). Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants, 11(11), 2121. https://doi.org/10.3390/antiox11112121