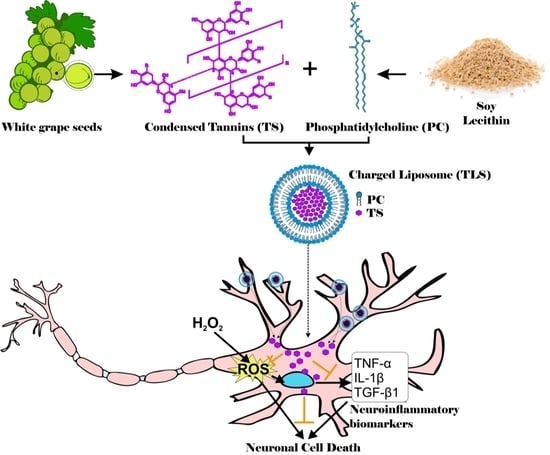
Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Liposome Preparation and Tannins Encapsulation
2.2. Mean Particle Size (MPS), Polydispersity Index (PDI), and z-Potential (ξ)
2.3. Proximal Composition
2.4. Quantification of Total Phenols
2.5. HPLC-UV
2.6. Trolox Equivalent Antioxidant Capacity Assay
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Intracellular ROS Assay
2.10. RNA Isolation, cDNA Synthesis and RT-qPCR Analysis of Neuroinflammatory Biomarkers
2.11. Statistical Analysis
3. Results
3.1. Lipid-Encapsulated Tannins Characterization
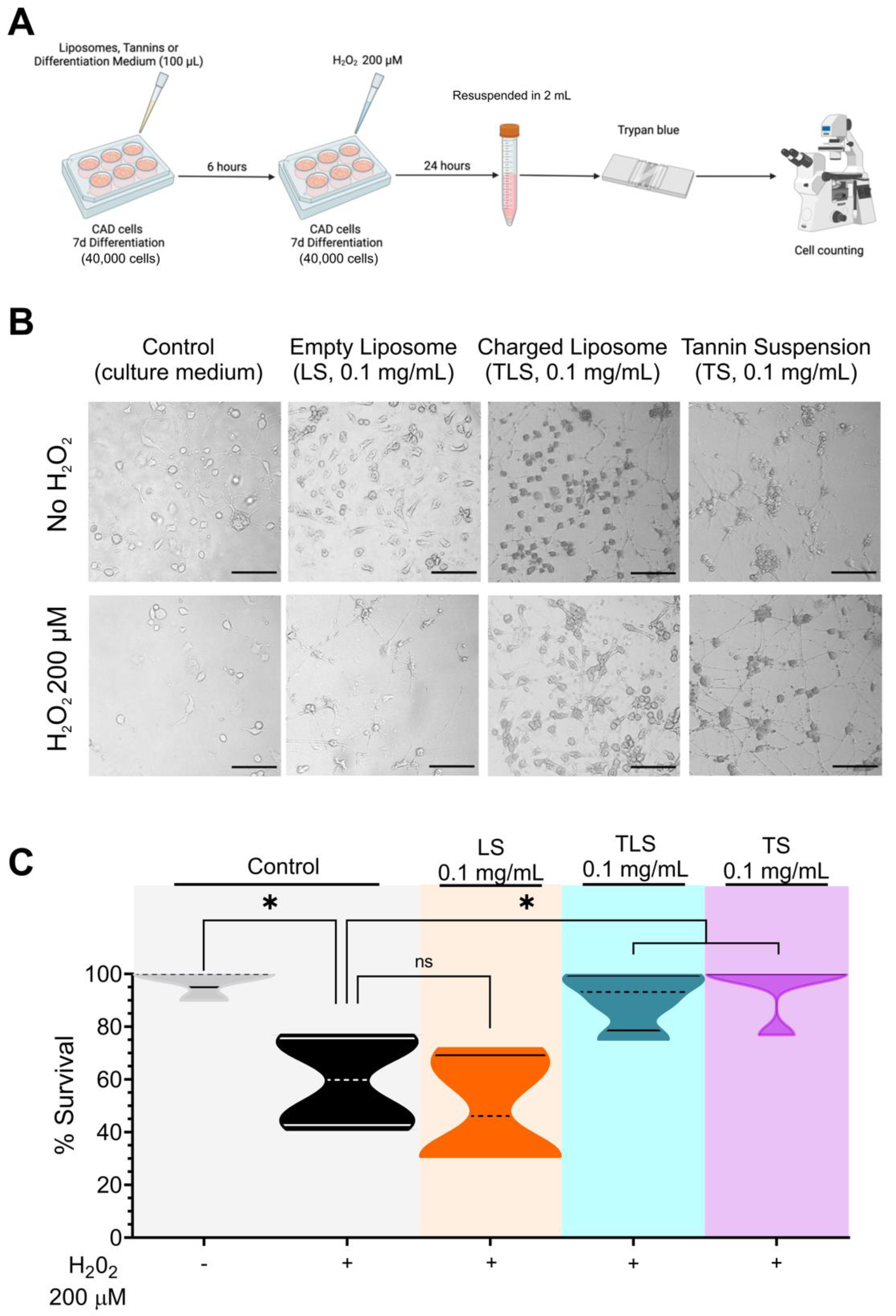
3.2. Tannins and Charged Liposomes Prevent Hydrogen Peroxide-Induced Cell Death
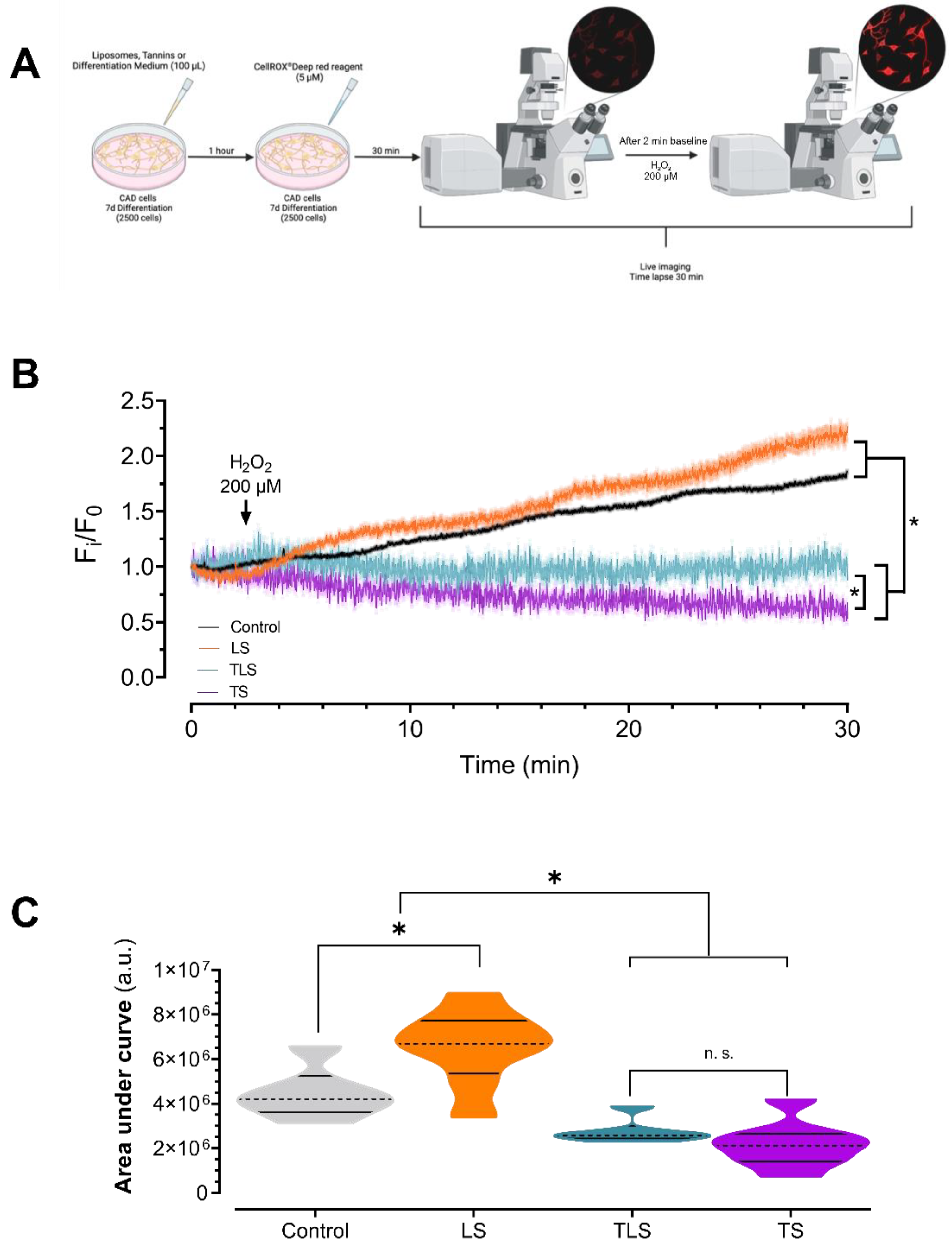
3.3. Tannins and Charged Liposomes Prevented Intracellular ROS Production after Hydrogen Peroxide Trearment
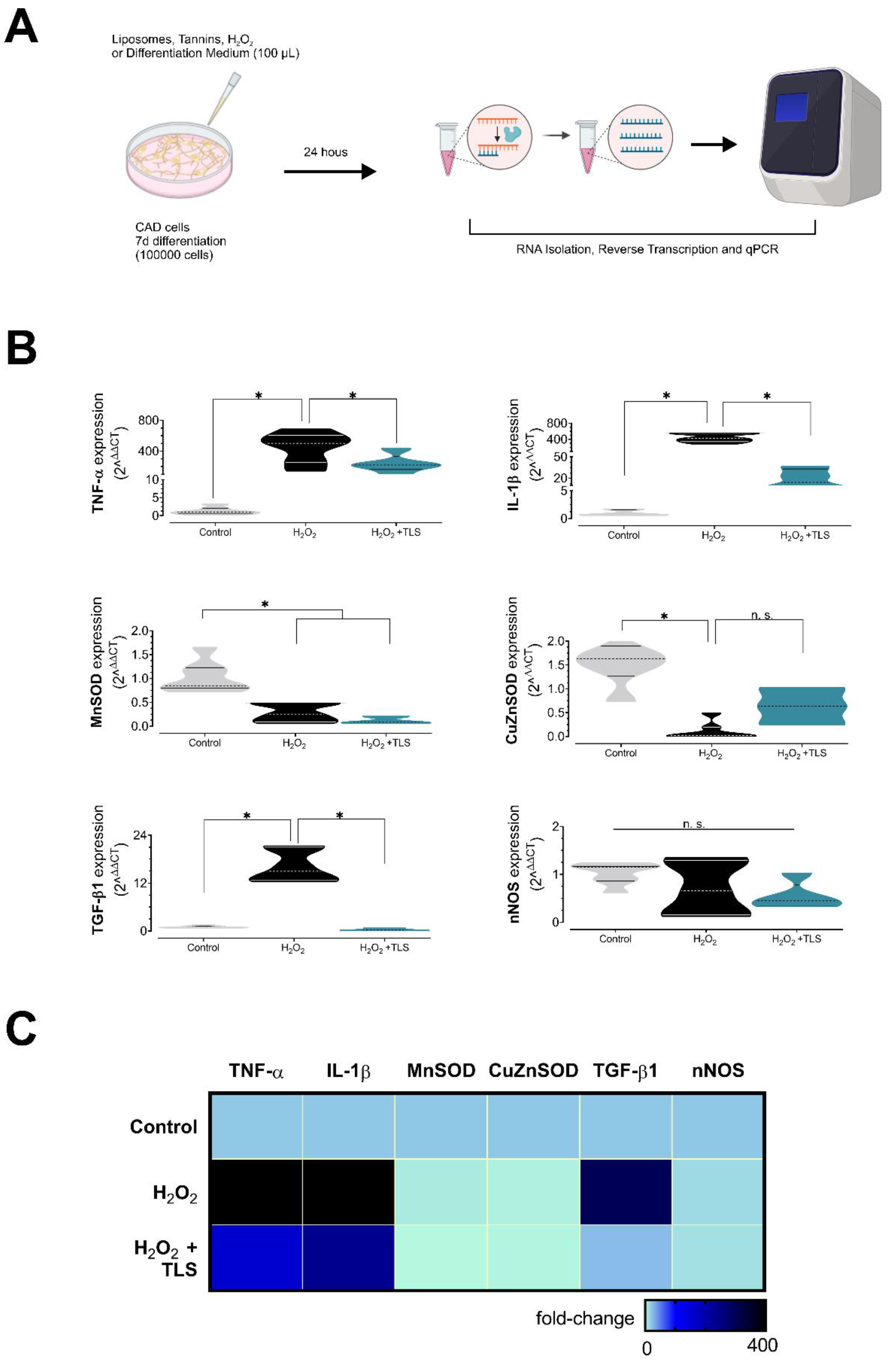
3.4. Lipid-Encapsulated Grape Tannins Protects Neurons against ROS-Induced Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef]
- Díaz, H.S.; Toledo, C.; Andrade, D.C.; Marcus, N.J.; Del Rio, R. Neuroinflammation in heart failure: New insights for an old disease. J. Physiol. 2020, 598, 33–59. [Google Scholar] [CrossRef]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef]
- Lehnardt, S.; Massillon, L.; Follett, P.; Jensen, F.E.; Ratan, R.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 8514–8519. [Google Scholar] [CrossRef]
- Zhu, Y.; Carvey, P.M.; Ling, Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006, 1090, 35–44. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox. Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sleiman, P.M.; Muqit, M.M.K.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Filosa, S.; Di Meo, F.; Crispi, S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front. Pharmacol. 2022, 13, 876614. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef] [PubMed]
- Roumes, H.; Sanchez, S.; Benkhaled, I.; Fernandez, V.; Goudeneche, P.; Perrin, F.; Pellerin, L.; Guillard, J.; Bouzier-Sore, A.K. Neuroprotective Effect of Eco-Sustainably Extracted Grape Polyphenols in Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 773. [Google Scholar] [CrossRef] [PubMed]
- El Gaamouch, F.; Liu, K.; Lin, H.Y.; Wu, C.; Wang, J. Development of grape polyphenols as multi-targeting strategies for Alzheimer’s disease. Neurochem. Int. 2021, 147, 105046. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 74–95. [Google Scholar] [CrossRef]
- Somers, T.J.P. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Baseer, A.; Irum Khan, S.; Akram, M.; Khan, S.; Ahmad, N.; Farooq, U.; Kanwal Nawaz, N.; Shaheen, S.; et al. Tannic acid inhibits lipopolysaccharide-induced cognitive impairment in adult mice by targeting multiple pathological features. Int. Immunopharmacol. 2022, 110, 108970. [Google Scholar] [CrossRef]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012, 287, 6912–6927. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Miroshnyk, I.; Habib, M.J.; Brausch, J.F.; Hussain, M.D. Enhanced Dissolution and Oral Bioavailability of Piroxicam Formulations: Modulating Effect of Phospholipids. Pharmaceutics 2010, 2, 339–350. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, X.; Lu, Y.; Shi, S.; Yang, D.; Yao, T. New Strategy for Reducing Tau Aggregation Cytologically by a Hairpinlike Molecular Inhibitor, Tannic Acid Encapsulated in Liposome. ACS Chem. Neurosci. 2020, 11, 3623–3634. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; De Pasquale, D.; Battaglini, M.; di Leo, N.; Degl’Innocenti, A.; Belenli Gümüş, M.; Drago, F.; Ciofani, G. Tannic Acid–Iron Complex-Based Nanoparticles as a Novel Tool against Oxidative Stress. ACS Appl. Mater. Interfaces 2022, 14, 15927–15941. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, J.K.; McMillian, M.; Chikaraishi, D.M. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 1997, 17, 1217–1225. [Google Scholar] [CrossRef]
- Alemán, A.; Mastrogiacomo, I.; López-Caballero, M.E.; Ferrari, B.; Montero, M.P.; Gómez-Guillén, M.C. A Novel Functional Wrapping Design by Complexation of ε-Polylysine with Liposomes Entrapping Bioactive Peptides. Food Bioproc. Tech. 2016, 9, 1113–1124. [Google Scholar] [CrossRef]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Giménez, B.; Cantero-López, P.; Vega, R.; Osorio, F.A. Effect of lyophilization on the physicochemical and rheological properties of food grade liposomes that encapsulate rutin. Food Res. Int. 2020, 130, 108967. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Bianchi, S.; Kroslakova, I.; Mayer, I. Determination of Molecular Structures of Condensed Tannins from Plant Tissues Using HPLC-UV Combined with Thiolysis and MALDI-TOF Mass Spectrometry. Bio-Protoc 2016, 6, e1975. [Google Scholar] [CrossRef]
- Compaoré, M.; Meda, R.N.-T.; Bakasso, S.; Vlase, L.; Kiendrebeogo, M. Antioxidative, anti-inflammatory potentials and phytochemical profile of Commiphora africana (A. Rich.) Engl. (Burseraceae) and Loeseneriella africana (Willd.) (Celastraceae) stem leaves extracts. Asian Pac. J. Trop. Biomed. 2016, 6, 665–670. [Google Scholar] [CrossRef]
- Díaz, H.S.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Pereyra, K.V.; Díaz-Jara, E.; Marcus, N.J.; Rio, R.D. Inhibition of Brainstem Endoplasmic Reticulum Stress Rescues Cardiorespiratory Dysfunction in High Output Heart Failure. Hypertension 2021, 77, 718–728. [Google Scholar] [CrossRef]
- Díaz-Jara, E.; Díaz, H.S.; Rios-Gallardo, A.; Ortolani, D.; Andrade, D.C.; Toledo, C.; Pereyra, K.V.; Schwarz, K.; Ramirez, G.; Ortiz, F.C.; et al. Exercise training reduces brainstem oxidative stress and restores normal breathing function in heart failure. Free Radic. Biol. Med. 2021, 172, 470–481. [Google Scholar] [CrossRef]
- Czaplicka, M.; Parypa, K.; Szewczuk, A.; Gudarowska, E.; Rowińska, M.; Zubaidi, M.A.; Nawirska-Olszańska, A. Assessment of Selected Parameters for Determining the Internal Quality of White Grape Cultivars Grown in Cold Climates. Appl. Sci. 2022, 12, 5534. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, I.T.; Wu, W.L.; Lin, W.N.; Yang, C.M. Adenosine triphosphate regulates NADPH oxidase activity leading to hydrogen peroxide production and COX-2/PGE2 expression in A549 cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L401–L412. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Carson, M.J.; Thrash, J.C.; Walter, B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef]
- Lawrimore, C.J.; Crews, F.T. Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol. Clin. Exp. Res. 2017, 41, 939–954. [Google Scholar] [CrossRef]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Defaux, A.; Braissant, O.; Cagnon, L.; Zurich, M.-G. Methods to assess neuroinflammation. Curr. Protoc. Toxicol. 2011, 50, 12.19.1–12.19.20. [Google Scholar] [CrossRef] [PubMed]
| Analysis | TS | TLS |
|---|---|---|
| Particle size Mean particle size (MPS) Polydispersity index (PDI) Z-potential (ζ) Proximal Analysis | 742.7 ± 5.30 nm 0.67 ± 0.03 −13.50 ± 1.70 mV Content (g/100 g) | 309.9 ± 5.90 nm 0.41 ± 0.02 −21.40 ± 1.70 mV Content (g/100 g) |
| Moisture Ash | 95.06 0.57 | 96.64 0.57 |
| Protein | 0.08 | 0.08 |
| Lipids | 0.48 | 0.18 |
| N.N.E | 2.64 | 2.53 |
| Kcal/100 g | 11.39 | 12.04 |
| GAE (µg/mL) | GAE (µg/mL) | |
| Total polyphenols | 78.34 ± 0.12 * | 30.23 ± 0.14 |
| TEAC (µmol TE/mL) | TEAC (µmol TE/mL) | |
| Antioxidant activity (ABTS) | 2017.51 ± 238.57 * | 9.10 ± 6.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, H.S.; Ríos-Gallardo, A.; Ortolani, D.; Díaz-Jara, E.; Flores, M.J.; Vera, I.; Monasterio, A.; Ortiz, F.C.; Brossard, N.; Osorio, F.; et al. Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants 2022, 11, 1928. https://doi.org/10.3390/antiox11101928
Díaz HS, Ríos-Gallardo A, Ortolani D, Díaz-Jara E, Flores MJ, Vera I, Monasterio A, Ortiz FC, Brossard N, Osorio F, et al. Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants. 2022; 11(10):1928. https://doi.org/10.3390/antiox11101928
Chicago/Turabian StyleDíaz, Hugo S., Angélica Ríos-Gallardo, Domiziana Ortolani, Esteban Díaz-Jara, María José Flores, Ignacio Vera, Angela Monasterio, Fernando C. Ortiz, Natalia Brossard, Fernando Osorio, and et al. 2022. "Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation" Antioxidants 11, no. 10: 1928. https://doi.org/10.3390/antiox11101928
APA StyleDíaz, H. S., Ríos-Gallardo, A., Ortolani, D., Díaz-Jara, E., Flores, M. J., Vera, I., Monasterio, A., Ortiz, F. C., Brossard, N., Osorio, F., & Río, R. D. (2022). Lipid-Encapsuled Grape Tannins Prevent Oxidative-Stress-Induced Neuronal Cell Death, Intracellular ROS Accumulation and Inflammation. Antioxidants, 11(10), 1928. https://doi.org/10.3390/antiox11101928