“Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Pressure and Anthropometric Measurements
2.3. Diagnosis of the Metabolic Syndrome
2.4. Laboratory Tests
2.5. Statistical Analysis
3. Results
3.1. Characteristic of the Study Group
3.2. Oxidative Stress Parameters
3.3. The Results of PCA Analysis
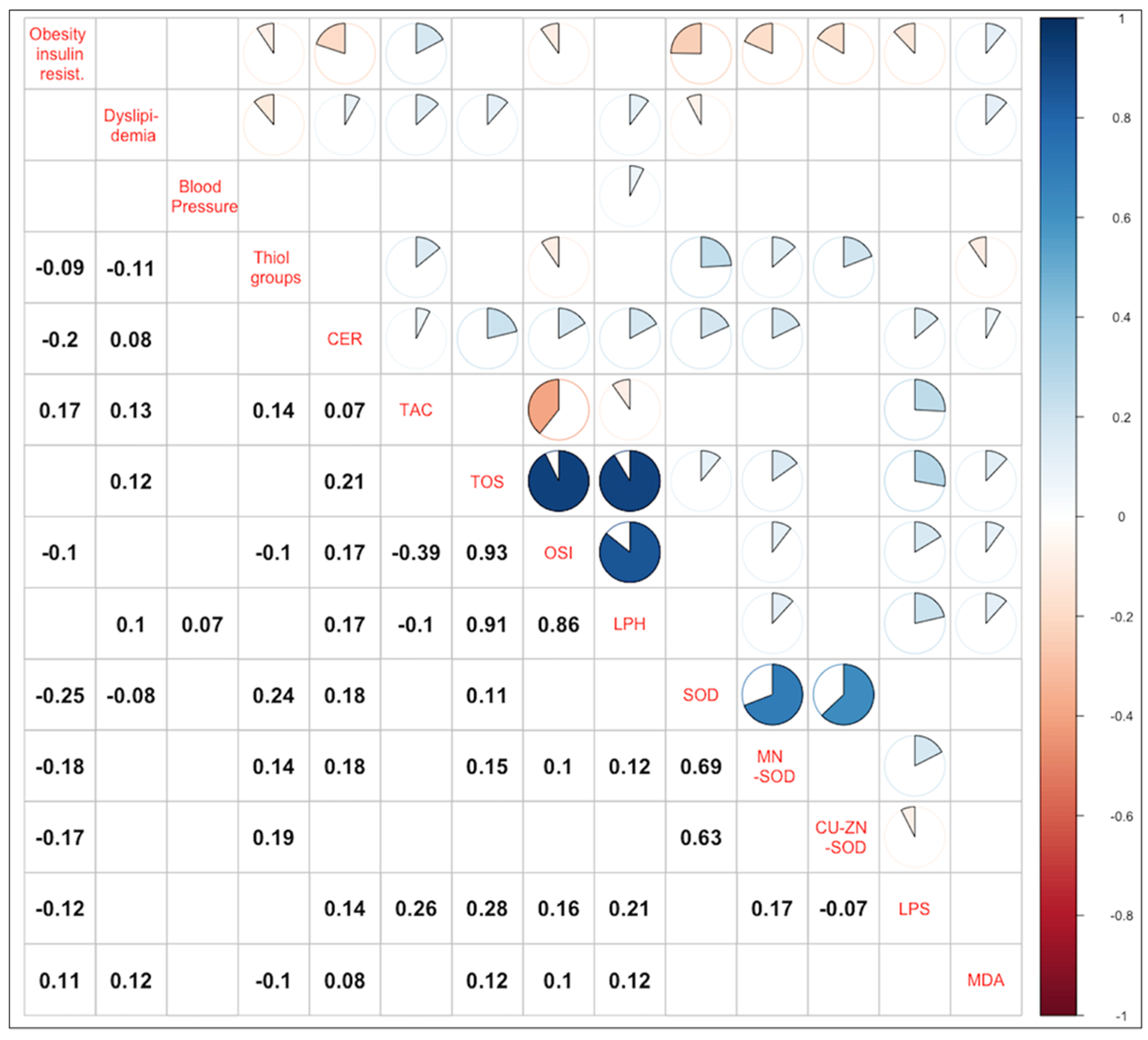
3.4. Correlations of the Main Components and Parameters of Oxidative Stress
4. Discussion
4.1. Obesity and Insulin Resistance and Oxidative Stress
4.2. Dyslipidemia and Oxidative Stress
4.3. Arterial Hypertension and Oxidative Stress
4.4. Metabolic Syndrome and Oxidative Stress
4.5. Principal Component Analysis in Research on the Metabolic Syndrome
4.6. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| 8-iso-PGF2α | 8-iso-prostaglandin F2α |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMPK | adenosine monophosphate-activated protein kinase |
| AST | aspartate aminotransferase |
| apoA | apolipoprotein A |
| apoB | apolipoprotein B |
| BMI | body mass index |
| BP | blood pressure |
| CAD | coronary artery disease |
| CAT | catalase |
| CER | ceruloplasmin |
| CuZnSOD | copper- and zinc-containing superoxide dismutase |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| EWA | equal-weighted average |
| FH | family history |
| GGT | gamma-glutamyl transpeptidase |
| H | height |
| HbA1c | glycated hemoglobin |
| HC | hip circumference |
| HDL | high-density lipoprotein |
| HDL-C | high-density lipoprotein cholesterol blood concentration |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| hsCRP | high-sensitive C-reactive protein |
| IFG | impaired fasting glucose |
| IGT | impaired glucose tolerance |
| IR | insulin resistance |
| LDH | lactate dehydrogenase |
| LDL | low-density lipoprotein |
| LDL-C | low-density lipoprotein cholesterol blood concentration |
| Lp(a) | lipoprotein(a) |
| LPH | lipid hydroperoxides |
| LPS | lipofuscin |
| MAGNETIC | Metabolic and genetic profiling of young adults with and without a family history of premature coronary heart disease |
| MDA | malondialdehyde |
| MHNW | metabolically healthy normal weight |
| MHO | metabolically healthy obese |
| MMP | matrix metalloproteinase |
| MnSOD | manganese-containing superoxide dismutase |
| MS | metabolic syndrome |
| MUO | metabolically unhealthy obese |
| NOX4 | nicotinamide adenine dinucleotide phosphate oxidase 4 |
| OS | oxidative stress |
| OSI | oxidative stress index |
| PCA | principal component analysis |
| PSH | thiol groups concentration per gram protein |
| RC | rotated component |
| SBP | systolic blood pressure |
| SNP | single nucleotide polymorphisms |
| SOD | superoxide dismutase |
| T2D | type 2 diabetes |
| TAC | total antioxidant capacity |
| TC | total cholesterol blood concentration |
| TG | triglyceride blood concentration |
| TOS | total oxidative status |
| TP | total protein |
| TSH | thyrotropin |
| UA | uric acid |
| VLDL | very-low-density lipoprotein |
| W | weight |
| WBC | number of white blood cells per unit of blood volume |
| WC | waist circumference |
| WHR | waist-to-hip ratio |
References
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr. Diab. Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Liu, X.; Li, M.; Wu, B.; Li, Y.; Liang, Y.; Shao, X.; Holthöfer, H.; Zou, H. Insulin resistance and metabolic syndrome in normal-weight individuals. Endocrine 2014, 46, 496–504. [Google Scholar] [CrossRef]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Viner, R.M.; Segal, T.Y.; Lichtarowicz-Krynska, E.; Hindmarsh, P. Prevalence of the insulin resistance syndrome in obesity. Arch. Dis. Child. 2005, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
- De Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Toschke, A.M.; Andler, W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 2007, 92, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, S.; Ziga, S.; Matic, V.; Bokun, T.; Radic, B.; Lucijanic, M.; Marusic, S.; Babic, Z.; Grgurevic, I. Ultrasound grade of liver steatosis is independently associated with the risk of metabolic syndrome. Can. J. Gastroenterol. Hepatol. 2018, 2018, 8490242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Qiao, Q.H.; Zhang, S.C.; Chen, Y.H.; Chao, G.Q.; Fang, L.Z. Metabolic syndrome and gallstone disease. World J. Gastroenterol. 2012, 18, 4215–4220. [Google Scholar] [CrossRef]
- Morrison, C.D.; Brannigan, R.E. Metabolic syndrome and infertility in men. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 507–515. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, V.Q.H.; Truong, Q.V.; Le, D.D.; Le, V.N.S.; Cao, N.T. Metabolic syndrome and insulin resistance syndrome among infertile women with polycystic ovary syndrome: A cross-sectional study from central Vietnam. Endocrinol. Metab. 2018, 33, 447–458. [Google Scholar] [CrossRef]
- Dentali, F.; Squizzato, A.; Ageno, W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin. Thromb. Hemost. 2009, 35, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, T.; Osadnik, K.; Pawlas, N.; Strzelczyk, J.; Kasperczyk, J.; Poloński, L.; Gąsior, M. Metabolic and genetic profiling of young adults with and without a family history of premature coronary heart disease (MAGNETIC). Study design and methodology. Arch. Med. Sci. 2019, 15, 590–597. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Kasperczyk, S.; Osadnik, T.; Pawlas, N. Oxidative stress in association with metabolic health and obesity in young adults. Oxid. Med. Cell. Longev. 2021, 2021, 9987352. [Google Scholar] [CrossRef] [PubMed]
- Fagerland, M.W. t-tests, non-parametric tests, and large studies—A paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Liang, Y.; Naruse, K.; Takahashi, K. Systematic understanding of pathophysiological mechanisms of oxidative stress-related conditions-diabetes mellitus, cardiovascular diseases, and ischemia-reperfusion injury. Front. Cardiovasc. Med. 2021, 8, 649785. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef]
- Fuentes-Venado, C.E.; Terán-Pérez, G.; Espinosa-Hernández, V.M.; Martínez-Herrera, E.; Segura-Uribe, J.J.; Mercadillo, R.E.; Pinto-Almazán, R.; Guerra-Araiza, C. Nutritional status influences oxidative stress and insulin resistance in preschool children. Metab. Syndr. Relat. Disord. 2021, 19, 513–523. [Google Scholar] [CrossRef]
- Kanikowska, D.; Kanikowska, A.; Swora-Cwynar, E.; Grzymisławski, M.; Sato, M.; Bręborowicz, A.; Witowski, J.; Korybalska, K. Moderate caloric restriction partially improved oxidative stress markers in obese humans. Antioxidants 2021, 10, 1018. [Google Scholar] [CrossRef]
- Metere, A.; Graves, C.E.; Pietraforte, D.; Casella, G. The effect of sleeve gastrectomy on oxidative stress in obesity. Biomedicines 2020, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Maurici, J.; Amigó, N.; Cuello, E.; Bermúdez, M.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Pardina, E. Bariatric surgery decreases oxidative stress and protein glycosylation in patients with morbid obesity. Eur. J. Clin. Investig. 2020, 50, e13320. [Google Scholar] [CrossRef]
- Wang, Z.; Bourgonje, A.R.; Groen, H.; Abdulle, A.E.; Cantineau, A.E.P.; van Oers, A.M.; van Dammen, L.; Bulthuis, M.L.C.; Wekker, V.; Mol, B.W.J.; et al. The effect of lifestyle intervention on systemic oxidative stress in women with obesity and infertility: A post-hoc analysis of a randomized controlled trial. J. Clin. Med. 2021, 10, 4243. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. Alterations in concentration/activity of superoxide dismutases in context of obesity and selected single nucleotide polymorphisms in genes: SOD1, SOD2, SOD3. Int. J. Mol. Sci. 2020, 21, 5069. [Google Scholar] [CrossRef] [PubMed]
- Góth, L.; Rass, P.; Páy, A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004, 8, 141–149. [Google Scholar] [CrossRef]
- Heit, C.; Marshall, S.; Singh, S.; Yu, X.; Charkoftaki, G.; Zhao, H.; Orlicky, D.J.; Fritz, K.S.; Thompson, D.C.; Vasiliou, V. Catalase deletion promotes prediabetic phenotype in mice. Free Radic. Biol. Med. 2017, 103, 48–56. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Bae, J.H.; Im, S.S.; Hwang, I.; Ha, H.; Song, D.K. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch. 2019, 471, 829–843. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Im, S.S.; Bae, J.H.; Song, D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 101749. [Google Scholar] [CrossRef]
- Jia, X.J.; Liu, L.X.; Tian, Y.M.; Wang, R.; Lu, Q. The correlation between oxidative stress level and intra-abdominal fat in obese males. Medicine 2019, 98, e14469. [Google Scholar] [CrossRef]
- Mohseni, R.; Arab Sadeghabadi, Z.; Goodarzi, M.T.; Teimouri, M.; Nourbakhsh, M.; Razzaghy Azar, M. Evaluation of Mn-superoxide dismutase and catalase gene expression in childhood obesity: Its association with insulin resistance. J. Pediatr. Endocrinol. Metab. 2018, 31, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska-Gęsiak, S.; Stołtny, D.; Brożek, A.; Muc-Wierzgoń, M.; Wysocka, E. Are insulin-resistance and oxidative stress cause or consequence of aging. Exp. Biol. Med. 2020, 245, 1260–1267. [Google Scholar] [CrossRef]
- Dzięgielewska-Gęsiak, S.; Wysocka, E.; Michalak, S.; Nowakowska-Zajdel, E.; Kokot, T.; Muc-Wierzgoń, M. Role of lipid peroxidation products, plasma total antioxidant status, and Cu-, Zn-superoxide dismutase activity as biomarkers of oxidative stress in elderly prediabetics. Oxid. Med. Cell. Longev. 2014, 2014, 987303. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.M.; Ziaei, R.; Maracy, M.R. Association of serum ceruloplasmin level with obesity: Some components of metabolic syndrome and high-sensitive C-reactive protein in Iran. J. Obes. 2012, 2012, 951093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguilar, M.J.; González-Jiménez, E.; Antelo, A.; Perona, J.S. Insulin resistance and inflammation markers: Correlations in obese adolescents. J. Clin. Nurs. 2013, 22, 2002–2010. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.Y.; Kim, J.Y.; Choi, C.S.; Kim, Y.I.; Chung, Y.E.; Lee, M.S.; Hong, S.K.; Lee, K.U. Elevated serum ceruloplasmin levels in subjects with metabolic syndrome: A population-based study. Metabolism 2002, 51, 838–842. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V.; Tinkov, A.A. Serum copper, zinc, and iron levels, and markers of carbohydrate metabolism in postmenopausal women with prediabetes and type 2 diabetes mellitus. J. Trace Elem. Med. Biol. 2017, 43, 46–51. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of oxLDL and its impact on cardiovascular health: Focus on atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Anderson, R.A.; Evans, M.L.; Ellis, G.R.; Graham, J.; Morris, K.; Jackson, S.K.; Lewis, M.J.; Rees, A.; Frenneaux, M.P. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis 2001, 154, 475–483. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Dong, Q.; Liu, G.; Gao, Y.; Li, X.L.; Jin, J.L.; Li, J.J.; Guo, Y.L. Improvement of oxidative stress status by lipoprotein apheresis in Chinese patients with familial hypercholesterolemia. J. Clin. Lab. Anal. 2020, 34, e23161. [Google Scholar] [CrossRef] [PubMed]
- Kubong, L.N.; Nya Biapa, P.C.; Chetcha, B.; Yanou-Njintang, N.; Moor Ama, V.J.; Pieme, C.A. Relationship between higher atherogenic index of plasma and oxidative stress of a group of patients living with sickle cell anemia in Cameroon. Adv. Hematol. 2020, 2020, 9864371. [Google Scholar] [CrossRef]
- Azzazi, Y.; Mostafa, W.Z.; Sayed, K.S.; Alhelf, M.; Safwat, M.; Mahrous, A.; El Lawindi, M.; Ragab, N. Support for increased cardiovascular risk in non-segmental vitiligo among Egyptians: A hospital-based, case-control study. Pigment Cell Melanoma Res. 2021, 34, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Simic, D.V.; Mimic-Oka, J.; Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Opacic, M.; Matic, D.; Ivanovic, B.; Simic, T. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J. Hum. Hypertens. 2006, 20, 149–155. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Taranta-Janusz, K.; Wasilewska, A.; Kossakowska, A.; Zalewska, A. A case-control study of salivary redox homeostasis in hypertensive children. Can salivary uric acid be a marker of hypertension? J. Clin. Med. 2020, 9, 837. [Google Scholar] [CrossRef]
- Takahashi, K.; Nammour, T.M.; Fukunaga, M.; Ebert, J.; Morrow, J.D.; Roberts, L.J., II; Hoover, R.L.; Badr, K.F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Investig. 1992, 90, 136–141. [Google Scholar] [CrossRef]
- Fukunaga, M.; Makita, N.; Roberts, L.J., II; Morrow, J.D.; Takahashi, K.; Badr, K.F. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol. 1993, 264, C1619–C1924. [Google Scholar] [CrossRef]
- Prado, A.F.; Batista, R.I.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix metalloproteinases and arterial hypertension: Role of oxidative stress and nitric oxide in vascular functional and structural alterations. Biomolecules 2021, 11, 585. [Google Scholar] [CrossRef]
- Janczura, M.; Rosa, R.; Dropinski, J.; Gielicz, A.; Stanisz, A.; Kotula-Horowitz, K.; Domagala, T. The associations of perceived and oxidative stress with hypertension in a cohort of police officers. Diabetes Metab. Syndr. Obes. 2021, 14, 1783–1797. [Google Scholar] [CrossRef]
- Korkmaz, G.G.; Altınoglu, E.; Civelek, S.; Sozer, V.; Erdenen, F.; Tabak, O.; Uzun, H. The association of oxidative stress markers with conventional risk factors in the metabolic syndrome. Metabolism 2013, 62, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, H.; Yu, J.; He, S.; Li, P.; Ma, C.; Zhang, H.; Xu, L.; Ping, F.; Li, W.; et al. Triglyceride is independently correlated with insulin resistance and islet beta cell function: A study in population with different glucose and lipid metabolism states. Lipids Health Dis. 2020, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Vichaibun, V.; Khananurak, K.; Sophonnithiprasert, T. Comparative analysis of plasma total antioxidant capacity in patients with hyperglycemia and hyperglycemia plus dyslipidemia. Diabetes Metab. Syndr. 2019, 13, 90–94. [Google Scholar] [CrossRef]
- Cardona, F.; Túnez, I.; Tasset, I.; Montilla, P.; Collantes, E.; Tinahones, F.J. Fat overload aggravates oxidative stress in patients with the metabolic syndrome. Eur. J. Clin. Investig. 2008, 38, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic-Sreckovic, V.; Colak, E.; Djordjevic, P.; Gostiljac, D.; Sreckovic, B.; Popovic, S.; Canovic, F.; Ilic, M.; Obrenovic, R.; Vukcevic, V.; et al. Prothrombogenic factors and reduced antioxidative defense in children and adolescents with pre-metabolic and metabolic syndrome. Clin. Chem. Lab. Med. 2007, 45, 1140–1144. [Google Scholar] [CrossRef]
- Darroudi, S.; Fereydouni, N.; Tayefi, M.; Ahmadnezhad, M.; Zamani, P.; Tayefi, B.; Kharazmi, J.; Tavalaie, S.; Heidari-Bakavoli, A.; Azarpajouh, M.R.; et al. Oxidative stress and inflammation, two features associated with a high percentage body fat, and that may lead to diabetes mellitus and metabolic syndrome. Biofactors 2019, 45, 35–42. [Google Scholar] [CrossRef]
- Vávrová, L.; Kodydková, J.; Zeman, M.; Dušejovská, M.; Macášek, J.; Staňková, B.; Tvrzická, E.; Zák, A. Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes. Facts 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Ziobro, A.; Duchnowicz, P.; Mulik, A.; Koter-Michalak, M.; Broncel, M. Oxidative damages in erythrocytes of patients with metabolic syndrome. Mol. Cell. Biochem. 2013, 378, 267–273. [Google Scholar] [CrossRef]
- Koter, M.; Franiak, I.; Strychalska, K.; Broncel, M.; Chojnowska-Jezierska, J. Damage to the structure of erythrocyte plasma membranes in patients with type-2 hypercholesterolemia. Int. J. Biochem. Cell. Biol. 2004, 36, 205–215. [Google Scholar] [CrossRef]
- Loos, R.J.; Katzmarzyk, P.T.; Rao, D.C.; Rice, T.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rankinen, T.; Bouchard, C.; HERITAGE Family Study. Genome-wide linkage scan for the metabolic syndrome in the HERITAGE Family Study. J. Clin. Endocrinol. Metab. 2003, 88, 5935–5943. [Google Scholar] [CrossRef]
- Chang, C.H.; Yen, C.H.; Chen, M.Y. Using principal component analysis to develop a single-parameter screening tool for metabolic syndrome. BMC Public Health 2010, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.F.; Carrington, M.J. A metabolic syndrome severity score: A tool to quantify cardio-metabolic risk factors. Prev. Med. 2016, 88, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, H.; Akbarzade, Z.; Djafari, F.; Shab-Bidar, S. Association of nutrient patterns and metabolic syndrome and its components in adults living in Tehran, Iran. J. Diabetes Metab. Disord. 2020, 19, 1071–1079. [Google Scholar] [CrossRef]
- Hailili, G.; Chen, Z.; Tian, T.; Fu, W.H.; Pei, H.L.; Mahan, Y.; Luo, T.; Alimu, D.; Wang, L.; Zhang, G.Z.; et al. Dietary patterns and their associations with the metabolic syndrome and predicted 10-year risk of CVD in northwest Chinese adults. Br. J. Nutr. 2021, 126, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tang, X.; Liu, Y.; Li, Y.; He, B. Dietary patterns and metabolic syndrome among urbanized Tibetans: A cross-sectional study. Environ. Res. 2021, 200, 111354. [Google Scholar] [CrossRef] [PubMed]
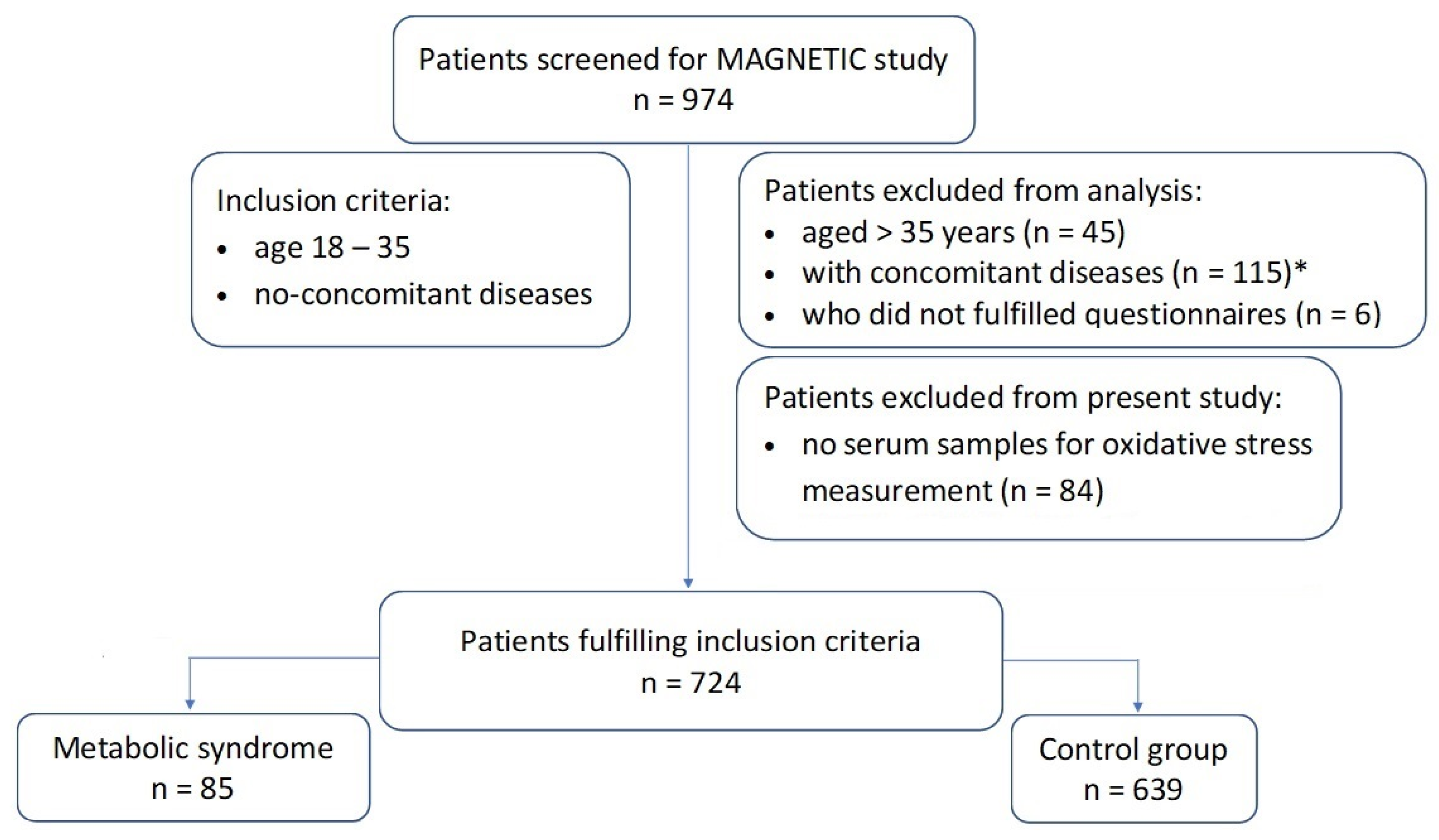
| Variable | Total Sample | Metabolic Syndrome | p-Value † | |
|---|---|---|---|---|
| Yes | No | |||
| N (%) | 724 (100) | 85 (11.7) | 639 (88.3) | |
| Age [years] | 27.9 ± 4.51 | 30.91 ± 3.34 | 27.51 ± 4.49 | <0.0001 |
| Current vs. never and former smoking (%) | 157 (21.9) | 26 (31.0) | 131 (20.7) | 0.04 |
| Higher education vs. other types of education (%) | 402 (55.5) | 46 (54.1) | 356 (55.7) | 0.25 |
| Financial situation above average vs. at or below average (%) | 178 (24.6) | 17 (20.0) | 161 (25.2) | 0.16 |
| Living conditions average or modest vs. good or very good (%) | 313 (43.3) | 41 (48.2) | 272 (42.6) | 0.55 |
| Physical activity low or average vs. high (%) | 522 (72.1) | 74 (87.1) | 448 (70.1) | 0.0002 |
| Daily hours of sleep 6 or less vs. above 6 (%) | 254 (35.2) | 35 (41.2) | 219 (34.4) | 0.4 |
| Place of residence: village or city below 20,000 residents vs. city above 20,000 residents (%) | 253 (34.9) | 33 (38.8) | 220 (34.4) | 0.83 |
| Physical examination | ||||
| BMI [kg/m2] | 24.31 ± 4.40 | 30.91 ± 4.54 | 23.43 ± 3.56 | <0.0001 |
| Waist men [cm] (n = 404) | 88.01 ± 10.82 | 101.76 ± 11.81 | 85.84 ± 8.9 | <0.0001 |
| Waist women [cm] (n = 309) | 74.22 ± 11.4 | 97.85 ± 11.12 | 72.05 ± 8.66 | <0.0001 |
| WHR | 0.83 ± 0.09 | 0.93 ± 0.08 | 0.83 ± 0.09 | <0.0001 |
| SBP [mmHg] | 126.51 ± 13.97 | 138.64 ± 14.96 | 124.9 ± 13.01 | <0.0001 |
| DBP [mmHg] | 78.59 ± 10.52 | 87.53 ± 11.43 | 77.4 ± 9.81 | <0.0001 |
| Variable | Total Sample | Metabolic Syndrome | p-Value † | |
|---|---|---|---|---|
| Yes | No | |||
| N (%) | 724 (100) | 85 (11.7) | 639 (88.3) | |
| Lipids | ||||
| Cholesterol [mmol/L] | 4.94 ± 1.02 | 5.62 ± 1.06 | 4.84 ± 0.98 | <0.0001 |
| LDL-C [mmol/L] | 2.96 ± 0.94 | 3.66 ± 0.89 | 2.87 ± 0.9 | <0.0001 |
| Apolipoprotein B [g/L] | 0.93 ± 0.43 | 1.18 ± 0.28 | 0.9 ± 0.43 | <0.0001 |
| HDL-C [mmol/L] | 1.58 ± 0.44 | 1.13 ± 0.3 | 1.64 ± 0.42 | <0.0001 |
| HDL-C [%] | 33.33 ± 10.78 | 20.81 ± 6.66 | 34.99 ± 10.11 | <0.0001 |
| Apolipoprotein A [g/L] | 1.62 ± 0.32 | 1.45 ± 0.29 | 1.64 ± 0.31 | <0.0001 |
| Triglycerides [mmol/L] | 1.18 ± 1.02 | 2.65 ± 2.07 | 0.99 ± 0.55 | <0.0001 |
| Lp(a) [nmol/L] | 41.86 ± 63.28 | 49.14 ± 64.16 | 40.89 ± 63.15 | 0.27 |
| Liver cells | ||||
| AST [IU/L] | 23.59 ± 21.01 | 27.85 ± 14.35 | 23.02 ± 21.69 | 0.008 |
| ALT [IU/L] | 25.45 ± 23.6 | 41.22 ± 28.03 | 23.36 ± 22.14 | <0.0001 |
| GGT [IU/L] | 26.55 ± 29.82 | 55.4 ± 57.35 | 22.71 ± 21.19 | <0.0001 |
| LDH [IU/L] | 173.91 ± 38.45 | 188.33 ± 27.03 | 171.99 ± 39.35 | <0.0001 |
| Bilirubin [μmol/L] | 11.43 ± 6.35 | 10.71 ± 6 | 11.53 ± 6.39 | 0.24 |
| ALP [IU/L] | 64.65 ± 19.58 | 73.06 ± 16.98 | 63.54 ± 19.64 | <0.0001 |
| Glucose metabolism | ||||
| Glucose [mmol/L] | 5.01 ± 0.45 | 5.45 ± 0.54 | 4.95 ± 0.41 | <0.0001 |
| HbA1c [%] | 4.99 ± 0.26 | 5.16 ± 0.26 | 4.95 ± 0.26 | <0.0001 |
| Total protein [g/L] | 75.24 ± 5.14 | 74.89 ± 3.7 | 75.29 ± 5.3 | 0.38 |
| Remaining lab test results | ||||
| WBC [×109 /L] | 5.85 ± 1.49 | 6.58 ± 1.63 | 5.75 ± 1.44 | <0.0001 |
| Albumin [g/L] | 47.61 ± 3.32 | 47.18 ± 3.25 | 47.66 ± 3.32 | 0.20 |
| Homocysteine [μmol/L] | 11.69 ± 4.43 | 12.96 ± 7.54 | 11.52 ± 3.82 | 0.09 |
| Fibrinogen [mg/dL] | 275.08 ± 64.65 | 305.77 ± 72.17 | 271.01 ± 62.52 | 0.0001 |
| hsCRP [mg/dL] | 1.77 ± 2.74 | 2.47 ± 2.65 | 1.68 ± 2.74 | 0.01 |
| Uric acid [μmol/L] | 310.11 ± 75.8 | 368.95 ± 67.52 | 302.29 ± 73.4 | <0.0001 |
| Cystatin C [mg/dL] | 0.81 ± 0.11 | 0.84 ± 0.12 | 0.8 ± 0.1 | 0.01 |
| Creatinine [μmol/L] | 78.1 ± 13.78 | 80.08 ± 13.95 | 77.83 ± 13.75 | 0.16 |
| TSH [mU/L] | 2.06 ± 1.11 | 2.23 ± 1.61 | 2.04 ± 1.03 | 0.29 |
| Vitamin D [ng/mL] | 22.51 ± 11.02 | 20.09 ± 11.06 | 22.83 ± 10.98 | 0.03 |
| Variable | Total Sample | Metabolic Syndrome | p-Value † | |
|---|---|---|---|---|
| Yes | No | |||
| N (%) | 724 (100) | 85 (11.7) | 639 (88.3) | |
| PSH [μmol/g protein] | 4.6 ± 0.77 | 4.4 ± 0.88 | 4.62 ± 0.77 | 0.03 |
| CER [mg/dL] | 44.04 ± 14.49 | 44.03 ± 14.47 | 44.04 ± 14.5 | 0.99 |
| TAC [mmol/L] | 1.07 ± 0.15 | 1.11 ± 0.17 | 1.06 ± 0.15 | 0.01 |
| TOS [μmol/L] | 11.91 ± 34.05 | 13.01 ± 37.6 | 11.76 ± 33.58 | 0.77 |
| OSI [%] | 1.13 ± 3.16 | 1.16 ± 3.12 | 1.13 ± 3.17 | 0.94 |
| LPH [μmol/L] | 5.9 ± 17.66 | 7.07 ± 21.71 | 5.74 ± 17.06 | 0.59 |
| SOD [NU/mL] | 20.72 ± 2.49 | 19.45 ± 2.43 | 20.89 ± 2.45 | <0.0001 |
| MnSOD [NU/mL] | 11.01 ± 1.84 | 10.57 ± 1.85 | 11.07 ± 1.83 | 0.02 |
| CuZnSOD [NU/mL] | 9.71 ± 1.93 | 8.86 ± 1.8 | 9.82 ± 1.93 | <0.0001 |
| LPS [RU/L] | 272.96 ± 138.26 | 255.93 ± 172.18 | 275.23 ± 133.11 | 0.32 |
| MDA [μmol/L] | 2.19 ± 2.4 | 2.3 ± 2.41 | 2.17 ± 2.41 | 0.64 |
| Parameters | Factor Loadings | ||
|---|---|---|---|
| RC1 “Obesity and Insulin Resistance” | RC2 “Dyslipidemia” | RC3 “Blood Pressure” | |
| BMI [kg/m2] | 0.82 | ||
| WC [cm] | 0.85 | ||
| SBP [mmHg] | 0.88 | ||
| DBP [mmHg] | 0.89 | ||
| TC [mmol/L] | 0.98 | ||
| LDL [mmol/L] | 0.89 | ||
| HDL [mmol/L] | −0.73 | ||
| TG [mmol/L] | 0.45 | 0.48 | |
| Glucose [mmol/L] | 0.59 | ||
| HbA1c [%] | 0.48 | ||
| Variance explained | 28% | 22% | 18% |
| Cumulative variance explained | 28% | 50% | 68% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2022, 11, 79. https://doi.org/10.3390/antiox11010079
Jakubiak GK, Osadnik K, Lejawa M, Osadnik T, Goławski M, Lewandowski P, Pawlas N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants. 2022; 11(1):79. https://doi.org/10.3390/antiox11010079
Chicago/Turabian StyleJakubiak, Grzegorz K., Kamila Osadnik, Mateusz Lejawa, Tadeusz Osadnik, Marcin Goławski, Piotr Lewandowski, and Natalia Pawlas. 2022. "“Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress" Antioxidants 11, no. 1: 79. https://doi.org/10.3390/antiox11010079
APA StyleJakubiak, G. K., Osadnik, K., Lejawa, M., Osadnik, T., Goławski, M., Lewandowski, P., & Pawlas, N. (2022). “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants, 11(1), 79. https://doi.org/10.3390/antiox11010079








