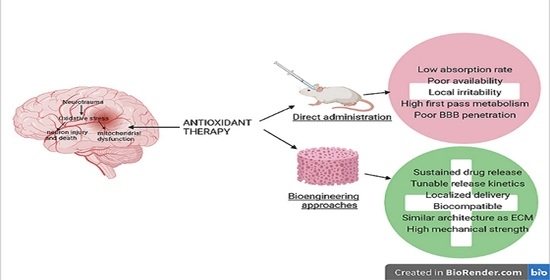
Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches
Abstract
:1. Introduction
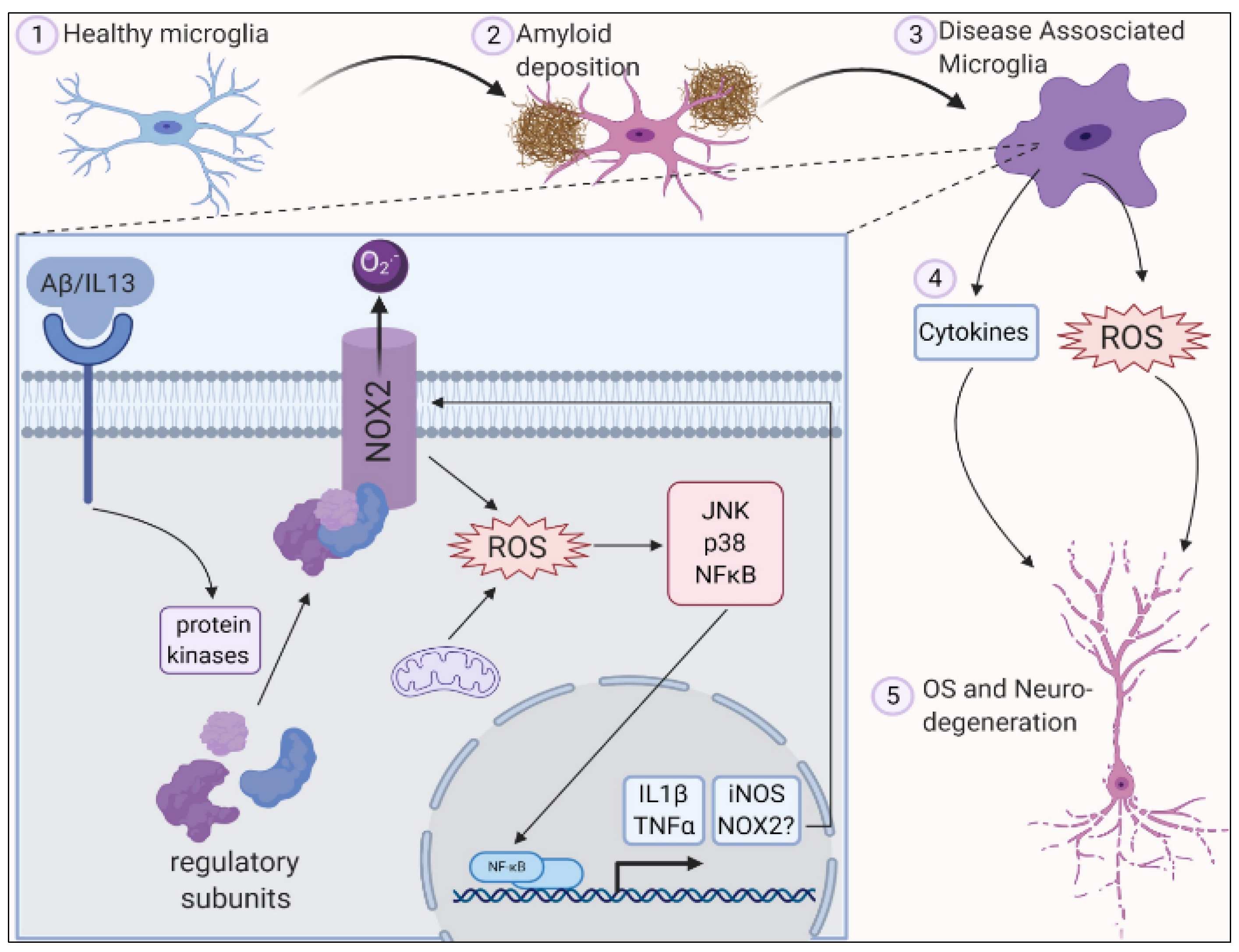
2. Role of Oxidative Stress in Central Nervous System
3. Role of Oxidative Stress in Neurodegenerative Diseases
4. Antioxidants in CNS and PNS
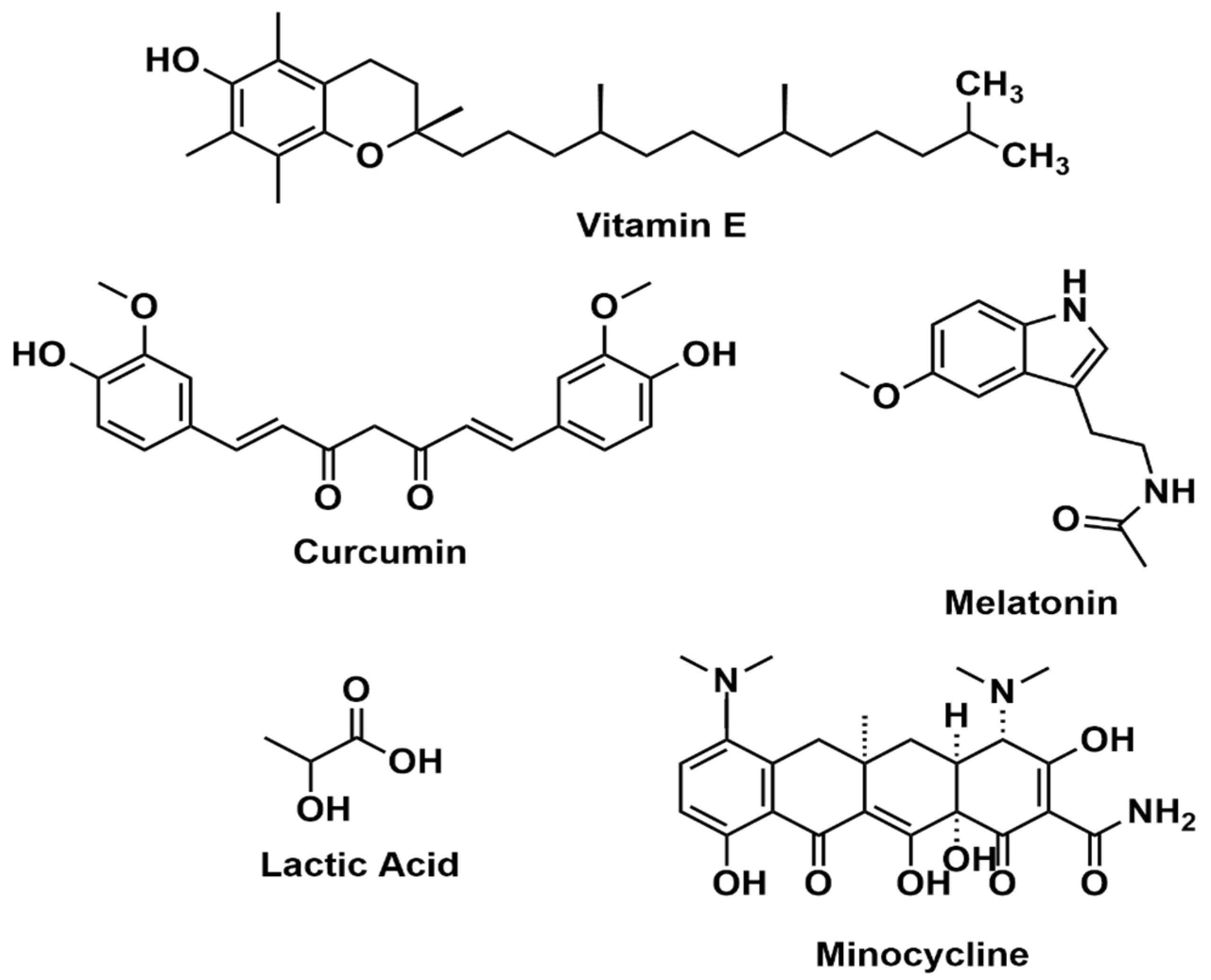
4.1. Vitamin E
4.2. Curcumin
4.3. Melatonin
4.4. Nanoceria
4.5. Lactic Acid
4.6. Hydroalcoholic Extract of the Red Propolis (HERP)
4.7. Minocycline
4.8. Nitrones
5. Bioengineering Approaches to Regenerating CNS and PNS
5.1. Antioxidants Derived from Natural Sources
5.2. Synthetically Obtained Antioxidants
6. Conclusions and the Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, N.S.; Tuveson, D.A. The Promise and Perils of Antioxidants for Cancer Patients. N. Engl. J. Med. 2014, 371, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Chapter 1: Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; RSC Publishing: London, UK, 2016; pp. 1–21. ISBN 978-1-78262-220-8. [Google Scholar]
- Agarwal, V.; Chatterjee, K. Recent Advances in the Field of Transition Metal Dichalcogenides for Biomedical Applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and Physiological Role of Reactive Oxygen Species—The Good, the Bad and the Ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Indrakumar, S.; Chatterjee, K.; Agarwal, V.; Bose, S. ‘Template-Free’ Hierarchical MoS2 Foam as a Sustainable ‘Green’ Scavenger of Heavy Metals and Bacteria in Point of Use Water Purification. Nanoscale Adv. 2020, 2, 2824–2834. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive Oxygen Species and Cancer: A Complex Interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Nadeem, A.; Masood, A.; Siddiqui, N. Oxidant—Antioxidant Imbalance in Asthma: Scientific Evidence, Epidemiological Data and Possible Therapeutic Options. Adv. Respir. Dis. 2008, 2, 215–235. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F. Antioxidants and Atherosclerotic Heart Disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef]
- Lu, L.Y.; Ou, N.; Lu, Q.-B. Antioxidant Induces DNA Damage, Cell Death and Mutagenicity in Human Lung and Skin Normal Cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milbourn, H.R.; Toomey, L.M.; Gavriel, N.; Gray, C.G.G.; Gough, A.H.; Fehily, B.; Giacci, M.K.; Fitzgerald, M. Limiting Oxidative Stress Following Neurotrauma with a Combination of Ion Channel Inhibitors. Discov. Med. 2017, 23, 361–369. [Google Scholar] [PubMed]
- Giacci, M.K.; Bartlett, C.A.; Smith, N.M.; Iyer, K.S.; Toomey, L.M.; Jiang, H.; Guagliardo, P.; Kilburn, M.R.; Fitzgerald, M. Oligodendroglia Are Particularly Vulnerable to Oxidative Damage after Neurotrauma In Vivo. J. Neurosci. 2018, 38, 6491–6504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxid. Redox Signal. 2021, 34, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, e2525967. [Google Scholar] [CrossRef]
- Lee, Y.M.; He, W.; Liou, Y.-C. The Redox Language in Neurodegenerative Diseases: Oxidative Post-Translational Modifications by Hydrogen Peroxide. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Halliwell, B. Role of Free Radicals in the Neurodegenerative Diseases: Therapeutic Implications for Antioxidant Treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Shah, R.; Farmer, L.A.; Zilka, O.; Kessel, A.T.M.V.; Pratt, D.A. Beyond DPPH: Use of Fluorescence-Enabled Inhibited Autoxidation to Predict Oxidative Cell Death Rescue. Cell Chem. Biol. 2019, 26, 1594–1607.e7. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Kraus, R.L.; Pasieczny, R.; Lariosa-Willingham, K.; Turner, M.S.; Jiang, A.; Trauger, J.W. Antioxidant Properties of Minocycline: Neuroprotection in an Oxidative Stress Assay and Direct Radical-Scavenging Activity. J. Neurochem. 2005, 94, 819–827. [Google Scholar] [CrossRef]
- Agarwal, V.; Tjandra, E.S.; Iyer, K.S.; Humfrey, B.; Fear, M.; Wood, F.M.; Dunlop, S.; Raston, C.L. Evaluating the Effects of Nacre on Human Skin and Scar Cells in Culture. Toxicol. Res. 2014, 3, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Yamato, M.; Egashira, T.; Utsumi, H. Application of in Vivo ESR Spectroscopy to Measurement of Cerebrovascular ROS Generation in Stroke. Free Radic. Biol. Med. 2003, 35, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent Advances in Reactive Oxygen Species Measurement in Biological Systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castro, P.; Vallejo, M.; San Román, M.F.; Ortiz, I. Insight on the Fundamentals of Advanced Oxidation Processes. Role and Review of the Determination Methods of Reactive Oxygen Species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione Peroxidase 4: A New Player in Neurodegeneration? Mol. Psychiatry 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, More than Just an Antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Arrigoni, O.; De Tullio, M.C. Ascorbic Acid: Much More than Just an Antioxidant. Biochim. Biophys. Acta 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-Lipoic Acid as a Biological Antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Dragsted, L.O. Antioxidant Actions of Polyphenols in Humans. Int. J. Vitam. Nutr. Res. 2003, 73, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced Sciatic Nerve Regeneration by Poly-L-Lactic Acid/Multi-Wall Carbon Nanotube Neural Guidance Conduit Containing Schwann Cells and Curcumin Encapsulated Chitosan Nanoparticles in Rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef]
- Singh, A.; Shiekh, P.A.; Das, M.; Seppälä, J.; Kumar, A. Aligned Chitosan-Gelatin Cryogel-Filled Polyurethane Nerve Guidance Channel for Neural Tissue Engineering: Fabrication, Characterization, and In Vitro Evaluation. Biomacromolecules 2019, 20, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Marcos, R.; Hernández, A. Nanoceria Acts as Antioxidant in Tumoral and Transformed Cells. Chem. Biol. Interact. 2018, 291, 7–15. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Sergeeva, V.; Kraevaya, O.; Ershova, E.; Kameneva, L.; Malinovskaya, E.; Dolgikh, O.; Konkova, M.; Voronov, I.; Zhilenkov, A.; Veiko, N.; et al. Antioxidant Properties of Fullerene Derivatives Depend on Their Chemical Structure: A Study of Two Fullerene Derivatives on HELFs. Oxid. Med. Cell. Longev. 2019, 2019, 4398695. [Google Scholar] [CrossRef]
- Türkan, F.; Taslimi, P.; Saltan, F.Z. Tannic Acid as a Natural Antioxidant Compound: Discovery of a Potent Metabolic Enzyme Inhibitor for a New Therapeutic Approach in Diabetes and Alzheimer’s Disease. J. Biochem. Mol. Toxicol. 2019, 33, e22340. [Google Scholar] [CrossRef]
- Moreno-García, A.; Kun, A.; Calero, M.; Calero, O. The Neuromelanin Paradox and Its Dual Role in Oxidative Stress and Neurodegeneration. Antioxidants 2021, 10, 124. [Google Scholar] [CrossRef]
- Floyd, R.A.; Kopke, R.D.; Choi, C.-H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as Therapeutics. Free Radic. Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Głowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Oset-Gasque, M.J. Synthesis and Neuroprotective Properties of N-Substituted C-Dialkoxyphosphorylated Nitrones. ACS Omega 2019, 4, 8581–8587. [Google Scholar] [CrossRef] [Green Version]
- Chioua, M.; Salgado-Ramos, M.; Diez-Iriepa, D.; Escobar-Peso, A.; Iriepa, I.; Hadjipavlou-Litina, D.; Martínez-Alonso, E.; Alcázar, A.; Marco-Contelles, J. Novel Quinolylnitrones Combining Neuroprotective and Antioxidant Properties. ACS Chem. Neurosci. 2019, 10, 2703–2706. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Martínez-Alonso, E.; Chioua, M.; Escobar-Peso, A.; Gonzalo-Gobernado, R.; Montaner, J.; Marco-Contelles, J.; Alcázar, A. Quinolinyl Nitrone RP19 Induces Neuroprotection after Transient Brain Ischemia. ACS Chem. Neurosci. 2017, 8, 2202–2213. [Google Scholar] [CrossRef]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P.; Caan, B.J. Antioxidant Supplement Use after Breast Cancer Diagnosis and Mortality in the Life After Cancer Epidemiology (LACE) Cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; de Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-de Jong, J.C. Total Dietary Antioxidant Capacity, Individual Antioxidant Intake and Breast Cancer Risk: The Rotterdam Study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural Antioxidants and Hypertension: Promise and Challenges. Cardiovasc. Ther. 2010, 28, 20–32. [Google Scholar] [CrossRef]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A Review of the Interaction among Dietary Antioxidants and Reactive Oxygen Species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M. Unraveling the Truth about Antioxidants: Mitohormesis Explains ROS-Induced Health Benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef]
- Milisav, I.; Ribarič, S.; Poljsak, B. Antioxidant Vitamins and Ageing. In Biochemistry and Cell Biology of Ageing: Part I Biomedical Science; Harris, J.R., Korolchuk, V.I., Eds.; Subcellular Biochemistry; Springer: Singapore, 2018; pp. 1–23. ISBN 9789811328350. [Google Scholar]
- Palacio, C.; Mooradian, A.D. Clinical Trials and Antioxidant Outcomes. In Oxidative Stress and Antioxidant Protection; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; pp. 493–506. ISBN 978-1-118-83243-1. [Google Scholar]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A Review of the Antioxidant Potential of Medicinal Plant Species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A Comparative Study between Natural and Synthetic Antioxidants: Evaluation of Their Performance after Incorporation into Biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Stokes, P.; Belay, R.E.; Ko, E.Y. Synthetic Antioxidants. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 543–551. ISBN 978-3-030-32300-4. [Google Scholar]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Bayir, H. Reactive Oxygen Species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef] [PubMed]
- Brown David, I.; Griendling Kathy, K. Regulation of Signal Transduction by Reactive Oxygen Species in the Cardiovascular System. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharm. Exp. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative Stress in Cancer and Fibrosis: Opportunity for Therapeutic Intervention with Antioxidant Compounds, Enzymes, and Nanoparticles. Redox Biol. 2016, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Brain Metabolism in Health, Aging, and Neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid Peroxidation Triggers Neurodegeneration: A Redox Proteomics View into the Alzheimer Disease Brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Anantharaman, M.; Tangpong, J.; Keller, J.N.; Murphy, M.P.; Markesbery, W.R.; Kiningham, K.K.; St Clair, D.K. Beta-Amyloid Mediated Nitration of Manganese Superoxide Dismutase: Implication for Oxidative Stress in a APPNLH/NLH X PS-1P264L/P264L Double Knock-in Mouse Model of Alzheimer’s Disease. Am. J. Pathol. 2006, 168, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, J.; Epand, R.F.; Epand, R.M. Tocopherols and Tocotrienols in Membranes: A Critical Review. Free Radic. Biol. Med. 2008, 44, 739–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Ulatowski, L.M. Vitamin E: Mechanism of Transport and Regulation in the CNS. IUBMB Life 2019, 71, 424–429. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Weihrauch, J.L. Vitamin E Content of Foods. J. Am. Diet Assoc. 1979, 75, 647–665. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Andersson, R. A Multivariate Study of the Correlation between Tocopherol Content and Fatty Acid Composition in Vegetable Oils. J. Am. Oil Chem. Soc. 1997, 74, 375–380. [Google Scholar] [CrossRef]
- Hosomi, A.; Goto, K.; Kondo, H.; Iwatsubo, T.; Yokota, T.; Ogawa, M.; Arita, M.; Aoki, J.; Arai, H.; Inoue, K. Localization of Alpha-Tocopherol Transfer Protein in Rat Brain. Neurosci. Lett. 1998, 256, 159–162. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, X. Vitamin E Reduces the Extent of Mouse Brain Damage Induced by Combined Exposure to Formaldehyde and PM2.5. Ecotoxicol. Environ. Saf. 2019, 172, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Ziembicka, D.; Żendzian-Piotrowska, M.; Maciejczyk, M. The Impact of High-Fat Diet on Mitochondrial Function, Free Radical Production, and Nitrosative Stress in the Salivary Glands of Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, e2606120. [Google Scholar] [CrossRef] [Green Version]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-Fat Diet-Induced Metabolic Syndrome and Oxidative Stress in Obese Rats Are Ameliorated by Yogurt Supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, S.M.; Kim, I.Y.; Sung, H.; Seong, J.K.; Moon, M.H. High-Fat Diet-Induced Lipidome Perturbations in the Cortex, Hippocampus, Hypothalamus, and Olfactory Bulb of Mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 980–990. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Hasan, Z.A.; Khabour, O.F.; Mayyas, F.A.; Al Yacoub, O.N.; Banihani, S.A.; Alomari, M.A.; Alrabadi, N.N. Vitamin E Modifies High-Fat Diet-Induced Reduction of Seizure Threshold in Rats: Role of Oxidative Stress. Physiol. Behav. 2019, 206, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-Oxidative Effects of Curcumin on Immobilization-Induced Oxidative Stress in Rat Brain, Liver and Kidney. Biomed. Pharm. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ferreira, E.; Sisa, C.; Bright, S.; Fautz, T.; Harris, M.; Contreras Riquelme, I.; Agwu, C.; Kurulday, T.; Mistry, B.; Hill, D.; et al. Curcumin: Novel Treatment in Neonatal Hypoxic-Ischemic Brain Injury. Front. Physiol. 2019, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.D.; Manning, H.J.; Robertson, N.J.; Ma, D.; Edwards, A.D.; Hagberg, H.; Maze, M. Preconditioning and Postinsult Therapies for Perinatal Hypoxic-Ischemic Injury at Term. Anesthesiology 2010, 113, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, C.; Brudin, L.; Wanby, A.-S.; Blomberg, M. Ante- and Intrapartum Risk Factors for Neonatal Hypoxic Ischemic Encephalopathy. J. Matern. Fetal. Neonatal. Med. 2018, 31, 1595–1601. [Google Scholar] [CrossRef]
- Rocha-Ferreira, E.; Phillips, E.; Francesch-Domenech, E.; Thei, L.; Peebles, D.M.; Raivich, G.; Hristova, M. The Role of Different Strain Backgrounds in Bacterial Endotoxin-Mediated Sensitization to Neonatal Hypoxic–Ischemic Brain Damage. Neuroscience 2015, 311, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Hope, P.L.; Costello, A.M.; Cady, E.B.; Delpy, D.T.; Tofts, P.S.; Chu, A.; Hamilton, P.A.; Reynolds, E.O.; Wilkie, D.R. Cerebral Energy Metabolism Studied with Phosphorus NMR Spectroscopy in Normal and Birth-Asphyxiated Infants. Lancet 1984, 2, 366–370. [Google Scholar] [CrossRef]
- Penrice, J.; Lorek, A.; Cady, E.B.; Amess, P.N.; Wylezinska, M.; Cooper, C.E.; D’Souza, P.; Brown, G.C.; Kirkbride, V.; Edwards, A.D.; et al. Proton Magnetic Resonance Spectroscopy of the Brain during Acute Hypoxia-Ischemia and Delayed Cerebral Energy Failure in the Newborn Piglet. Pediatr. Res. 1997, 41, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhao, F.; Wang, H.; Qu, Y.; Mu, D. Nitric Oxide Synthase in Hypoxic or Ischemic Brain Injury. Rev. Neurosci. 2015, 26, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.-K. Reactive Oxygen Species and Inhibitors of Inflammatory Enzymes, NADPH Oxidase, and INOS in Experimental Models of Parkinson’s Disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible Nitric Oxide Synthase (INOS) in Tumor Biology: The Two Sides of the Same Coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The Dual Role of Inducible Nitric Oxide Synthase in Myocardial Ischemia/Reperfusion Injury: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, e8364848. [Google Scholar] [CrossRef] [Green Version]
- Licinio, J.; Prolo, P.; McCann, S.M.; Wong, M.-L. Brain INOS: Current Understanding and Clinical Implications. Mol. Med. Today 1999, 5, 225–232. [Google Scholar] [CrossRef]
- Wong, M.L.; Rettori, V.; al-Shekhlee, A.; Bongiorno, P.B.; Canteros, G.; McCann, S.M.; Gold, P.W.; Licinio, J. Inducible Nitric Oxide Synthase Gene Expression in the Brain during Systemic Inflammation. Nat. Med. 1996, 2, 581–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yüce, S.; Cemal Gökçe, E.; Işkdemir, A.; Koç, E.R.; Cemil, D.B.; Gökçe, A.; Sargon, M.F. An Experimental Comparison of the Effects of Propolis, Curcumin, and Methylprednisolone on Crush Injuries of the Sciatic Nerve. Ann. Plast. Surg. 2015, 74, 684–692. [Google Scholar] [CrossRef]
- Shokouhi, G.; Tubbs, R.S.; Shoja, M.M.; Hadidchi, S.; Ghorbanihaghjo, A.; Roshangar, L.; Farahani, R.M.; Mesgari, M.; Oakes, W.J. Neuroprotective Effects of High-Dose vs. Low-Dose Melatonin after Blunt Sciatic Nerve Injury. Childs Nerv. Syst. 2008, 24, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gül, S.; Celik, S.E.; Kalayci, M.; Taşyürekli, M.; Cokar, N.; Bilge, T. Dose-Dependent Neuroprotective Effects of Melatonin on Experimental Spinal Cord Injury in Rats. Surg. Neurol. 2005, 64, 355–361. [Google Scholar] [CrossRef]
- Baydas, G.; Reiter, R.J.; Nedzvetskii, V.S.; Yaşar, A.; Tuzcu, M.; Ozveren, F.; Canatan, H. Melatonin Protects the Central Nervous System of Rats against Toluene-Containing Thinner Intoxication by Reducing Reactive Gliosis. Toxicol. Lett. 2003, 137, 169–174. [Google Scholar] [CrossRef]
- Eller, N.; Netterstrøm, B.; Laursen, P. Risk of Chronic Effects on the Central Nervous System at Low Toluene Exposure. Occup. Med. 1999, 49, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The Effects of Toluene on the Central Nervous System. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, N.L.; Kleinschmidt-DeMasters, B.K.; Davis, K.A.; Dreisbach, J.N.; Hormes, J.T.; Filley, C.M. Toluene Abuse Causes Diffuse Central Nervous System White Matter Changes. Ann. Neurol. 1988, 23, 611–614. [Google Scholar] [CrossRef]
- Moonen, G.; Rogister, B.; Leprince, P.; Rigo, J.; Delrée, P.; Lefebvre, P.; Schoenen, J. Neurono-Glial Interactions and Neural Plasticity. Prog. Brain Res. 1990, 86, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Demır, M.; Cicek, M.; Eser, N.; Yoldaş, A.; Sısman, T. Effects of Acute Toluene Toxicity on Different Regions of Rabbit Brain. Anal. Cell. Pathol. 2017, 2017, 2805370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, M.M. The Central Nervous System and Exposure to Toluene: A Risk Characterization. Environ. Res. 1997, 72, 1–7. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.-F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-Catalytic Ceria Nanoparticles Offer Neuroprotection to Adult Rat Spinal Cord Neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate—A Signal Coordinating Cell and Systemic Function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Herz, H.; Blake, D.R.; Grootveld, M. Multicomponent Investigations of the Hydrogen Peroxide- and Hydroxyl Radical-Scavenging Antioxidant Capacities of Biofluids: The Roles of Endogenous Pyruvate and Lactate. Relevance to Inflammatory Joint Diseases. Free Radic. Res. 1997, 26, 19–35. [Google Scholar] [CrossRef]
- Lampe, K.J.; Namba, R.M.; Silverman, T.R.; Bjugstad, K.B.; Mahoney, M.J. Impact of Lactic Acid on Cell Proliferation and Free Radical-Induced Cell Death in Monolayer Cultures of Neural Precursor Cells. Biotechnol. Bioeng. 2009, 103, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free Radical Scavenging and Antioxidant Effects of Lactate Ion: An in Vitro Study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar] [CrossRef]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian Red Propolis: Unreported Substances, Antioxidant and Antimicrobial Activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian Red Propolis—Chemical Composition and Botanical Origin. Evid.-Based Complement. Altern. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Moise, A.R.; Bobiş, O. Baccharis Dracunculifolia and Dalbergia Ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- de Carvalho, F.M.d.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Caramão Bastos, E.; et al. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef]
- Dantas Silva, R.P.; Machado, B.A.S.; de Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, Antimicrobial, Antiparasitic, and Cytotoxic Properties of Various Brazilian Propolis Extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- Barbosa, R.A.; Nunes, T.L.G.M.; Nunes, T.L.G.M.; da Paixão, A.O.; Belo Neto, R.; Moura, S.; Albuquerque Junior, R.L.C.; Cândido, E.A.F.; Padilha, F.F.; Quintans-Júnior, L.J.; et al. Hydroalcoholic Extract of Red Propolis Promotes Functional Recovery and Axon Repair after Sciatic Nerve Injury in Rats. Pharm. Biol. 2016, 54, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Arai, M.A.; Koryudzu, K.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Naturally Occurring Ngn2 Promoter Activators from Butea Superba. Mol. Biosyst. 2013, 9, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Q.; Jin, Z.-Y.; Li, G.-H. Biochanin A Protects Dopaminergic Neurons against Lipopolysaccharide-Induced Damage through Inhibition of Microglia Activation and Proinflammatory Factors Generation. Neurosci. Lett. 2007, 417, 112–117. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an Antibiotic. Br. J. Pharm. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogden, R.N.; Speight, T.M.; Avery, G.S. Minocycline: A Review of Its Antibacterial and Pharmacokinetic Properties and Therapeutic Use. Drugs 1975, 9, 251–291. [Google Scholar] [CrossRef]
- Yrjänheikki, J.; Tikka, T.; Keinänen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A Tetracycline Derivative, Minocycline, Reduces Inflammation and Protects against Focal Cerebral Ischemia with a Wide Therapeutic Window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.M.; Choi, A.O.; Maysinger, D.; Winnik, F.M. Minocycline Block Copolymer Micelles and Their Anti-Inflammatory Effects on Microglia. Macromol. Biosci. 2010, 10, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquette, D.W. Minocycline Microspheres: A Complementary Medical-Mechanical Model for the Treatment of Chronic Periodontitis. Compend. Contin. Educ. Dent. 2002, 23, 15–21. [Google Scholar] [PubMed]
- Kashi, T.S.J.; Eskandarion, S.; Esfandyari-Manesh, M.; Marashi, S.M.A.; Samadi, N.; Fatemi, S.M.; Atyabi, F.; Eshraghi, S.; Dinarvand, R. Improved Drug Loading and Antibacterial Activity of Minocycline-Loaded PLGA Nanoparticles Prepared by Solid/Oil/Water Ion Pairing Method. Int. J. Nanomed. 2012, 7, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Papa, S.; Rossi, F.; Ferrari, R.; Mariani, A.; De Paola, M.; Caron, I.; Fiordaliso, F.; Bisighini, C.; Sammali, E.; Colombo, C.; et al. Selective Nanovector Mediated Treatment of Activated Proinflammatory Microglia/Macrophages in Spinal Cord Injury. ACS Nano 2013, 7, 9881–9895. [Google Scholar] [CrossRef]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early Modulation of Pro-Inflammatory Microglia by Minocycline Loaded Nanoparticles Confers Long Lasting Protection after Spinal Cord Injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Wang, Z.; Nong, J.; Shultz, R.B.; Zhang, Z.; Kim, T.; Tom, V.J.; Ponnappan, R.K.; Zhong, Y. Local Delivery of Minocycline from Metal Ion-Assisted Self-Assembled Complexes Promotes Neuroprotection and Functional Recovery after Spinal Cord Injury. Biomaterials 2017, 112, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Deletraz, A.; Zéamari, K.; Hua, K.; Combes, M.; Villamena, F.A.; Tuccio, B.; Callizot, N.; Durand, G. Substituted α-Phenyl and α-Naphthlyl-N-Tert-Butyl Nitrones: Synthesis, Spin-Trapping, and Neuroprotection Evaluation. J. Org. Chem. 2020, 85, 6073–6085. [Google Scholar] [CrossRef]
- Marco-Contelles, J. Recent Advances on Nitrones Design for Stroke Treatment. J. Med. Chem. 2020, 63, 13413–13427. [Google Scholar] [CrossRef]
- Deletraz, A.; Tuccio, B.; Roussel, J.; Combes, M.; Cohen-Solal, C.; Fabre, P.-L.; Trouillas, P.; Vignes, M.; Callizot, N.; Durand, G. Para-Substituted α-Phenyl-N-Tert-Butyl Nitrones: Spin-Trapping, Redox and Neuroprotective Properties. ACS Omega 2020, 5, 30989–30999. [Google Scholar] [CrossRef]
- Gruber, N.; Orelli, L.; Minnelli, C.; Mangano, L.; Laudadio, E.; Mobbili, G.; Stipa, P. Amidinoquinoxaline-Based Nitrones as Lipophilic Antioxidants. Antioxidants 2021, 10, 1185. [Google Scholar] [CrossRef]
- German, B.-G.; Carolina, A.; Claudio, O.-A.; Maria, C.Z.-L.; Mercedes, G.; Hugo, C. Nitrones: A Potential New Alternative as Therapeutic Agents. Curr. Org. Chem. 2017, 21, 2062–2067. [Google Scholar]
- Cancela, S.; Canclini, L.; Mourglia-Ettlin, G.; Hernández, P.; Merlino, A. Neuroprotective Effects of Novel Nitrones: In Vitro and in Silico Studies. Eur. J. Pharmacol. 2020, 871, 172926. [Google Scholar] [CrossRef]
- Griffin, M.F.; Malahias, M.; Hindocha, S.; Khan, W.S. Peripheral Nerve Injury: Principles for Repair and Regeneration. Open Orthop. J. 2014, 8, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Elke, Y.; Guillaume, L.; Veerle, S.; Sofie, G.; Vincent, T.; Sophie, J. The Neuroinflammatory Role of Schwann Cells in Disease. Neurobiol. Dis. 2013, 55. [Google Scholar] [CrossRef]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Review: Bioengineering Approach for the Repair and Regeneration of Peripheral Nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.; Zou, J.; Chen, X.; Munshi, A.; Smith, N.M.; Agarwal, V.; Hodgetts, S.I.; Plant, G.W.; Bakker, A.J.; Harvey, A.R.; et al. Hierarchical Patterning of Multifunctional Conducting Polymer Nanoparticles as a Bionic Platform for Topographic Contact Guidance. ACS Nano 2015, 9, 1767–1774. [Google Scholar] [CrossRef]
- Li, B.; Agarwal, V.; Ho, D.; Vede, J.-P.; Iyer, K.S. Systematic Assessment of Surface Functionality on Nanoscale Patterns for Topographic Contact Guidance of Cells. New J. Chem. 2018, 42, 7237–7240. [Google Scholar] [CrossRef]
- Macaya, D.; Spector, M. Injectable Hydrogel Materials for Spinal Cord Regeneration: A Review. Biomed. Mater. 2012, 7, 012001. [Google Scholar] [CrossRef]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic Neural Scaffolds: A Crucial Step towards Optimal Peripheral Nerve Regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef]
- Mahumane, G.D.; Kumar, P.; Toit, L.C.d.; Choonara, Y.E.; Pillay, V. 3D Scaffolds for Brain Tissue Regeneration: Architectural Challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Krishnan, U.M.; Sethuraman, S. PLGA Nanofibers Blended with Designer Self-Assembling Peptides for Peripheral Neural Regeneration. Mater. Sci. Eng. C 2016, 62, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Subramanian, A.; Krishnan, U.M.; Kaimal, S.S.; Sethuraman, S. Self-Assembling Peptide Nanostructures on Aligned Poly(Lactide-Co-Glycolide) Nanofibers for the Functional Regeneration of Sciatic Nerve. Nanomedicine 2017, 12, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Nune, M.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Peptide Nanostructures on Nanofibers for Peripheral Nerve Regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 1059–1070. [Google Scholar] [CrossRef]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D Bioprinting Applications in Neural Tissue Engineering for Spinal Cord Injury Repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110741. [Google Scholar] [CrossRef]
- Merzlyak, A.; Indrakanti, S.; Lee, S.-W. Genetically Engineered Nanofiber-Like Viruses for Tissue Regenerating Materials. Nano Lett. 2009, 9, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging Nanotechnology Approaches in Tissue Engineering for Peripheral Nerve Regeneration. Nanomedicine 2011, 7, 50–59. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of Biomaterial Scaffold for Nerve Tissue Engineering: Biomaterial Mediated Neural Regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Agarwal, V.; Bradshaw, M.; Chen, X.; Smith, S.M.; Raston, C.L.; Iyer, K.S. Nitrate Removal from Liquid Effluents Using Microalgae Immobilized on Chitosan Nanofiber Mats. Green Chem. 2012, 14, 2682–2685. [Google Scholar] [CrossRef]
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 1209. [Google Scholar] [CrossRef] [PubMed]
- Boido, M.; Ghibaudi, M.; Gentile, P.; Favaro, E.; Fusaro, R.; Tonda-Turo, C. Chitosan-Based Hydrogel to Support the Paracrine Activity of Mesenchymal Stem Cells in Spinal Cord Injury Treatment. Sci. Rep. 2019, 9, 6402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Song, H. Synthesis of Cerium Oxide Nanoparticles Loaded on Chitosan for Enhanced Auto-Catalytic Regenerative Ability and Biocompatibility for the Spinal Cord Injury Repair. J. Photochem. Photobiol. B Biol. 2019, 191, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Javad Hosseini, S.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Lord, M.S.; Lovell, E.; Wong, R.J.; Jung, M.S.; Oscar, D.; Mann, R.; Amal, R. Oxygen-Vacancy Engineering of Cerium-Oxide Nanoparticles for Antioxidant Activity. ACS Omega 2019, 4, 9473–9479. [Google Scholar] [CrossRef]
- Azizi, A.; Azizi, S.; Heshmatian, B.; Amini, K. Improvement of Functional Recovery of Transected Peripheral Nerve by Means of Chitosan Grafts Filled with Vitamin E, Pyrroloquinoline Quinone and Their Combination. Int. J. Surg. 2014, 12, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Amjed, N.; Bhatti, I.A.; Zia, K.M.; Iqbal, J.; Jamil, Y. Synthesis and Characterization of Stable and Biological Active Chitin-Based Polyurethane Elastomers. Int. J. Biol. Macromol. 2020, 154, 1149–1157. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-Responsive Polyurethane Fibrous Patches Loaded with Methylprednisolone (MP) for Restoring Structures and Functions of Infarcted Myocardium in Vivo. Biomaterials 2020, 232, 119726. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Moattari, F.; Kouchesfahani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Curcumin and Biodegradable Membrane Promote Nerve Regeneration and Functional Recovery After Sciatic Nerve Transection in Adult Rats. Ann. Plast. Surg. 2018, 81, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Santos, D.K.D.; Barros, B.R.d.S.; Aguiar, L.M.d.S.; da Cruz Filho, I.J.; de Lorena, V.M.B.; de Melo, C.M.L.; Napoleão, T.H. Immunostimulatory and Antioxidant Activities of a Lignin Isolated from Conocarpus Erectus Leaves. Int. J. Biol. Macromol. 2020, 150, 169–177. [Google Scholar] [CrossRef]
- de Melo, C.M.L.; da Cruz Filho, I.J.; de Sousa, G.F.; de Souza Silva, G.A.; do Nascimento Santos, D.K.D.; da Silva, R.S.; de Sousa, B.R.; de Lima Neto, R.G.; do Carmo Alves de Lima, M.; de Moraes Rocha, G.J. Lignin Isolated from Caesalpinia Pulcherrima Leaves Has Antioxidant, Antifungal and Immunostimulatory Activities. Int. J. Biol. Macromol. 2020, 162, 1725–1733. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Zhao, H.; Guo, T.; Wu, W.; Jin, Y. Structure-Antioxidant Activity Relationship of Active Oxygen Catalytic Lignin and Lignin-Carbohydrate Complex. Int. J. Biol. Macromol. 2019, 139, 21–29. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and Degradable Antioxidant Poly(ε-Caprolactone)-Lignin Nanofiber Membrane for Effective Osteoarthritis Treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/Poly(Butylene Succinate) Composites with Antioxidant and Antibacterial Properties for Potential Biomedical Applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Renault, H.; Werck-Reichhart, D.; Weng, J.-K. Harnessing Lignin Evolution for Biotechnological Applications. Curr. Opin. Biotechnol. 2019, 56, 105–111. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Its Integration into Metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Saudi, A.; Amirpour, N.; Jahromi, M.; Najafabadi, S.S.; Kazemi, M.; Rafienia, M.; Salehi, H. Application of Electrospun Polycaprolactone Fibers Embedding Lignin Nanoparticle for Peripheral Nerve Regeneration: In Vitro and in Vivo Study. Int. J. Biol. Macromol. 2020, 159, 154–173. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/Lignin Nanofibers as an Antioxidant Scaffold for the Growth of Neuron and Schwann Cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Samadian, H.; Vaez, A.; Ehterami, A.; Salehi, M.; Farzamfar, S.; Sahrapeyma, H.; Norouzi, P. Sciatic Nerve Regeneration by Using Collagen Type I Hydrogel Containing Naringin. J. Mater. Sci. Mater. Med. 2019, 30, 107. [Google Scholar] [CrossRef]
- Rienecker, S.B.; Mostert, A.B.; Schenk, G.; Hanson, G.R.; Meredith, P. Heavy Water as a Probe of the Free Radical Nature and Electrical Conductivity of Melanin. J. Phys. Chem. B 2015, 119, 14994–15000. [Google Scholar] [CrossRef]
- Abbas, M.; D’Amico, F.; Morresi, L.; Pinto, N.; Ficcadenti, M.; Natali, R.; Ottaviano, L.; Passacantando, M.; Cuccioloni, M.; Angeletti, M.; et al. Structural, Electrical, Electronic and Optical Properties of Melanin Films. Eur. Phys. J. E Soft Matter. 2009, 28, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, L.; Manini, P.; Altamura, D.; Giannini, C.; Tassini, P.; Maglione, M.G.; Minarini, C.; Pezzella, A. Evidence of Unprecedented High Electronic Conductivity in Mammalian Pigment Based Eumelanin Thin Films After Thermal Annealing in Vacuum. Front. Chem. 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M. Melanin Biopolymers: Tailoring Chemical Complexity for Materials Design. Angew. Chem. Int. Ed. Engl. 2020, 59, 11196–11205. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, M.; Kocot, A.; Tajber, L. Photoconductivity of Synthetic Dopa-Melanin Polymer. J. Photochem. Photobiol. B 2002, 66, 201–206. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The Potential Role of Antioxidants in Mitigating Skin Hyperpigmentation Resulting from Ultraviolet and Visible Light-Induced Oxidative Stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces Glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef] [PubMed]
- De Cássia, R. Goncalves, R.; Pombeiro-Sponchiado, S.R. Antioxidant Activity of the Melanin Pigment Extracted from Aspergillus Nidulans. Biol. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar] [CrossRef] [Green Version]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin Incorporated Electroactive and Antioxidant Silk Fibroin Nanofibrous Scaffolds for Nerve Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Zhao, X.; Song, J.; Cheng, Y.; Fang, Z.; Ouyang, Y.; Yuan, W.-E.; Fan, C. 3D Melatonin Nerve Scaffold Reduces Oxidative Stress and Inflammation and Increases Autophagy in Peripheral Nerve Regeneration. J. Pineal Res. 2018, 65, e12516. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.B.; Gehrke, S.A.; Koo, S.; Allegrini, S.; Rogero, S.O.; Ikeda, T.I.; Cruz, Á.S.; Shinohara, E.H.; Yoshimoto, M. An Alternative to Nerve Repair Using an Antioxidant Compound: A Histological Study in Rats. J. Mater. Sci. Mater. Med. 2015, 26, 5340. [Google Scholar] [CrossRef]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/Nanoceria Nanocomposite Fibers as Antioxidant Scaffolds for Neuronal Regeneration. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, Q.; Zhao, X.; Li, H.; Yuan, W.-E.; Fan, C. Asymmetrical 3D Nanoceria Channel for Severe Neurological Defect Regeneration. iScience 2019, 12, 216–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xie, C.; Wang, P.; Wang, X.; Wang, C.; Xun, X.; Lin, C.; Huang, Z.; Cheng, Y.; Li, L.; et al. An Elastic Gel Consisting of Natural Polyphenol and Pluronic for Simultaneous Dura Sealing and Treatment of Spinal Cord Injury. J. Control. Release 2020, 323, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Chen, X.; Guo, W.; Fu, C.; Pan, S. Theanine-Modified Graphene Oxide Composite Films for Neural Stem Cells Proliferation and Differentiation. J. Nanomater. 2020, 2020, e3068173. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-Aging Perspective. Front. Bioeng. Biotechnol. 2019, 7, 447. [Google Scholar] [CrossRef] [Green Version]
- Seven, E.S.; Zhou, Y.; Seven, Y.B.; Mitchell, G.S.; Leblanc, R.M. Crossing Blood-Brain Barrier with Carbon Quantum Dots. FASEB J. 2019, 33, 785.8. [Google Scholar] [CrossRef]
- Villalva, M.D.; Agarwal, V.; Ulanova, M.; Sachdev, P.S.; Braidy, N. Quantum Dots as a Theranostic Approach in Alzheimer’s Disease: A Systematic Review. Nanomedicine 2021, 16, 1595–1611. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Kesidou, D.; Moura, F.; Felli, E.; Song, W. Redox-Responsive Nanobiomaterials-Based Therapeutics for Neurodegenerative Diseases. Small 2020, 16, e1907308. [Google Scholar] [CrossRef]
- Li, C.-W.; Li, L.-L.; Chen, S.; Zhang, J.-X.; Lu, W.-L. Antioxidant Nanotherapies for the Treatment of Inflammatory Diseases. Front. Bioeng. Biotechnol. 2020, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid. Med. Cell. Longev. 2018, 2018, e1767265. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwana, N.; Agarwal, V.; Nune, M. Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants 2022, 11, 72. https://doi.org/10.3390/antiox11010072
Rizwana N, Agarwal V, Nune M. Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants. 2022; 11(1):72. https://doi.org/10.3390/antiox11010072
Chicago/Turabian StyleRizwana, Nasera, Vipul Agarwal, and Manasa Nune. 2022. "Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches" Antioxidants 11, no. 1: 72. https://doi.org/10.3390/antiox11010072
APA StyleRizwana, N., Agarwal, V., & Nune, M. (2022). Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants, 11(1), 72. https://doi.org/10.3390/antiox11010072