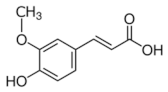
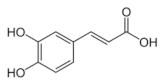
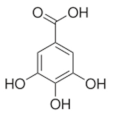
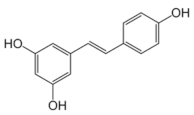
Abstract
Lactobacilli are well-studied bacteria that can undergo oxidative selective pressures by plant phenolic compounds (PPCs) in plants, during some food fermentations or in the gastrointestinal tract of animals via dietary inputs. Lactobacilli are known to be more tolerant to PPCs than other bacterial groups and, therefore, must have mechanisms to cope with the effects of these metabolites. In this review, we intend to present what is currently known about the basics beyond the responses of Lactobacillus spp. to individual PPCs. We review the molecular mechanisms that are engaged in the PPC-modulated responses studied to date in these bacteria that have been mainly characterized by system-based strategies, and we discuss their differences and similarities. A wide variety of mechanisms are induced to increase the oxidative stress response highlighting the antimicrobial nature of PPCs. However other uncovered mechanisms that are involved in the response to these compounds are reviewed, including the capacity of PPCs to modulate the expression of molecular functions used by lactobacilli to adapt to host environments. This shows that these phytochemicals can act as more than just antimicrobial agents in the dual interaction with lactobacilli.
1. Introduction
Plant phenolic compounds (PPCs) are a class of phytochemicals with high diverse structural complexity. According to animal and in vitro studies that evidenced antioxidant, radical-scavenging, and antimutagenic properties, long-term consumption of different PPCs has been correlated with chronic disease prevention [1,2]. An important role attributed to PPCs is defense functionality against microbiological threats. Antimicrobials usually feature the overproduction of reactive oxygen species (ROS) to exert their activity [3]; therefore, oxidative stress can be at the basis of the antimicrobial action of PPCs. Owing to their antimicrobial properties PPCs may exert broad effects on microorganisms that can result in rapid shifts and long-term modification in plant and animal microbiomes. In this regard, several studies have shown that supplementation with food substrates rich in PPCs variates the structure of the gut microbiota, commonly resulting in alteration of the Firmicutes to Bacteroidetes ratio and leading to Lactobacillus spp. as one of the predominant bacterial populations [4,5,6,7,8]. In addition, supplementation with individual PPCs can also exert growth-promoting effects on Lactobacillus spp., which has been observed for flavonols [9,10], flavanols [11,12], hydroxybenzoic acids [13], flavonones, flavones and isoflavones [14], anthocyanins [15], hydrolyzable tannins [16], and stilbenes [17].
Improved knowledge on the molecular bases that govern the reciprocal polyphenol-microbiota interactions is important in two ways. On one hand, this knowledge is crucial to understand how PPCs shape host–microbial communities, on which host fitness partly depends. On the other hand, this knowledge is necessary to decipher the biochemical pathways that bacteria, in the context of symbiosis with their hosts, have evolved to metabolize PPCs and provide these compounds in their bioavailable and bioactive forms [2,18].
Although PPCs can exert broad effects on bacteria and shape host–microbial communities impacting host fitness, many knowledge gaps still remain on how bacterial physiology and functionality are influenced upon exposure to PPCs. In this regard, molecular techniques may help to increase our understanding of the mechanisms underlying the bacterial responses to PPCs. Approaches considering the use of individual PPCs appear to be more suitable than the application of plant substrates containing a mixture of PPCs which may limit a mechanistic understanding of the microbe–PPC interaction. Lactobacillus spp. are more tolerant to PPCs than other bacterial groups and are part of the microbiomes of humans [19], animals [20], and plants [21,22]; therefore, they are suitable to study the molecular interaction with PPCs.
This review summarizes the progress in our understanding of the molecular mechanisms underlying the responses of Lactobacillus spp. to individual PPCs. The differences and similarities between these responses are described and discussed.
2. Comparative Transcriptomic Responses of L. plantarum WCFS1 to Different PPCs
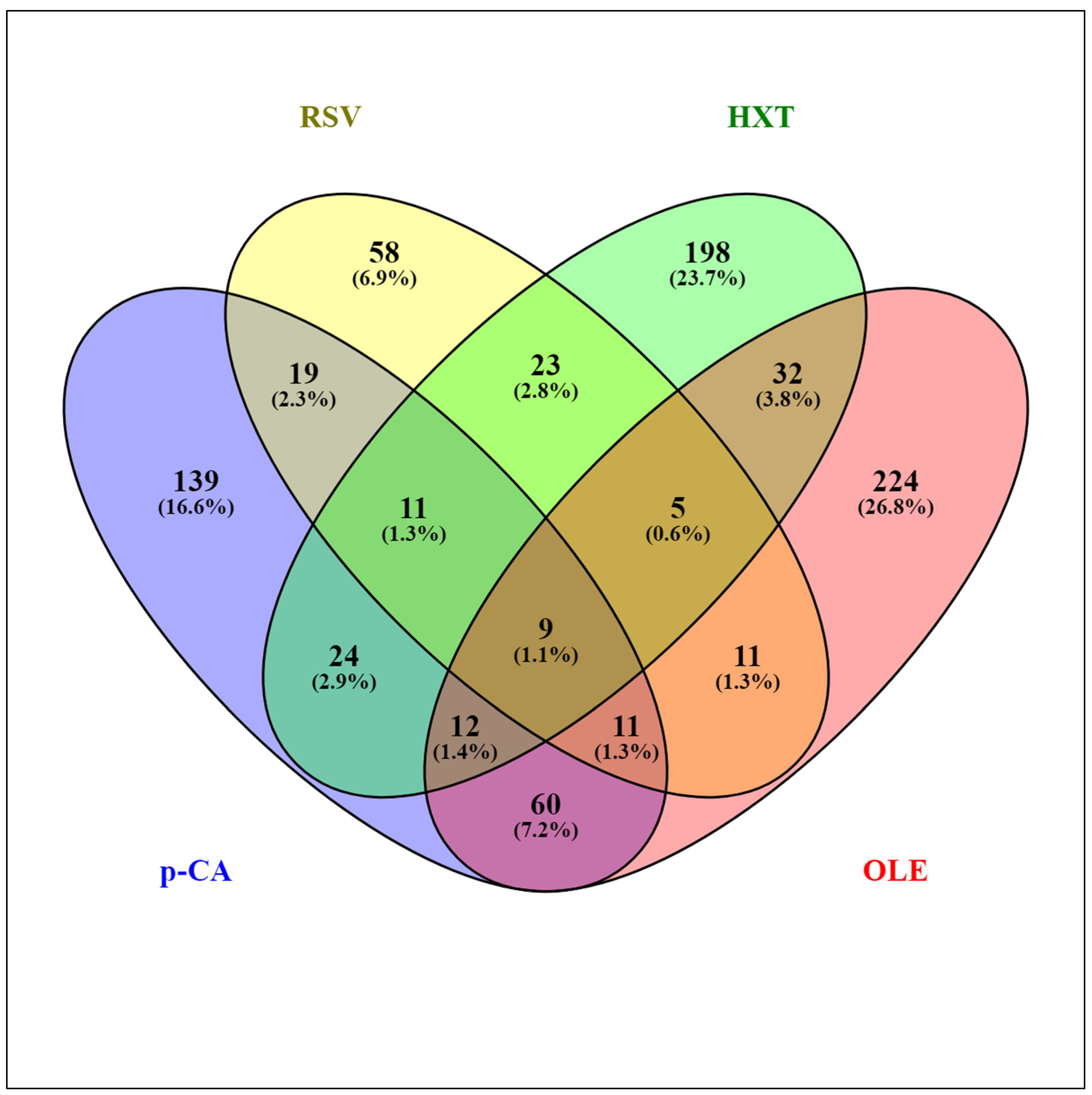
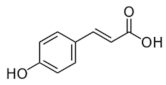
Several studies have addressed the transcriptomic responses of Lactobacillus spp. to individual PPCs [23,24,25,26,27,28,29]. The transcriptomes of the model strain Lactiplantibacillus plantarum WCFS1 exposed to different representative PPCs, including a hydroxycinnamic acid (p-coumaric acid (p-CA)) [25], a hydroxybenzoic acid (gallic acid (GA)) [26], a stilbene (resveratrol (RSV)) [27], a secoiridoid (oleuropein (OLE)) [28], and a phenylethanoid (hydroxytyrosol (HXT)) [29] have been revealed using DNA microarrays. The standardized experimental setup, applying stresses for 10 min to the same strain, permitted a reliable comparison among the different responses to these PPCs. The presence of PPCs affected hundreds of genes, and the largest transcriptome variations were observed upon exposure to OLE (358 genes involved), while exposure to GA had the least impact (40 affected genes). Figure 1 shows an overview of the extent of similarity among the responses of L. plantarum WCFS1 to p-CA, HXT, OLE, and RSV using the Venny 2.1 tool [30]. The assembled diagram shows the percentages of L. plantarum WCFS1 genes that overlap between the responses to these PPCs. When considering paired responses, the highest degree of coincidence was between OLE and p-CA where 25% and 32% of the genes, respectively, overlapped with respect to each other. The response to GA was also compared to the mentioned four phenolic compounds (not shown) displaying the best match with the response to p-CA (52.5% of the GA responsive genes were also regulated by p-CA). Comparative transcriptomic analysis of the specific responses to these individual PPCs revealed functional gene categories that highlighted the importance of adaptation of Lactobacillus cell surface properties, carbon and nitrogen metabolism, stress responsive pathways, and transport functions. The differentially expressed genes are incorporated and discussed in more detail as part of the sections below.
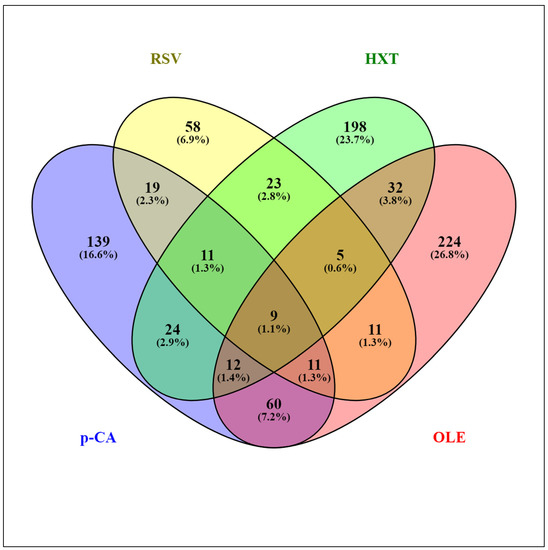
Figure 1.
Venn diagram assembled from Lactiplantibacillus plantarum WCFS1 genes differentially expressed in response to various plant phenolic compounds: p-coumaric acid (p-CA), resveratrol (RSV), hydroxytyrosol (HXT), and oleuropein (OLE).
3. Oxidative Stress Responses Modulated by PPCs
Since antimicrobials usually feature the overproduction of reactive oxygen species (ROS) to exert their activity [3], oxidative stress responses can be expected when microbes interact with PPCs. Accordingly, Lactobacillus exposed to PPCs differentially expressed genes and proteins required to cope with oxidative stress (Table 1).

Table 1.
Genes and proteins of Lactiplantibacillus plantarum WCFS1 induced by plant phenolic compounds that are responsive to oxidative stress.
Methionine sulfoxide reductase, glutathione reductase, components of the thioredoxin (Trx)–thioreductase (TrxR) system, and a broad set of genes dedicated to methionine (Met) biosynthesis were transcriptionally upregulated in L. plantarum in response to p-CA [25]. This response is required to counter thiol-specific oxidative stress, and it is consistent with the pro-oxidative stress arising from p-CA autoxidation [31]. Methionine residues in proteins can be exploited by bacteria as ROS scavengers to protect proteins and lipids from oxidation [32,33]. Genes responsive to thiol-specific oxidative stress are induced by HXT in L. plantarum WCFS1, including methionine sulfoxide reductase, glutathione reductase, and a regulator of disulfide bond formation [29].
The stilbene RSV absolutely requires copper, a redox-active metal, to generate RSV-derived ROS. To counter this potential pro-oxidant behavior, L. plantarum responds to RSV by inducing a multicopper oxidase and CopR [27], a transcription factor that senses the copper status of the cytoplasm and controls the expression of copper export ATPases [34]. In agreement with this role, CopR has been suggested to play an important role in elevating resistance to H2O2 stress in L. plantarum [35]. In addition, RSV induces two zinc ABC transporters, a metal that competes with and antagonizes copper. One of these L. plantarum transporters (lp_0464) was also induced by OLE [28], and the corresponding L. brevis homologous permease (lvis_0472) was induced in cells exposed to ferulic acid [23] (Table 1). L. plantarum downregulates a Fe2+ transporter mntH3 [36] upon HXT stress [29], which would diminish hydroxyl radical production arising from the Fenton reaction of this metal with H2O2. According to these profiles, management of the transport of transition metals participating in the Fenton-like chemistry seems a strategy used by Lactobacillus spp. to prevent an increase of the oxidative stress generated by PPCs.
Enzymes inactivating toxic oxygen radicals, which are part of the first defense against oxygen damage, were also implicated in the response to some PPCs (Table 1). The gene coding for the NADH peroxidase (npr2) was overexpressed by L. plantarum in the presence of OLE [28] or HXT [29]. A proteomic study revealed that the NPR2 enzyme was also upregulated in the presence of tannic acid [37]. Catalase (kat), which also detoxifies H2O2, was induced in L. plantarum upon HXT stress [29]. In addition, pyruvate oxidase (pox), an enzyme linked to oxidative stress resistance, was transcriptionally induced by OLE [28] or HXT [29].
The differential expression of the cysE–metC–cysK operon constituted a common response, except for GA, to PPCs in L. plantarum (Table 1). In Lactococcus lactis (a close relative of L. plantarum), the metC–cysK operon is well correlated with robustness toward oxidative stress [38]. In L. plantarum, the cysE–metC–cysK operon was downregulated upon p-CA [25] or RSV stress [27] but upregulated in the presence of OLE [28] or HXT [29]. The metC and cysK genes encode cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), respectively, which are the microbial orthologs of the mammalian CSE and CBS. These are the most important H2S-generating enzymes in many bacteria [39], including L. plantarum, where CBS and CSE also produce H2S efficiently [40,41]. This gas has been shown to protect bacteria from the lethal effects of ROS formed from antibiotics [39].
Induction of the L. plantarum cysE–metC–cysK operon observed upon OLE or HXT stress indicates higher H2S generation. This profile was concurrent with the induction of NADH peroxidase, pyruvate oxidase, and catalase (Table 1). Protection against H2O2 provided by these enzymes and H2S agrees with the capacity of both phenolic compounds to generate H2O2.
In contrast, the cysE–metC–cysK operon was downregulated in the presence of p-CA [25]. Even though the cysE–metC–cysK operon is involved in methionine biosynthesis from cystathionine, p-CA still induced L. plantarum genes to rescue methionine biosynthesis via the alternative sulfhydrylase pathway [25], highlighting the role of this amino acid as an ROS scavenger in proteins.
4. Genotoxic Stress Responses
Some phenolic compounds could cause genotoxic stress as judged by the induction of genes and proteins that are induced by DNA damage. Tannic acid strongly overexpressed the RecA protein [37], which activates the SOS response upon DNA damage [42]. RSV induced up to eight genes of the pyrimidine biosynthetic route (required for the polymerization of DNA during repair) and genes linked to DNA repair mechanisms, including two xerC homologs, a DNA-3-methyladenine glycosylase II, and mfd and pth, a gene pair required to couple DNA repair and transcription [27]. The expression of genes encoding all components of the L. plantarum RNA degradosome was regulated by HXT [29], indicating that, along with DNA damage, RNA can also undergo oxidative stress in presence of some PPCs.
5. General Stress Pathways Activated in Response to PPCs
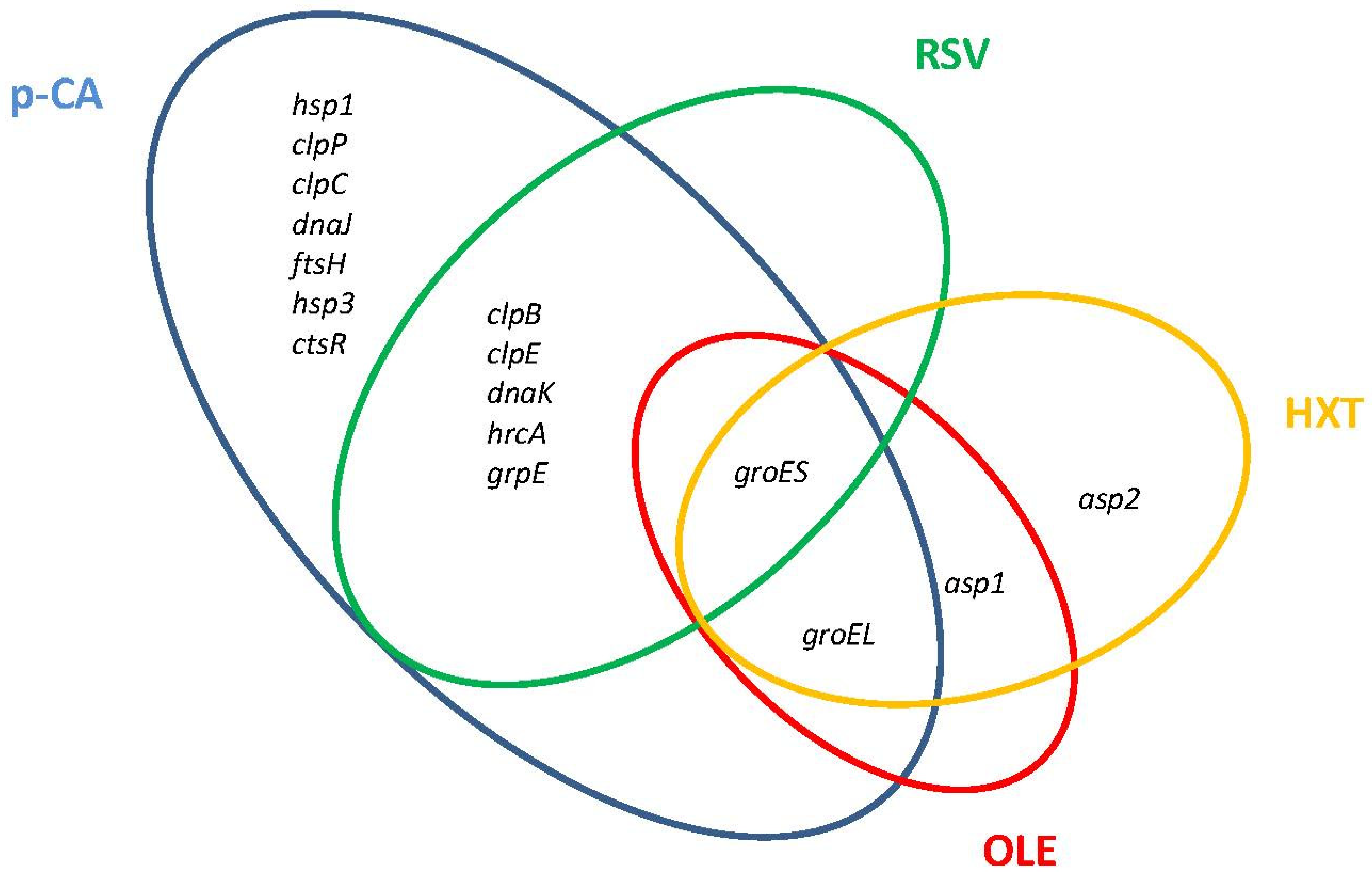
Major molecular chaperone and protease systems that accomplish essential functions to control protein quality under stress conditions are induced by Lactobacillus in the presence of PPCs (Figure 2).
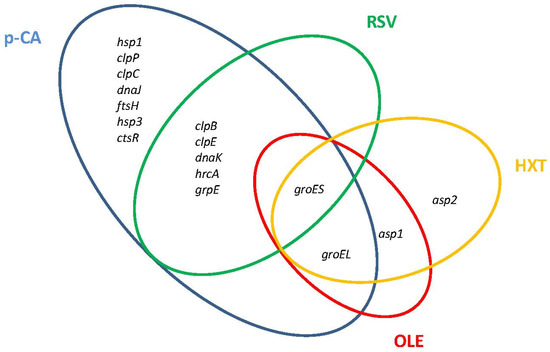
Figure 2.
Venn diagram assembled from Lactiplantibacillus plantarum WCFS1 general stress-related genes differentially upregulated in response to individual plant phenolic compounds. p-CA, p-coumaric acid; RSV, resveratrol; OLE, oleuropein; HXT, hydroxytyrosol.
The DnaK system (DnaK chaperone, co-chaperone DnaJ, and nucleotide exchange factor GrpE) is one of the chaperones involved in the molecular responses to PPCs. In L. plantarum WCFS1, components of the DnaK system were induced in the presence of p-CA or RSV (Figure 2). Another chaperone responsive to PPCs is the refolding GroES/GroEL system. GroES was transcriptionally induced in L. plantarum WCFS1 by p-CA, RSV, OLE, and HXT, while groEL was induced by these PPCs except by p-CA (Figure 2). Proteomic studies have shown that GroES is also notably induced at high tannic acid concentrations in L. plantarum [43], while GroEL is induced in response to tannic acid in Lentilactobacillus hilgardii [44] or the flavonol glycoside rutin in Lactobacillus acidophilus NCFM [45].
Several members of the Clp ATP family of proteases were induced by PPCs in Lactobacillus. Transcriptomic and proteomic studies have shown that the ClpP protease is induced by p-CA in L. plantarum WCFS1 [25], Lacticaseibacillus casei BL23 [24], and by rutin in L. acidophilus NCFM. The ClpC and ClpE proteases, which can associate with the ClpP protease in proteolytic complexes, are induced by p-CA in L. plantarum WCFS1 (Figure 2), whereas ClpE is induced by rutin in L. acidophilus NCFM. The expression of clpE, but not of clpP, was induced by RSV in L. plantarum WCFS1 [27] indicating that ClpE acts here as molecular chaperone [46]. In addition, L. acidophilus NCFM induced the two-component proteasome (HslV protease/HslU ATPase) in presence of rutin [45].
Other stress-related genes, including heat (hsp) and alkaline (asp) shock protein-encoding genes, also contribute to phenolic compound resistance in Lactobacillus. Levilactobacillus brevis cells exposed to ferulic acid (a hydroxycinnamic acid) [23] induced an hsp gene highly similar to the hsp genes induced by p-CA in L. plantarum. L. acidophilus NCFM also induced an hsp protein (GrpE) in the presence of rutin [45]. The asp1 and asp2 genes are induced in L. plantarum by OLE [28] and HXT [29], and the Asp1 protein is induced by tannic acid in L. plantarum [37]. Interestingly the homologous L. plantarum asp2 gene of L. casei (asp23) contributes to gentamicin resistance in this microorganism [47].
Transcriptomic studies have revealed that the stringent response (SR), a conserved bacterial response that transcriptionally affects general stress responses [48,49], was involved in the molecular adaptation of L. plantarum WCFS1 to several PPCs. Several sets of genes owing to SR, including ribosome, nucleotide, and fatty-acid biosynthesis genes, were downregulated in L. plantarum WCFS1 by p-CA [25], OLE [28], or HXT [29]. In L. brevis, genes encoding proteins responsible for transcription, translation, and key proteins involved in cell division were strongly downregulated in the presence of ferulic acid [23]. Differential expression of L. plantarum genes coding for enzymes directly involved in the metabolism of (p)ppGpp (the alarmone assumed to trigger the SR), including small translational GTPases and (p)ppGpp synthases, were observed in the presence of HXT [29]. The (p)ppGpp metabolism is interconnected with GTP metabolism and regulates the intracellular concentrations of this nucleotide which, in turn, modulate the biosynthesis of the alarmone [49]. Genes related to GTP biosynthetic and GTP-consuming pathways were differentially regulated by p-CA [25], OLE [28], and HXT [29], indicating that GTP regulation is involved in the response to these PPCs, and that maintaining GTP levels within a range is essential for viability under different environmental conditions (Kriel et al. 2012).
It is worth noting that neither the chaperone/Clp machinery nor the SR were involved in the molecular response of L. plantarum to GA [26], indicating that the capacity to cause stress to Lactobacillus differs among PPCs.
6. Detoxification Mechanisms of PPCs in Lactobacillus
6.1. Metabolic Pathways for the Detoxification of PPCs
A means to overcome the toxicity of some PPCs is to metabolize them into less toxic compounds. The metabolic routes for the metabolism of PPCs that have been characterized at the molecular level to date in Lactobacillus, mainly hydroxycinnamic and hydroxybenzoic acids, were recently reviewed [50] and are not discussed here.
6.2. Drug Efflux and ABC-Transport Systems
The expression of Lactobacillus genes encoding several putative ABC-type and MFS drug extrusion systems are markedly modulated by different PPCs, which can contribute to directly counteract the toxic effects of these compounds.
As shown in Table 2, L. plantarum strongly induced two drug extrusion systems in presence of p-CA [25] or RSV [27]. One of these systems (lp_0989 to lp_0992) is a multidrug efflux pump of the MFS superfamily. The L. brevis homolog of lp_0991 (the multidrug transporter), lvis_1917, is also strongly induced by ferulic acid [23]. The other induced extrusion system is an ABC-type multidrug resistance (MDR) system encoded by genes lp_2393 to lp_2395. The permease of this system (lp_2394) is homologous to the LmrA MDR transporter from Lactococcus lactis [51], which efficiently extrudes drugs out of the cell.

Table 2.
Drug efflux and ABC transport systems of Lactiplantibacillus plantarum WCFS1 differentially expressed in presence of plant phenolic compounds.
Interestingly, an ABC-type transport system from L. plantarum encoded by the lp_2739 and lp_2740 genes was strongly downregulated by all PPCs compounds examined (p-CA, GA, RSV, HXT, and OLE) (Table 2). The permease of this system (lp_2740) displays a domain architecture that agrees with BceAB-type transporters [52], and it is homologous to the BceAB-like transporter OrABC from L. casei BL23 [24]. This type of Bce-like module plays crucial roles to sense and detoxify antimicrobial peptides (AMPs) [53] via import and subsequent intracellular enzymatic inactivation. However, the strong downregulation of this Bce-like module suggests that it does not take active part in resistance to PPCs, leaving its physiological significance to be established.
7. Metabolic Adaptations of Lactobacillus spp. to PPCs
7.1. Nitrogen Metabolism
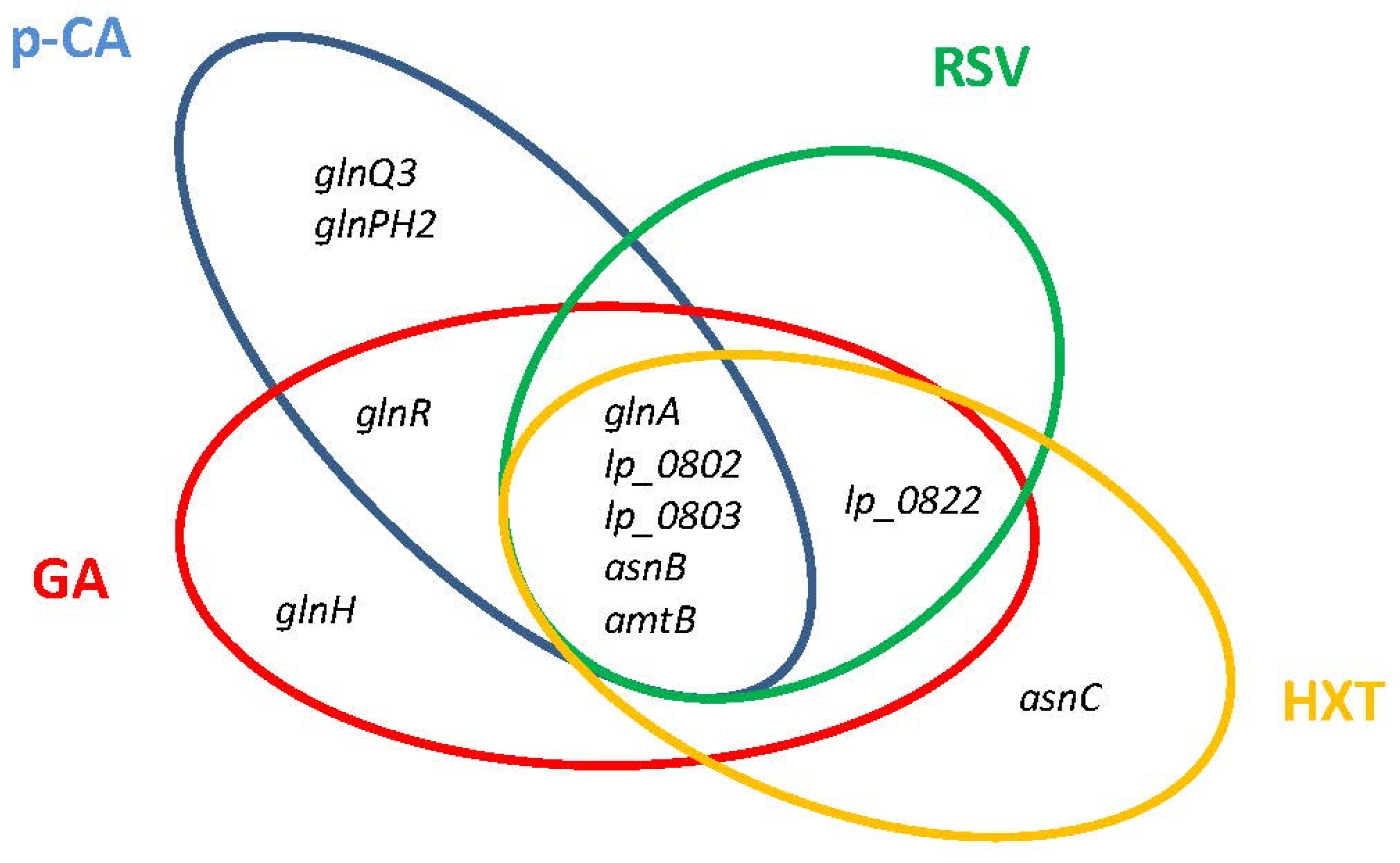
The L. plantarum transcriptome responses to GA [26], RSV [27], HXT [29], and p-CA [25] share a conserved profile that entail the downregulation of genes involved in nitrogen metabolism (Figure 3). These genes, all under the control of GlnR regulator [54], include the glutamine (Gln) synthetase (glnA), aspartate ammonia lyase which produces ammonium (asnB), a Gln ABC transporter (lp_0802 and lp_0803), and the ammonium transporter protein (amtB). The observed profile agrees with a tight control of intracellular ammonia levels, the previously proposed regulatory function of GlnR in Lactobacillus, which would be achieved by constraining the import of ammonia/amino-containing compounds and controlling the production of intracellular ammonia and glutamine [55]. The regulation of nitrogen metabolism triggered by PPCs could contribute to control the bacterial access to host glutamine, which is a hub for nitrogen metabolism in plants and bacteria [56] and can be important for plant–bacterial combinations. It is to note that OLE, a PPC proposed to conduct a close association between L. plantarum and Oleaceae plant hosts [28], does not modulate the GlnR regulon which could facilitate nitrogen transfer from the plant to the microbial symbiont, an essential requirement for symbiotic relationships. This knowledge opens the possibility to use suitable PPCs to better control the bacterial access to host glutamine. In the case of some human pathogens, preventing access to host glutamine is required to attenuate its virulence [57,58].
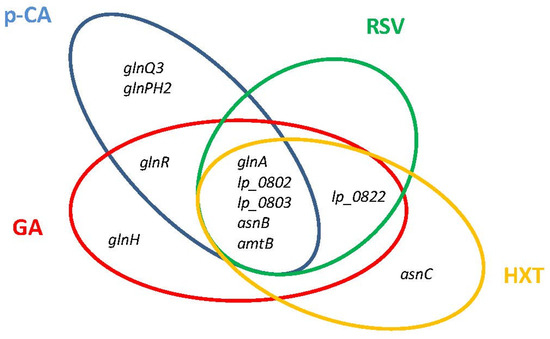
Figure 3.
Venn diagram assembled from Lactiplantibacillus plantarum WCFS1 GlnR regulon genes involved in nitrogen metabolism that are differentially downregulated in response to individual plant phenolic compounds. p-CA, p-coumaric acid; RSV, resveratrol; HXT, hydroxytyrosol; GA, gallic acid.
7.2. Sugar and Energy Metabolism
L. plantarum altered the expression of genes or proteins involved in the transport and metabolism of different carbon sources in response to PPCs.
L. plantarum decreased the expression of glucose permease and increased the expression of genes involved in the malolactic fermentation (MLF) pathway in the presence of p-CA. This metabolic adaptation generates energy via chemiosmotic mechanisms and increases the intracellular pH to reduce acid stress. A similar transcriptomic reprogramming was observed during the fermentation by L. plantarum of vegetable and fruit juices rich in hydroxycinnamic acids [59,60]. Upregulation of genes involved in the MLF pathway has also been observed during the response of L. brevis to ferulic acid [23].
Genes encoding transketolase (tkta), phosphoglycerate mutase (pgm), and phosphoketolase (xpkA) were upregulated under p-CA stress [25]. This profile suggests accumulation of glycolytic intermediates such as 3-phosphoglycerate and 2-phosphoglycerate, a metabolic adaptation characteristic of carbohydrate starvation conditions that is supported by uspA induction, which encodes a universal stress protein leading to a continuous growth-arrest state [61]. The Pgm and UspA proteins were also induced under tannic acid stress [37], suggesting similar metabolic adaptations.
OLE transcriptionally reprogrammed the expression of transporters from the phosphotransferase (PTS) system of L. plantarum [28] including the induction of the mannitol and fructose PTS systems which are the main soluble components of olive source and sink tissues. This expression profile would promote sugar transfer from the host and support the proposed role of OLE as a signaling molecule in the association between Lactobacillus and the plant host [28].
L. plantarum WCFS1 encodes an N-acetyl glucosamine (GlcNAc) transporter (nagE) that is downregulated in presence of p-CA, OLE, and RSV [25,27,28]. However, the expression of nag genes involved in GlcNAc catabolism was not affected by these PPCs. The concurrent downregulation of genes involved in the production of cell-wall and capsular polysaccharide constituents suggests that nagE downregulation is related to cell-wall remodeling, as GlcNAc may act as a building block for biosynthesis of these cellular components.
Unlike p-CA, OLE, and RSV, the transcriptomic signature of GA showed increased expression of genes required for GlcNAc utilization, including those coding for glucosamine-6-P isomerase (nagB) and a putative PTS transporter of the [GlcNAc]2 disaccharide (lp_2954) suggesting that GA promotes GlcNAc utilization by L. plantarum WCFS1 [26].
7.3. Gallic Acid: A Source of Chemiosmotic Energy to Lactobacillus
DNA microarray experiments and physiological analysis established that GA triggered a specific metabolic adaptation in L. plantarum: the transport and catabolism of GA was self-inducible and supplied chemiosmotic energy to this microorganism [26]. GA triggered a huge transcriptional induction of the GA transporter (gacP) and the three gallate decarboxylase subunits (lpdB, lpdC, lpdD) [26]. The uptake of monoanionic GA by gacP was coupled to the extrusion of the GA decarboxylation product, uncharged pyrogallol [26]. Membrane potential and internal pH measurements showed that the transport process and the cytosolic H+ ions consumed during gallic acid decarboxylation by the gallate decarboxylase generated ∆pH and ∆Ψ gradients, thus increasing the proton motive force (PMF) over the cell membrane and the intracellular pH to reduce acid stress [26].
8. Membrane and Cell-Wall Modifications in Response to PPCs
8.1. Cell-Wall Modifications
Lactobacillus regulates the expression of genes and proteins involved in the biosynthesis of all types of macromolecular components of the cell envelope in response to PPCs, including peptidoglycan (PG), teichoic acids (TAs), and capsular polysaccharides (cps).
Modification of the biosynthesis of PG precursors is a known adaptation to stress conditions in Gram-positive bacteria [62,63]. Some PPCs such as tannic acid inflict injuries in the cell wall leading to rougher cell surfaces and leakages [64]. To circumvent the inhibitory effects of tannic acid, L. plantarum modifies its profile of PBPs (penicillin-binding proteins) (64), which are enzymes known to play a key role in PG biosynthesis. In addition, L. plantarum respond to TA aggression by overproducing LdhD (d-lactate dehydrogenase) and DapF (diaminopimelate (DAP) epimerase), two proteins required for the biogenesis of the PG precursors d-lactate and meso-diaminopimelic acid (meso-DAP), respectively [37]. Similarly, L. hilgardii and L. acidophilus NCFM overexpressed LdhD under tannic acid [44] or rutin stress [45], respectively. In addition to LdhD, L. acidophilus NCFM overexpressed GlmU in the presence of rutin, this enzyme catalyzing the last steps of UDP-GlcNAc, one of the cell-wall peptidoglycan precursors.
p-CA injures and leads the bacterial membrane to become leaky [65]. The transcriptomic response of L. plantarum to p-CA [25] revealed overexpression of aad (d-alanyl-d-alanine dipeptidase) and hicD2 (d-hydroxyisocaproate dehydrogenase), which reportedly modify the biosynthesis of PG precursors [66]. This profile coincided with a marked decrease in the expression of genes encoding putative lytic glycosyl-transferases (lp_0302, lp_3014, lp_3015) and a muropetidase (lp_3421), all encompassed within the peptidoglycan hydrolase (PGH) complement of L. plantarum WCFS1 [67]. This transcriptome reprogramming does point to PG modification, reduction in PG turnover, and repression of cell lysis to counter the effects of p-CA [25].
The production levels and structure of wall teichoic acids (WTA) and lipoteichoic acids (LTA) from L. plantarum were likely affected by the presence of some PPCs as judged by the altered expression of genes and proteins responsible for their production. TagE6 (poly(glycerol-phosphate) α-glycosyltransferase), an enzyme involved in glucosyl substitution of poly(ribitol-5-P) WTA [68], was induced after exposure to tannic acid [37] and transcriptionally overexpressed in response to OLE [28] or HXT [29].
Induction of genes of the tarIJKL locus is a genetic marker of WTA backbone alditol switching in L. plantarum [68] as it is responsible for poly(ribitol-5-P) WTA production, an alternative WTA variant to the wild-type glycerol-containing backbone. The tarIJK and the tarK genes are transcriptionally induced in response to OLE [28] and HXT [29], respectively, suggesting that WTA backbone alditol switching occurs in response to these PPCs. Downregulation of tagD2 (lp_1248), which CDP-activates glycerol-P, was also observed in presence of HXT, further supporting WTA backbone alditol switching. In addition, downregulation of gtca1 which encodes an LTA glycosylation protein suggests changes in the decoration of LTAs exposed to HXT.
The capsular polysaccharide (cps) genes encode the enzymatic machinery involved in the different steps of the repeating unit synthesis, export, and polymerization of these macromolecules in lactic acid bacteria [69,70]. The decreased expression of cps genes was a common transcriptome response of L. plantarum WCFS1 to p-CA [25], RSV [27], and OLE [28]. These genes are organized in four cps gene clusters distributed along the genome of this microorganism. In the presence of p-CA, up to 16 out of all 36 cps genes that were encompassed within cps1, cps3, and cps4 gene clusters were downregulated. In the presence of OLE, 21 out of 36 cps genes were downregulated including genes from all cps1, cps2, cps3, and cps4 clusters. In the presence of RSV, three genes of the cps4 gene cluster and genes involved in the transport and metabolism of precursors for cps biosynthesis (mannose, glucosamine-6-P, and GlcNAc) were downregulated. It is also to note that CPS was not detectably accumulated over the outer surface of L. plantarum WCFS1 exposed to tannic acid [64].
8.2. Membrane Modifications
Proteomic and DNA microarray experiments revealed that PPCs markedly regulate the expression of genes and proteins involved in membrane lipid biosynthesis. Most L. plantarum genes from the fatty-acid (FA) biosynthesis (fab) locus were downregulated in the presence of p-CA [25] or OLE [28]. Decreased expression of the fab locus is indicative of membrane modifications in its FA composition, which is a common strategy in Lactobacillus spp. to counter different types of stress conditions [71,72,73,74]. L. casei BL23 or L. brevis upregulated one protein of the FAB pathway in response to p-CA [24] or ferulic acid [23], respectively.
Proteomic and transcriptomic studies have shown that L. plantarum WCFS1 downregulates a cyclopropane-fatty-acyl-phospholipid synthase (Cfa2) in the presence of TA [37] or OLE [28]. Cfa2 was also downregulated in E. coli in response to cranberry polyphenols [75] or naringerin [76]. This adaptation alters the cyclic to saturated membrane FA ratio (CFA/SFA) which influences the membrane fluidity. Combined downregulation of the fab locus and cfa2 suggests changes in membrane FA composition to counteract the damage inflicted by some PPCs in the Lactobacillus cell membrane.
Membrane phospholipid remodeling can also be involved in the adaptation of Lactobacillus to PPCs. The OLE-responsive transcriptome showed a set of genes coordinately expressed by L. plantarum in ways to increase the synthesis of sn-glycerol-3-P, the obligated glycerophospholipid precursor [28]. This modification may be important in the adaptation of Lactobacillus spp. to PPCs since membrane phospholipid alterations are crucial for bacterial adaptability to environmental stress [77].
Since a high number of genes encoding membrane proteins were overexpressed during the responses of L. brevis to ferulic acid [23] and L. plantarum to p-CA [25], membrane crowding with proteins has been proposed to stabilize the Lactobacillus membranes against hydroxycinnamic acids.
Genes encoding transporters of compatible solutes including glycine-betaine/carnitine/choline, glycerol, trehalose, or GABA were downregulated upon exposure of L. plantarum to p-CA [25] or HXT [29]. This can contribute to stabilize the membrane, as previously reported for trehalose in L. acidophilus [78]. Membrane damage can cause proton motive force (PMF) dissipation [79,80]. In Lactobacillus spp., this disturbance can be in part compensated for by an increased abundance of F1/F0 ATPase that pumps protons to the outside environment [81,82]. Upregulation of the L. plantarum F1/F0 ATPase components in the presence of HXT [29] suggests that this PPC causes PMF dissipation.
9. Role of PPCs in the Molecular Adaptation of Lactobacillus to Host Environments
PPCs can be released by plants to enable bidirectional interfaces with the environment. Within these interactions, the microbe signaling mediated by PPCs can be crucial for plant robustness. A known example is the microbe signaling by plant isoflavonoids to transcriptionally activate bacterial key genes for nodule formation that stimulate plant growth [83,84]. Benzoxazinoids can also acts as mediators of plant health by modulating the soil microbiome [85].
These cases illustrate the ability of some PPCs to shape the structure and function of plant host–microbial communities. This capacity may operate not only in setting up phytomicrobiomes but also to shape the microbiome structure and function across the animal kingdom given that PPCs are consumed in the diet. Because the host microbiome markedly influences the host phenotype, it is necessary to understand the direct effects of PPCs on microbes and the influence of PPCs on host–microbial interactions to establish a causal relationship between PCs exposure and effects on the host.
Molecular details on how PPCs influence the survival and function of Lactobacillus spp. in host environments remain largely unknown. This section touches on a few approaches that reveal how individual PPCs can modulate the expression of molecular traits from Lactobacillus spp. reportedly involved in the adaptive response to host environments or that have documented functions in the host–Lactobacillus molecular dialog.
9.1. Regulation of Molecular Functions Involved in the Survival of Lactobacillus to Gastrointestinal (GI) Tract Stress
Lactobacilli undergo different stress conditions during the passage through the GI tract. Interestingly, genes coding for different molecular functions involved in the survival of L. plantarum to the GI tract conditions were also responsive to PPCs (Table 3).

Table 3.
Genes and proteins of Lactiplantibacillus plantarum WCFS1 associated with improved survival against gastrointestinal tract-induced stress that are differentially expressed by plant phenolic compounds.
L. plantarum WCFS1 submitted to an orogastric–intestinal (OGI) simulator [86] induced a set of proteases (clpB, clpE, clpP, fstH), molecular chaperones (groEL, dnaK), and heat-shock proteins (hsp1, hsp2, hsp3). Figure 2 shows that all these genes were also induced by p-CA while some of them were also responsive to RSV, HXT, and OLE.
High osmolarity and contact with bile salts in the duodenum lead L. plantarum to induce a relatively high number of genes involved in cell envelope functions [87,88]. L. plantarum triggers similar responses when it contacts with tannic acid, p-CA, or OLE, including the regulation of the expression of genes involved in the biosynthesis of cell envelope constituents or that are major actors in cell-wall turnover (Table 3). Multidrug transporters may also play an important role for bile tolerance in Lactobacillus spp. [82,89]. In this regard, p-CA and RSV markedly induced L. plantarum genes encoding an efflux pump (lp_0990-lp_0992) that is also induced in the small intestine and proposed to be involved in the extrusion of bile salts [90] (Table 3). To counteract the high osmolarity stress in the small intestine, L. plantarum involves transporters of compatible solutes including glycine-betaine/carnitine/choline [91], which are also responsive to p-CA and HXT (Table 3).
In addition to acidic and high-osmolarity conditions, bacteria face oxidative stress at the mucosal surface of the colon [92]. As mentioned above, Lactobacillus activates different mechanisms to counter oxidative stress in the presence of PPCs (Table 3) which can cross-protect these bacteria in the colon.
In addition to these molecular mechanisms, more functions related to the survival of Lactobacillus in the GI tract can be individually controlled by p-CA, GA, TA, or RSV, as described in the next sections.
9.1.1. Gallic Acid
GA strongly induces the expression of lp_2940, a crucial gene for the persistence and survival of L. plantarum WCFS1 in the GI tract [93]. GA also induces genes involved in GlcNAc utilization. This amino sugar is part of the human intestinal mucus glycoproteins and can be used as a carbon source by Lactobacillus, particularly under bile stress [82]. The increased capacity to use GlcNAc together with the overexpression of genes coding for tannase and GA metabolism (see above) may confer competitive advantages to L. plantarum in the GI tract where nutrients are not in constant supply.
9.1.2. Tannic Acid
Four genes markedly induced in the GI tracts of human and mouse (argG, copA, ram2, and lp_2940) were upregulated upon exposure to tannic acid [64]. From these, copA and lp_2940 [90,94] are crucial for the persistence and survival of L. plantarum in the digestive tract. In addition, the penicillin-binding PBP2A protein, a biomarker negatively related with GI survival [95], was inactivated at the post-translational level by tannic acid suggesting enhanced GI survival reportedly associated with the inactivation of this function [64].
9.1.3. p-Coumaric Acid (p-CA)
L. plantarum treated with p-CA overexpressed a gene set also induced when this microorganism was perfused in the small intestine [90], which includes an alkaline shock protein (asp2), universal stress protein (uspA), several chorismate biosynthesis genes, and an amino-acid ABC transporter (lp_1744–1746).
9.1.4. Resveratrol
L. plantarum overexpressed the lp_3368 gene in the presence of RSV. This gene encodes a multidrug transporter potentially able to use deoxycholate as a substrate [96], a function consistent with its location in the vicinity of bsh3, a bile salt hydrolase-encoding gene [97]. Transport of secondary bile acids that are produced by BSH activity can govern bacterial fitness and host colonization [98], as well as alter host physiology [99].
The capacity of PPCs to modulate the expression of genes related to GI tract survival may be of utility to improve fitness of lactobacilli in this niche, more in view that gut robustness of individual strains may depend on differential gene expression levels rather than on the presence or absence of conserved genes [100].
9.2. Remodeling of the Cell Envelope Induced by PPCs: Potential Impact on the Communication Capacities of Lactobacillus with the Host
The Lactobacillus cell envelope is a source of molecules that act as key probiotic ligands. These molecules are known to interact with host receptors inducing signaling pathways that result in probiotic effects [87,91,92,101]. As mentioned above, the biosynthesis of cell-wall constituents (peptidoglycan, teichoic acids, polysaccharides, and proteins) is subjected to regulation by PPCs. Hence, PPCs emerge as new potential modulators of the cell surface properties from Lactobacillus spp.
9.2.1. Capsular Polysaccharides
The capacity of L. plantarum to synthesize CPS was markedly downregulated in the presence of different PPCs (see above and Table 4). CPSs may shield adhesion proteins and microorganism-associated molecular patterns (MAMPs) interacting with pattern recognition receptors (PRRs) of dendritic cells, which play key roles in innate and adaptive immunity. Accordingly, CPS downregulation has been found to be required for optimal adherence of L. rhamnosus GG to intestinal epithelial cells [102] (Lebeer et al. 2009), and deletion mutants in the four cps clusters of L. plantarum WCFS1 alter its immunomodulatory capacities by increasing the exposure of bacterial MAMPs to their host receptors to induce signaling cascades [69,103]. Therefore, decreased expression of cps genes driven by the exposure to some PPCs is likely to optimize the immunomodulatory and adhesion capacities of Lactobacillus.

Table 4.
Genes and proteins of Lactobacillus involved in the interaction with host cells that are differentially expressed by plant phenolic compounds.
9.2.2. Teichoic Acids
The different biochemical properties (chain length, backbone composition, or degree of glycosyl substitution) and production levels of cell wall teichoic acids (WTA) and lipoteichoic acids (LTA) are directly related to the inmunodulatory capacity of Lactobacillus spp. [104] and severely impact its capacity to communicate with their hosts [68]. The expression of several enzymes implicated in the biosynthesis or modification of L. plantarum teichoic acids that are associated with altered host responses, including TagE6, Tag D2, enzymes encoded by the tarIJKL locus, and the LTA glycosylation protein encoded by the gtca1 gene, were differently regulated by various PPCs, mainly OLE and HXT (Table 4).
According to these observations, exposure to these PPCs is expected to modify the signaling ability of L. plantarum and its capacity to communicate with their hosts. This notion is strongly supported in the case of OLE [28] as exposure to this PPC also modifies the expression of other documented immunomodulators from this microorganism, including components of the plantaricin and LamBDCA quorum-sensing systems [105,106,107,108].
9.2.3. Surface and Moonlighting Proteins
Analysis of differential whole-cell and surface proteomes of the probiotic L. acidophilus NCFM strain revealed that ferulic acid, reveratrol, tannic acid, and caffeic acid varied the abundance of moonlighting proteins engaged in adhesion [109] (Table 4). These profiles were associated with variations in the adhesive capacity of this strain triggered by these PPCs. The abundance of some of these moonlighting proteins including EF-P, pyruvate kinase, and EF-Tu correlated well with binding capabilities to HT-29 cells triggered by RSV or caffeic acid. Although adhesion capacities have not been tested in presence of rutin, L. acidophilus NCFM also overexpresses EF-P and pyruvate kinase (as well as GAPDH and EF-G) in the presence of this flavonol glycoside [45]. In this regard, the same positive correlation between rutin and L. acidophilus adhesive capacity could be expected.
However, it must be noted that abundance of GAPDH or EF-G did not fit with the good adhesion capacities of this strain stimulated by RSV or TA, respectively [109]. The expression of two moonlighting proteins implicated in adhesion, EF-GreA [110] (upregulated) and LuxS [111] (downregulated), was also of different tendency in response to tannic acid in L. plantarum WCFS1 [37] (Table 4). Overall, these results show that abundance of moonlighting proteins with potential adhesion capacity cannot always be directly correlated with improved adhesion induced by these PPCs, indicating that mutant approaches are necessary to complete these studies. Other candidate proteins with moonlighting functionality have been implicated in adhesion to host cells [112,113,114]. Among these, the oligopeptide-binding protein Opp [115] or some molecular chaperones [116,117,118], are markedly induced in presence of some PPCs. The oppA gene is markedly overexpressed in the presence of p-CA or OLE (Table 4). GroEL and small heat-shock proteins (HSPs) are induced by several PPCs (Table 4) and even though these proteins participate in the binding of L. johnsonii and L. plantarum WCFS1 to host cells, respectively, adhesion assays in the presence of PPCs and mutant approaches are also required to show which among these moonlighting proteins induced by specific PPCs improve the adhesion to host cells.
The msa gene from L. plantarum encodes a mannose-specific adhesion factor [67] that is overexpressed in the presence of RSV (Table 4). Since improved adhesion to mannose-containing intestinal cells is believed to be important for the health-promoting effects of probiotics [119], induction of msa by RSV may help in improving the adhesion capacity and probiotics effects of L. plantarum.
10. Concluding Remarks
Exposure to plant phenolic compounds leads to changes in the composition of plant and animal host microbiomes which can decisively contribute to host fitness and health. However, our understanding of the molecular mechanisms underlying the direct effects of PPCs on microbes, as well as on host–microbe interactions, is still largely unexplored, in terms of compounds, microbes, and hosts examined. These insights are necessary to substantiate a causal nexus between exposure to PPCs and its effects on microbes and the host.
Here, molecular research into the interaction between Lactobacillus spp. and individual PPCs was reviewed (Table 5). A variety of mechanisms are induced by PPCs to increase the oxidative stress response in these microorganisms which highlights the antimicrobial nature of these compounds. These responses, together with the induction of general stress responses, revealed that these compounds are perceived, but not always, as stressors by these microorganisms. To cope with this selective pressure, Lactobacillus relies, in addition to known metabolic pathways for PPC detoxification, on efflux pumps to extrude, at least, hydroxycinnamic acids and stilbenes out of the cell. Furthermore, exposure to different PPCs variably adjusts the capacity of lactobacilli for carbohydrate acquisition and metabolism. The adaptation of nitrogen metabolism in to different PPCs is conserved and oriented to control the import and production of ammonia/amino-containing compounds. These metabolic adaptations, together with the induction of oxidative and general stress responses, are important ecological fitness determinants that leave Lactobacillus better able to cope with the stress encountered in the host environment.

Table 5.
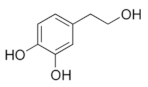
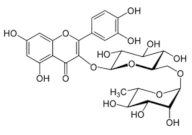
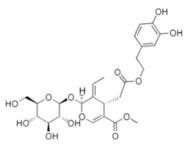
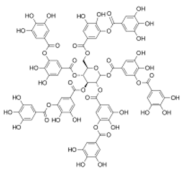
Structural formulas of plant phenolic compounds and techniques used to study the molecular responses of Lactobacillus spp. to these compounds.
The biosynthetic pathways for macromolecular components of the cell wall, which play key physiological roles in the communication of lactobacilli with the host environment and directly participate in host signaling, were markedly modulated by PPCs. In addition, some PPCs regulate the expression of specific molecular components with documented immunomodulatory capacities. PPCs, thus, emerge as new potential modulators of the cell surface and immunomodulatory properties of Lactobacillus spp. This finding opens new promising ways to target modifications with these natural products of cell surface properties that are associated with the physiological status of the host. The increased understanding of the Lactobacillus–PPC interaction provides molecular criteria to rationally improve the effector capacities and increase the success rates when these microorganisms are administered to improve plant growth or to benefit animal host health.
Author Contributions
Conceptualization, F.L.d.F., B.d.l.R. and R.M.; writing—original draft preparation, F.L.d.F.; writing—review and editing, F.L.d.F., B.d.l.R. and R.M.; funding acquisition, F.L.d.F., B.d.l.R. and R.M. All authors read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministerio de Economía, Industria and Competitividad under Grant numbers AGL2017-84614-C2-2-R and AGL2014-52911 (AEI/FEDER, UE) and CSIC under Grant number ICOOPB20412.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in review.
Conflicts of Interest
The authors report no conflicts of interest.
References
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. MOLI-SANI Study Investigators. Mediterranean diet, dietary polyphenols and low grade inflammation: Results from the MOLI-SANI study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, 310–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 781–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervert-Hernandez, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Curiel, J.A.; Muñoz, R.; López de Felipe, F. pH and dose-dependent effects of quercetin on the fermentation capacity of Lact. plantarum. LWT Food Sci. Technol. 2010, 43, 926–933. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- López de Felipe, F.; Curiel, J.A.; Muñoz, R. Improvement of the fermentation performance of Lactobacillus plantarum by the flavanol catechin is uncoupled from its degradation. J. Appl. Microbiol. 2010, 109, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberto, M.R.; Farías, M.E.; Manca De Nadra, M.C. Effect of gallic acid and catechin on Lactobacillus hilgardii 5w growth and metabolism of organic compounds. J. Agric Food Chem. 2001, 49, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Rad. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Hammes, W.; Hertel, C. The genera Lactobacillus and Carnobacterium. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 320–403. [Google Scholar] [CrossRef]
- Fhoula, I.; Najjari, A.; Turki, Y.; Jaballah, S.; Boudabous, A.; Ouzari, H. Diversity and antimicrobial properties of lactic acid bacteria isolated from rhizosphere of olive trees and desert truffles of Tunisia. Biome. Res. Int. 2013, 2013, 405708. [Google Scholar] [CrossRef] [Green Version]
- Pontonio, E.; Di Cagno, R.; Tarraf, W.; Filannino, P.; De Mastro, G.; Gobbetti, M. Dynamic and assembly of epiphyte and endophyte lactic acid bacteria during the life cycle of Origanum vulgare L. Front. Microbiol. 2018, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kao, K.C. Transcriptional analysis of Lactobacillus brevis to N-butanol and ferulic acid stress responses. PLoS ONE 2011, 6, e21438. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Sendra, A.; Landete, J.M.; Alcántara, C.; Zúñiga, M. Response of Lactobacillus casei BL23 to phenolic compounds. J. Appl. Microbiol. 2011, 111, 1473–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverón, I.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Genome-wide transcriptomic responses of a human isolate of Lactobacillus plantarum exposed to p-coumaric acid stress. Mol. Nutr. Food Res. 2012, 56, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Reverón, I.; de las Rivas, B.; Matesanz, R.; Muñoz, R.; López de Felipe, F. Molecular adaptation of Lactobacillus plantarum WCFS1 to gallic acid revealed by genome-scale transcriptomic signature and physiological analysis. Microb. Cell Fact. 2015, 14, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverón, I.; Plaza-Vinuesa, L.; Franch, M.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Transcriptome-based analysis in Lactobacillus plantarum WCFS1 reveals new insights into resveratrol effects at system level. Mol. Nutr. Food Res. 2018, 62, e1700992. [Google Scholar] [CrossRef]
- Santamaría, L.; Reverón, I.; Plaza-Vinuesa, L.; Oliveros, J.C.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Oleuropein transcriptionally primes Lactobacillus plantarum to interact with plant hosts. Front. Microbiol. 2019, 10, 2177. [Google Scholar] [CrossRef]
- Reverón, I.; Plaza-Vinuesa, L.; Santamaría, L.; Oliveros, J.C.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Transcriptomic evidence of molecular mechanisms underlying the response of Lactobacillus plantarum WCFS1 to hydroxytyrosol. Antioxidants 2020, 9, 442. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. (2007–2015). Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 25 July 2020).
- Simić, A.; Manojlović, D.; Segan, D.; Todorović, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Schindeldecker, M.; Moosmann, B. Protein-borne methionine residues as structural antioxidants in mitochondria. Amino Acids 2015, 47, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Gatz, S.; Wiesmüller, L. Take a break-resveratrol in action on DNA. Carcinogenesis 2008, 29, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yin, J.; Liu, J.; Xu, Q.; Lan, T.; Ren, F.; Hao, Y. The copper homeostasis transcription factor CopR is involved in H2O2 stress in Lactobacillus plantarum CAUH2. Front. Microbiol. 2017, 8, 2015. [Google Scholar] [CrossRef] [Green Version]
- Groot, M.N.; Klaassens, E.; de Vos, W.M.; Delcour, J.; Hols, P.; Kleerebezem, M. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology 2005, 151, 1229–1238. [Google Scholar] [CrossRef]
- Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Anglade, P.; Baraige, F.; Zagorec, M.; Champomier-Vergès, M.; Muñoz, R.; López de Felipe, F. Response of a Lactobacillus plantarum human isolate to tannic acid challenge assessed by proteomic analyses. Mol. Nutr. Food Res. 2011, 55, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.R.; Alkema, W.; Starrenburg, M.J.; Hugenholtz, J.; van Hijum, S.A.; Bron, P.A. Strain-dependent transcriptome signatures for robustness in Lactococcus lactis. PLoS ONE 2016, 11, e0167944. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Yoshida, T.; Izuhara-Kihara, H.; Noda, M.; Sugiyama, M. Crystallographic and mutational analyses of cystathionine β-synthase in the H2S-synthetic gene cluster in Lactobacillus plantarum. Protein Sci. 2017, 26, 763–783. [Google Scholar] [CrossRef]
- Matoba, Y.; Noda, M.; Yoshida, T.; Oda, K.; Ezumi, Y.; Yasutake, C.; Izuhara-Kihara, H.; Danshiitsoodol, N.; Kumaga, T.; Sugiyama, M. Catalytic specificity of the Lactobacillus plantarum cystathionine γ-lyase presumed by the crystallographic analysis. Sci. Rep. 2020, 10, 14886. [Google Scholar] [CrossRef]
- Roca, A.I.; Cox, M.M. The RecA protein, structure and function. Crit. Rev. Bioch. Mol. Biol. 1990, 25, 415–456. [Google Scholar] [CrossRef]
- Cecconi, D.; Cristofoletti, M.; Milli, A.; Antonioli, P.; Rinalducci, S.; Zolla, L.; Zapparoli, G. Effect of tannic acid on Lactobacillus plantarum wine strain during starvation: A proteomic study. Electrophoresis 2009, 30, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.; Rinalducci, S.; Zolla, L.; Antonioli, P.; Righetti, P.G.; Zapparoli, G. Effect of tannic acid on Lactobacillus hilgardii analysed by a proteomic approach. J. Appl. Microbiol. 2007, 102, 787–795. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Lippolis, R.; Sorrentino, A.; Liberti, S.; Fragnito, F.; Siciliano, R.A. Lactobacillus acidophilus-rutin interplay investigated by proteomics. PLoS ONE 2015, 10, e0142376. [Google Scholar]
- Zolkiewski, M. A camel passes through the eye of a needle: Protein unfolding activity of Clp ATPases. Mol. Microbiol. 2006, 61, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Cao, C.; Zhang, J.; Kwok, L.Y.; Zhang, H.; Chen, Y. Lactobacillus casei asp23 gene contributes to gentamycin resistance via regulating specific membrane-associated proteins. J. Dairy Sci. 2018, 101, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Kriel, A.; Bittner, A.N.; Kim, S.H.; Liu, K.; Tehranchi, A.K.; Zou, W.Y.; Rendon, S.; Chen, R.; Tu, B.P.; Wang, J.D. Direct regulation of GTP homeostasis by (p)ppGpp: A critical component of viability and stress resistance. Mol. Cell 2012, 48, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M.; Rodríguez, H.; Curiel, J.A.; de las Rivas, B.; López de Felipe, F.; Muñoz, M. Degradation of phenolic compounds found in olive products by Lactobacillus plantarum strains. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Victor, R., Preedy, R.R.W., Eds.; Academic Press: London, UK, 2021; pp. 133–144. ISBN 9780128195284. [Google Scholar]
- van Veen, H.W.; Venema, K.; Bolhuis, H.; Oussenko, I.; Kok, J.; Poolman, B.; Driessen, A.J.; Konings, W.N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 1996, 93, 10668–10672. [Google Scholar] [CrossRef] [Green Version]
- Revilla-Guarinos, A.; Gebhard, S.; Mascher, T.; Zúñiga, M. Defence against antimicrobial peptides: Different strategies in Firmicutes. Environ. Microbiol. 2014, 16, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Tian, X.L.; Versey, J.; Wishart, A.; Li, Y.H. The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob. Agents Chemother. 2010, 54, 3895–3906. [Google Scholar] [CrossRef] [Green Version]
- Groot Kormelink, T.; Koenders, E.; Hagemeijer, Y.; Overmars, L.; Siezen, R.J.; de Vos, W.M.; Francke, C. Comparative genome analysis of central nitrogen metabolism and its control by GlnR in the class Bacilli. BMC Genom. 2012, 13, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boogerd, F.C.; Ma, H.; Bruggeman, F.J.; van Heeswijk, W.C.; García-Contreras, R.; Molenaar, D.; Krab, K.; Westerhoff, H.V. AmtB-mediated NH3 transport in prokaryotes must be active and as a consequence regulation of transport by GlnK is mandatory to limit futile cycling of NH4(+)/NH3. FEBS Lett. 2011, 585, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, J.D.; Herskovits, A.A.; O’ Riordan, M.X.D. Metabolism of the Gram-Positive bacterial Pathogen Listeria monocytogenes. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Plumed-Ferrer, C.; Koistinen, K.M.; Tolonen, T.L.; Lehesranta, S.J.; Kärenlampi, S.O.; Mäkimattila, E.; Joutsjoki, V.; Virtanen, V.; von Wright, A. Comparative study of sugar fermentation and protein expression patterns of two Lactobacillus plantarum strains grown in three different media. Appl. Environ. Microbiol. 2008, 74, 5349–5358. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; de Angelis, M.; Gobbetti, M.; di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [Green Version]
- Kvint, K.; Nachin, L.; Diez, A.; Nystrom, T. The bacterial universal stress protein: Function and regulation. Curr. Opin. Microbiol. 2003, 6, 140–145. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Fact. 2014, 29, S9. [Google Scholar] [CrossRef] [Green Version]
- Rohde, M. The Gram-Positive bacterial cell wall. Microbiol. Spectrum 2019, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Reverón, I.; Rodríguez, H.; Campos, G.; Curiel, J.A.; Ascaso, C.; Carrascosa, A.V.; Prieto, A.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Tannic acid-dependent modulation of selected Lactobacillus plantarum traits linked to gastrointestinal survival. PLoS ONE 2013, 11, e66473. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Mainardi, J.L.; Villet, R.; Bugg, T.D.; Mayer, C. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol. Rev. 2008, 32, 386–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boekhorst, J.; Wels, M.; Kleerebezem, M.; Siezen, R.J. The predicted secretome of Lactobacillus plantarum WCFS1 sheds light on interactions with its environment. Microbiology 2006, 152, 3175–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, P.A.; Tomita, S.; van Swam, I., I; Remus, D.M.; Meijerink, M.; Wels, M.; Okada, S.; Wells, J.M.; Kleerebezem, M. Lactobacillus plantarum possesses the capability for wall teichoic acid backbone alditol switching. Microb. Cell Fact. 2012, 11, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remus, D.M.; van Kranenburg, R.; van Swam, I.; Taverne., N.I.; Bongers, R.S.; Wels, M.; Wells, J.M.; Bron, P.A.; Kleerebezem, M. Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling. Microb. Cell Fact. 2012, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Peng, Z.; Hu, M.; Xiao, Y.S.; Liu, Z.G.; Guan, Q.Q.; Xie, M.Y.; Xiong, T. Interactions between Lactobacillus plantarum NCU116 and its environments based on extracellular proteins and polysaccharides prediction by comparative analysis. Genomics 2020, 112, 3579–3587. [Google Scholar] [CrossRef]
- Parlindungan, E.; May, B.K.; Jones, O.A.H. Metabolic insights into the effects of nutrient stress on Lactobacillus plantarum B21. Front. Mol. Biosci. 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Fozo, E.M.; Kajfasz, J.K.; Quivey, R.G., Jr. Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol. Lett. 2004, 238, 291–295. [Google Scholar] [CrossRef]
- van Bokhorst-van de Veen, H.; Abee, T.; Tempelaars, M.; Bron, P.A.; Kleerebezem, M.; Marco, M.L. Short- and long-term adaptation to ethanol stress and its cross-protective consequences in Lactobacillus plantarum. Appl. Environ. Microbiol. 2011, 77, 5247–5256. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo., Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Qiu, X.; de los Reyes, B.G.; Lin, C.S.; Pan, Y. Application of cranberry concentrate (Vaccinium macrocarpon) to control Escherichia coli O157:H7 in ground beef and its antimicrobial mechanism related to the down-regulated slp, hdeA and cfa. Food Microbiol. 2009, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Zeng, X.A.; Wang, M.S.; Brennan, C.S.; Gong, D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. Biochim. Biophys. Acta Biomembr. 2018, 1860, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rowlett, V.W.; Mallampalli, V.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.; Barrangou, R.; Russell, W.M.; Klaenhammer, T.R. Characterisation of the tre locus and analysis of trehalose cryoprotection in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 2006, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, A.Y.; Font de Valdez, G.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [Green Version]
- Koskenniemi, K.; Laakso, K.; Koponen, J.; Kankainen, M.; Greco, D.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Surakka, A.; Salusjärvi, T.; et al. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteomics 2011, 10, M110.002741. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; Mathesius, U. The role of flavonoids in root–rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [Green Version]
- Oldroyd, G.E.D. Speak, friend, and enter: Signaling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Schandry, N.; Becker, C. Allelopathic plants: Models for studying plant-interkingdom interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bove, P.; Russo, P.; Capozzi, V.; Gallone, A.; Spano, G.; Fiocco, D. Lactobacillus plantarum passage through an oro-gastro-intestinal tract simulator: Carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol. Res. 2013, 168, 351–359. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; de Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [Green Version]
- Bron, P.A.; Marco, M.L.; Hoffer, S.M.; van Mullekom, E.; de Vos, W.M.; Kleerebezem, M. Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J. Bacteriol. 2004, 186, 7829–7835. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, P.A.; Grangette, C.; Mercenier, A.; de Vos, W.M.; Kleerebezem, M. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J. Bacteriol. 2004, 186, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Bron, P.A.; Molenaar, D.; de Vos, W.M.; Kleerebezem, M. DNA micro-array-based identification of bile-responsive genes in Lactobacillus plantarum. J. Appl. Microbiol. 2006, 100, 728–738. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.C. Analyzing Global Gene Expression of Lactobacillus plantarum in the Human Gastrointestinal Tract. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006; p. 147. [Google Scholar]
- Kleerebezem, M.; Hols, P.; Bernard, E.; Rolain, T.; Zhou, M.; Siezen, R.J.; Bron, P.A. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 2010, 34, 199–230. [Google Scholar] [CrossRef]
- Van den Nieuwboer, M.; van Hemert, S.; Claassen, E.; de Vos, W.M. Lactobacillus plantarum WCFS1 and its host interaction: A dozen years after the genome. Microb. Biotechnol. 2016, 9, 452–465. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L.; de Vries, M.C.; Wels, M.; Molenaar, D.; Mangell, P.; Ahrne, S.; de Vos, W.M.; Vaughan, E.E.; Kleerebezem, M. Convergence in probiotic Lactobacillus gut-adaptive responses in humans and mice. ISME J. 2010, 4, 1481–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, P.A.; Meijer, M.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Dynamics of competitive population abundance of Lactobacillus plantarum ivi gene mutants in faecal samples after passage through the gastrointestinal tract of mice. J. Appl. Microbiol. 2007, 103, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Van Bokhorst-van de Veen, H.; Lee, I.C.; Marco, M.L.; Wels, M.; Bron, P.A.; Kleerebezem, M. Modulation of Lactobacillus plantarum gastrointestinal robustness by fermentation conditions enables identification of bacterial robustness markers. PLoS ONE 2012, 7, e39053. [Google Scholar]
- Heng, J.; Zhao, Y.; Liu, M.; Liu, Y.; Fan, J.; Wang, X.; Zhao, Y.; Zhang., X.C. Substrate-bound structure of the E. coli multidrug resistance transporter MdfA. Cell Res. 2015, 25, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl. Environ. Microbiol. 2008, 74, 4719–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, M.H.; O’Flaherty, S.; Allen, G.; Rivera, A.J.; Stewart, A.K.; Barrangou, R.; Theriot, C.M. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl. Acad. Sci. USA 2021, 118, e2017709118. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Van Bokhorst-van de Veen, H.; van Swam, I., I; Wels, M.; Bron, P.A.; Kleerebezem, M. Congruent strain specific intestinal persistence of Lactobacillus plantarum in an intestine-mimicking in vitro system and in human volunteers. PLoS ONE 2012, 7, e44588. [Google Scholar] [CrossRef] [Green Version]
- Lebeer, S.; Vanderleyden, J.; de Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.; Francius, G.; Schoofs, G.; Lambrichts, I.; Dufrêne, Y.; Vanderleyden, J.; de Keersmaecker, S.C. Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl. Environ. Microbiol. 2009, 75, 3554–3563. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.C.; Caggianiello, G.; van Swam, I., I; Taverne, N.; Meijerink, M.; Bron, P.A.; Spano, G.; Kleerebezem, M. Strain-specific features of extracellular polysaccharides and their impact on Lactobacillus plantarum-host interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.; van Hemert, S.; Taverne, N.; Wels, M.; de Vos, P.; Bron, P.A.; Savelkoul, H.F.; van Bilsen, J.; Kleerebezem, M.; Wells, J.M. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS ONE 2010, 5, e10632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hemmert, S.; Meijerink, M.; Molenaar, D.; Bron, P.A.; de Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, H.U.; Delsoglio, M.; Brix, S.; Pessione, E.; Svensson, B. Plant polyphenols stimulate adhesion to intestinal mucosa and induce proteome changes in the probiotic Lactobacillus acidophilus NCFM. Mol. Nutr. Food Res. 2018, 62, 4. [Google Scholar] [CrossRef]
- Cui, G.; Wang, J.; Qi, X.; Su, J. Transcription elongation factor GreA plays a key role in cellular invasion and virulence of Francisella tularensis subsp. novicida. Sci. Rep. 2018, 8, 6895. [Google Scholar] [CrossRef]
- Buck, B.L.; Azcarate-Peril, M.A.; Klaenhammer, T.R. Role of autoinducer-2 on the adhesion ability of Lactobacillus acidophilus. J. Appl. Microbiol. 2009, 107, 269–279. [Google Scholar] [CrossRef]
- Wang, W.; Jeffery, C.J. An analysis of surface proteomics results reveals novel candidates for intracellular/surface moonlighting proteins in bacteria. Mol. Biosyst. 2016, 12, 1420–1431. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Olesen, S.V.; Prehn, K.; Lahtinen, S.J.; Brix, S.; Hachem, M.A.; Svensson, B. Mucin- and carbohydrate-stimulated adhesion and subproteome changes of the probiotic bacterium Lactobacillus acidophilus NCFM. Proteomics 2017, 163, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, K.; Ochiai, A.; Tsubokawa., D.; Ishihara, K.; Yamamoto, Y. Identification and characterization of sulphated carbohydrate-binding protein from Lactobacillus reuteri. PLoS ONE 2013, 8, e83703. [Google Scholar] [CrossRef]
- Espinosa-Urgel, M.; Salido, A.; Ramos, J.L. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J. Bacteriol. 2000, 182, 2363–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef] [PubMed]
- Bergonzelli, G.E.; Granato, D.; Pridmore, R.D.; Marvin-Guy, L.F.; Donnicola, D.; Corthesy-Theulaz, I.E. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: Potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 2006, 74, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, A.; Russo, P.; Capozzi, V.; Spano, G.; Fiocco, D. Knock out of sHSP genes determines some modifications in the probiotic attitude of Lactiplantibacillus plantarum. Biotechnol Lett. 2020, 43, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Glenting, J.; Holmstrøm, K.; Israelsen, H.; Vrang, A.; Antonsson, M.; Ahrné, S.; Madsen, S.M. Molecular switch controlling expression of the mannose-specific adhesin, msa, in Lactobacillus plantarum. Appl. Environ. Microbiol. 2019, 85, e02954-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).