In Vitro and In Vivo Studies of Anti-Lung Cancer Activity of Artemesia judaica L. Crude Extract Combined with LC-MS/MS Metabolic Profiling, Docking Simulation and HPLC-DAD Quantification
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Process
2.2. In Vitro Cytotoxic Activity
2.2.1. Cell Culture and MTT Cytotoxic Assay
2.2.2. Annexin V/PI Staifning and Cell Cycle Analysis
2.2.3. RT-PCR for the Apoptosis-Related Genes
2.3. In Vivo Experiment (Xenograft Model)
2.3.1. Animals
2.3.2. Study Design
2.3.3. Biochemical Investigation and Histopathological Examination
2.4. LC/Triple-TOF-MS/MS Metabolomic Analysis
2.5. Molecular Docking Simulations of the Metabolites Detected by LC/Triple-TOF-MS/MS Analysis
2.6. HPLC-DAD Analysis
2.6.1. Standard Compounds
2.6.2. Apparatus and Operating Conditions
2.6.3. Sample Preparation
2.6.4. Calibration Graphs and Calculations
3. Results and Discussion
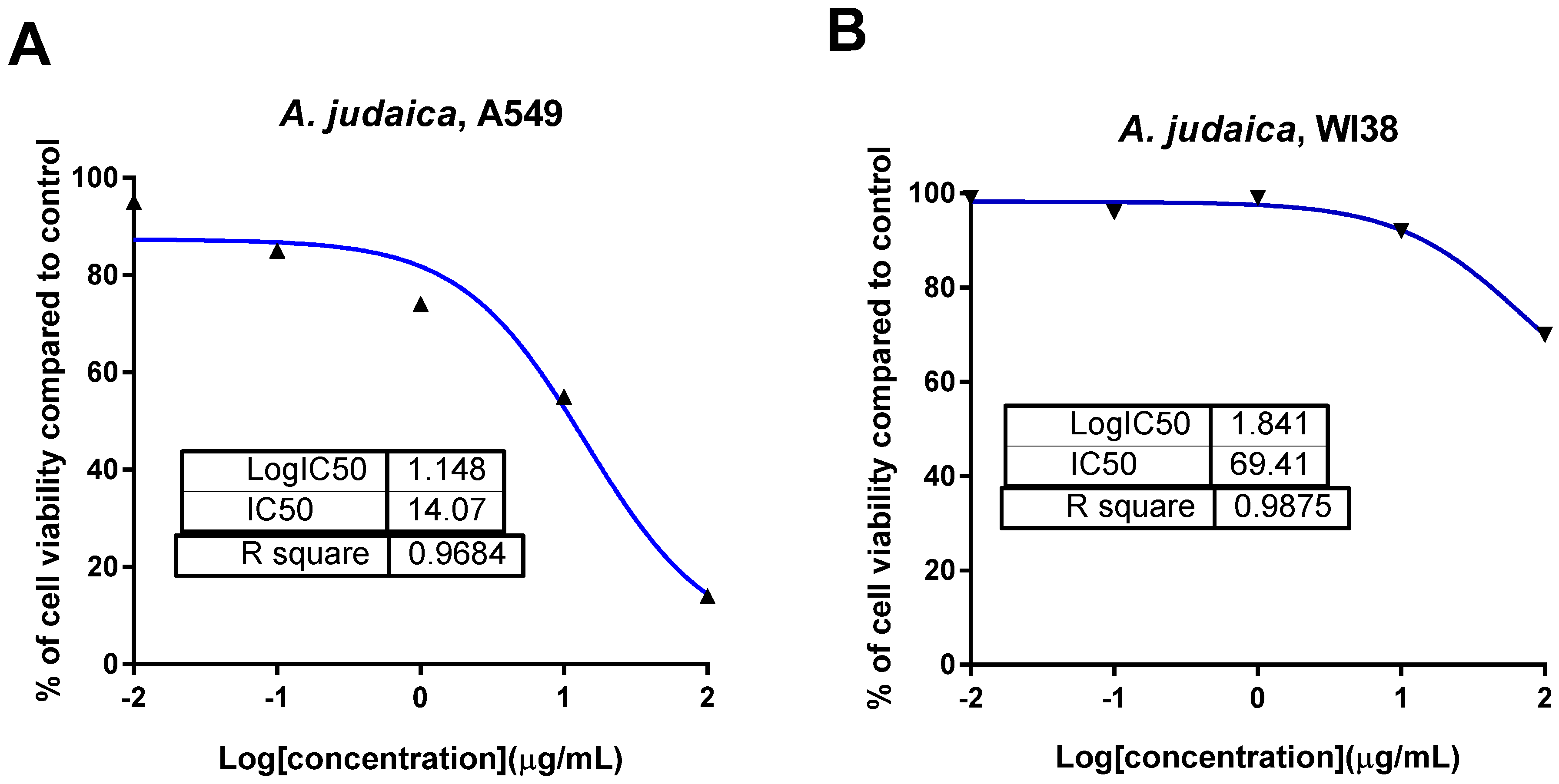
3.1. In Vitro Cytotoxic Activity of the Ethanolic Crude Extract of A. judaica L.
3.1.1. The Cytotoxic Activity of A. judaica L. Extract against A549 Cells Using MTT Assay
3.1.2. Effect of Crude Extract of A. judaica L. on Apoptosis Induction in A549 Cells Using Flow Cytometry
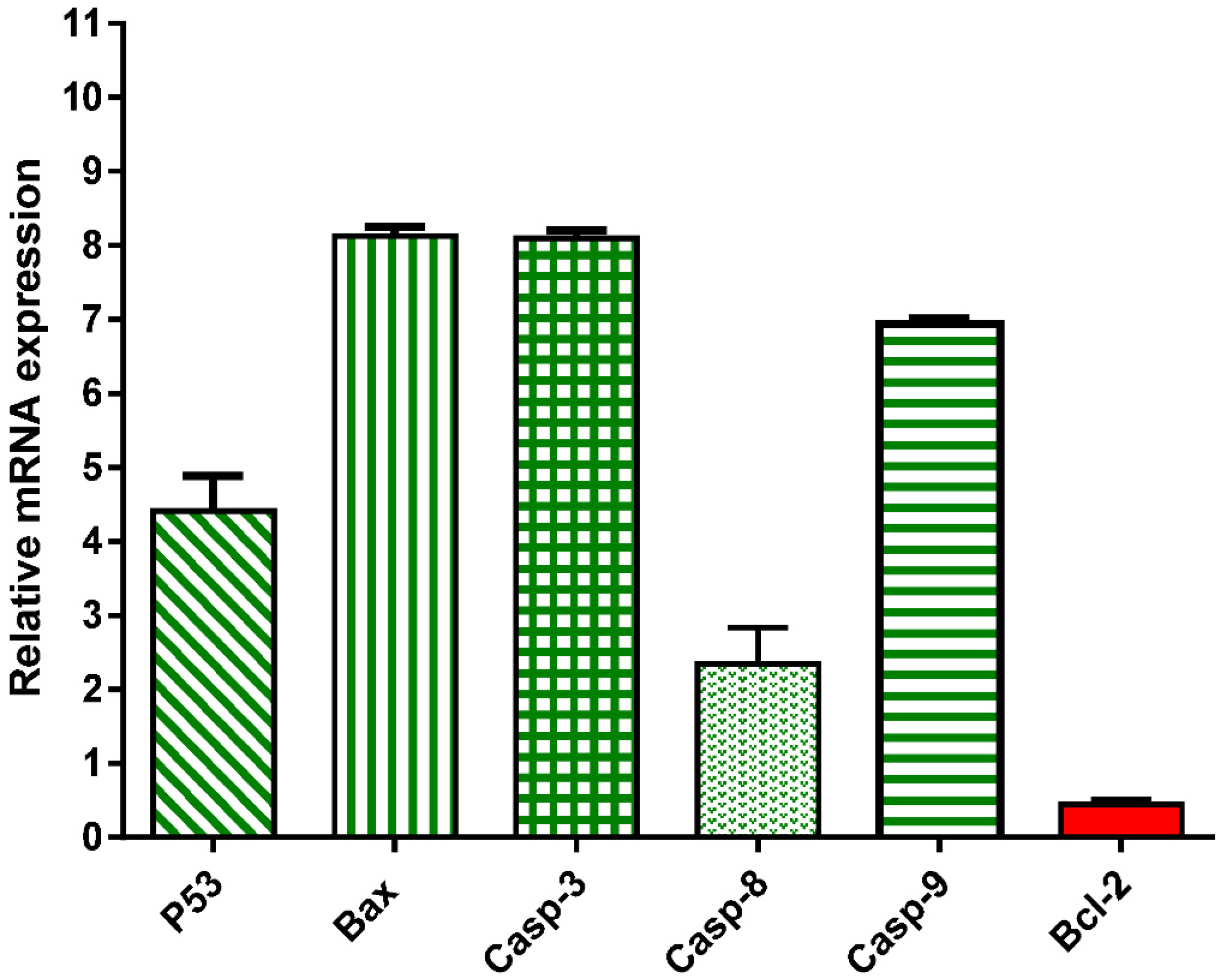
3.1.3. Effect of Crude Extract of A. judaica L. on mRNA Gene Expression of Apoptosis-related Genes
3.2. In Vivo Study (Xenograft Model)
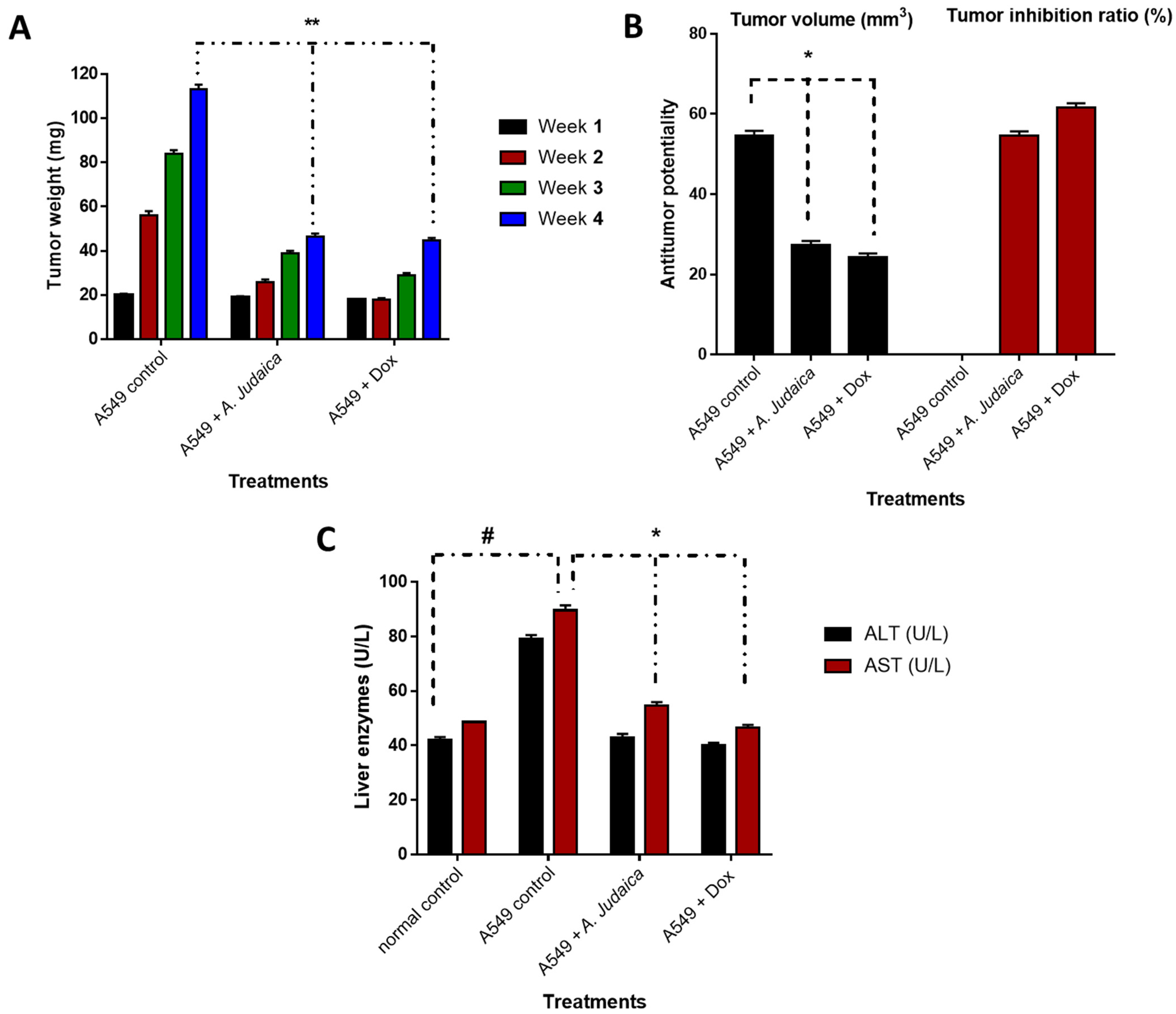
3.2.1. Effect of A. judaica Crude Extract on Tumor Mass Growth Xenografts
3.2.2. Biochemical Investigation and Histopathological Examination of Liver Tissues
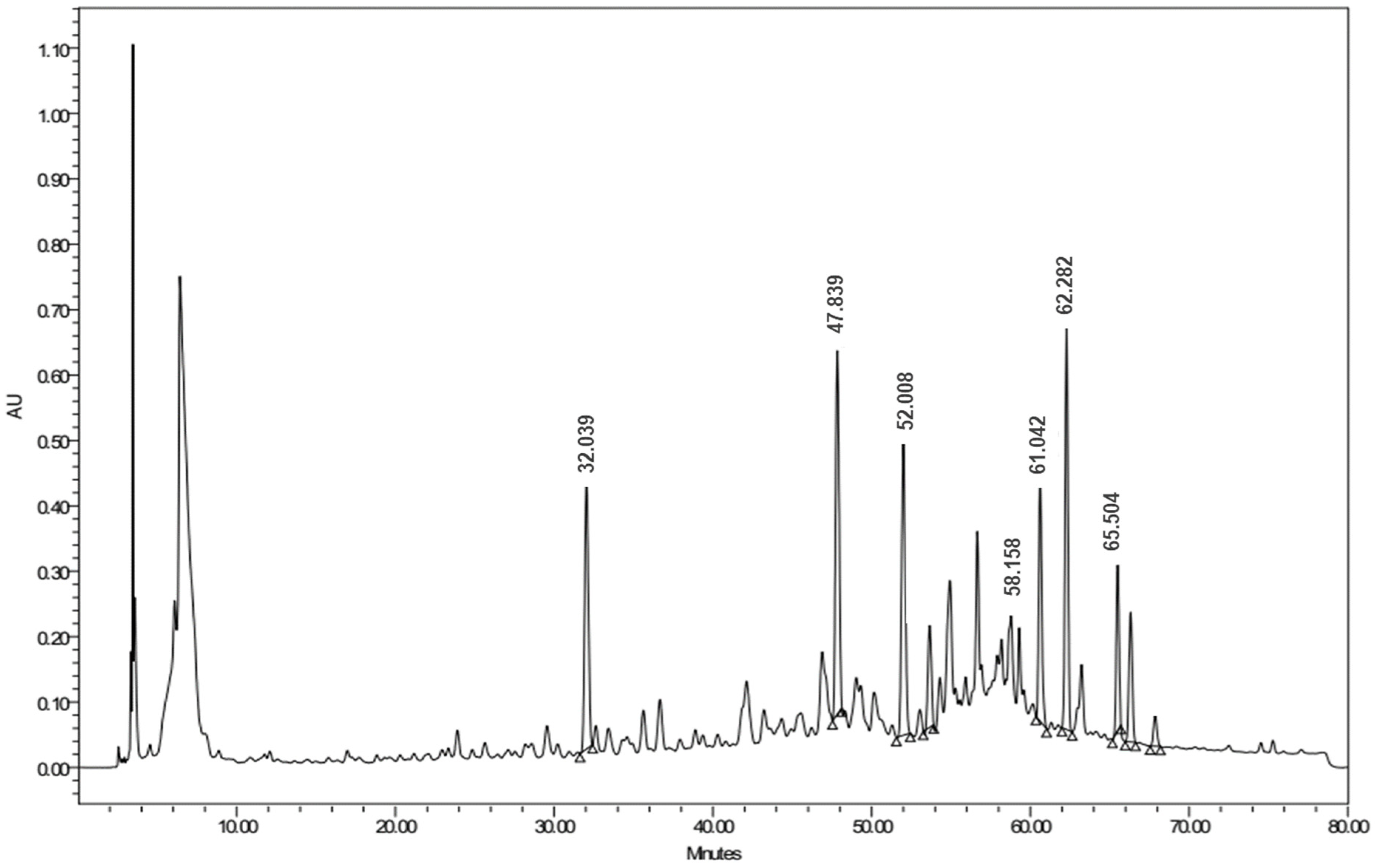
3.3. LC/Triple-TOF-MS/MS Metabolomic Analysis of Ethanolic Crude Extract of Artemisia judaica L.
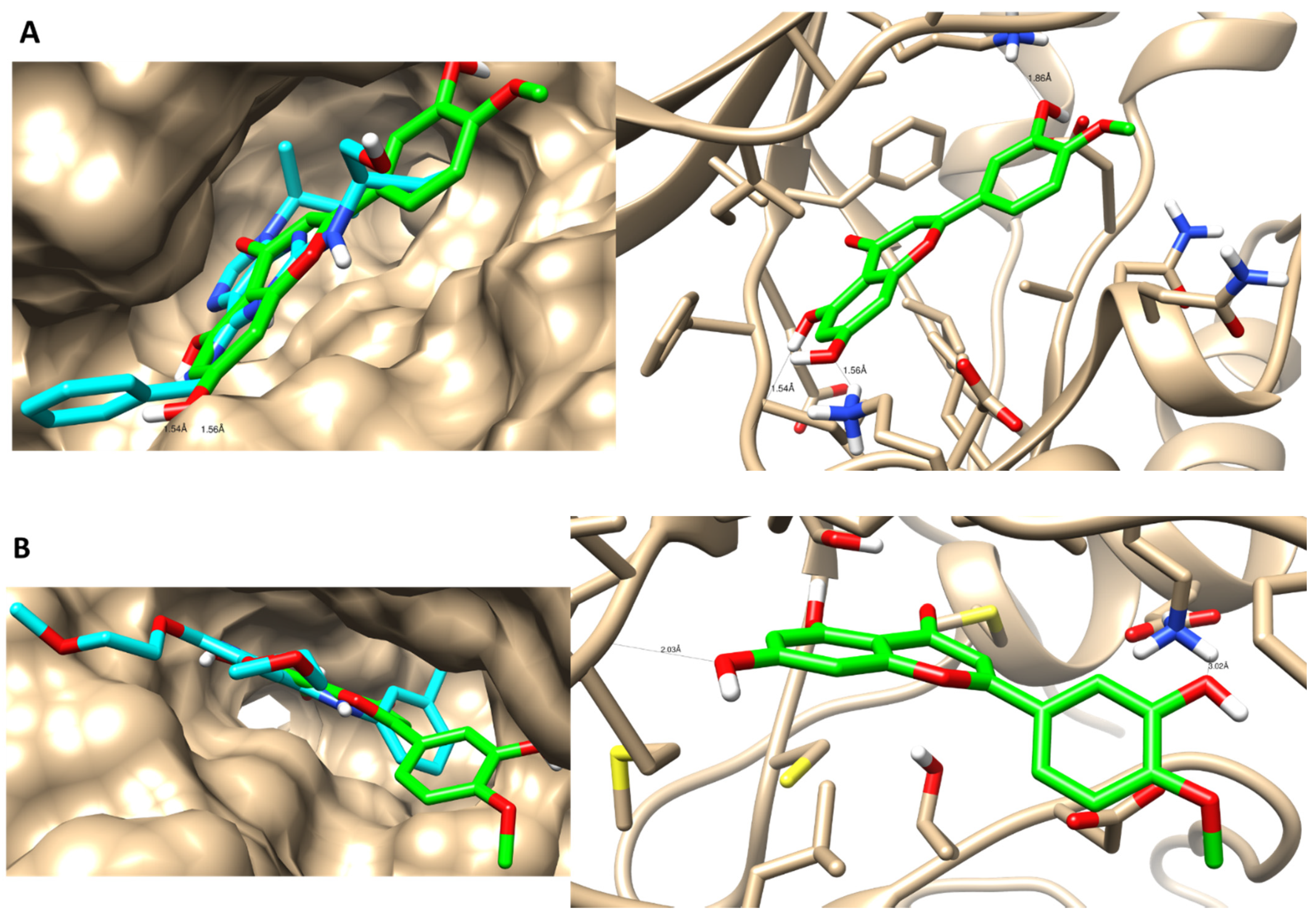
3.4. Molecular Docking Simulations
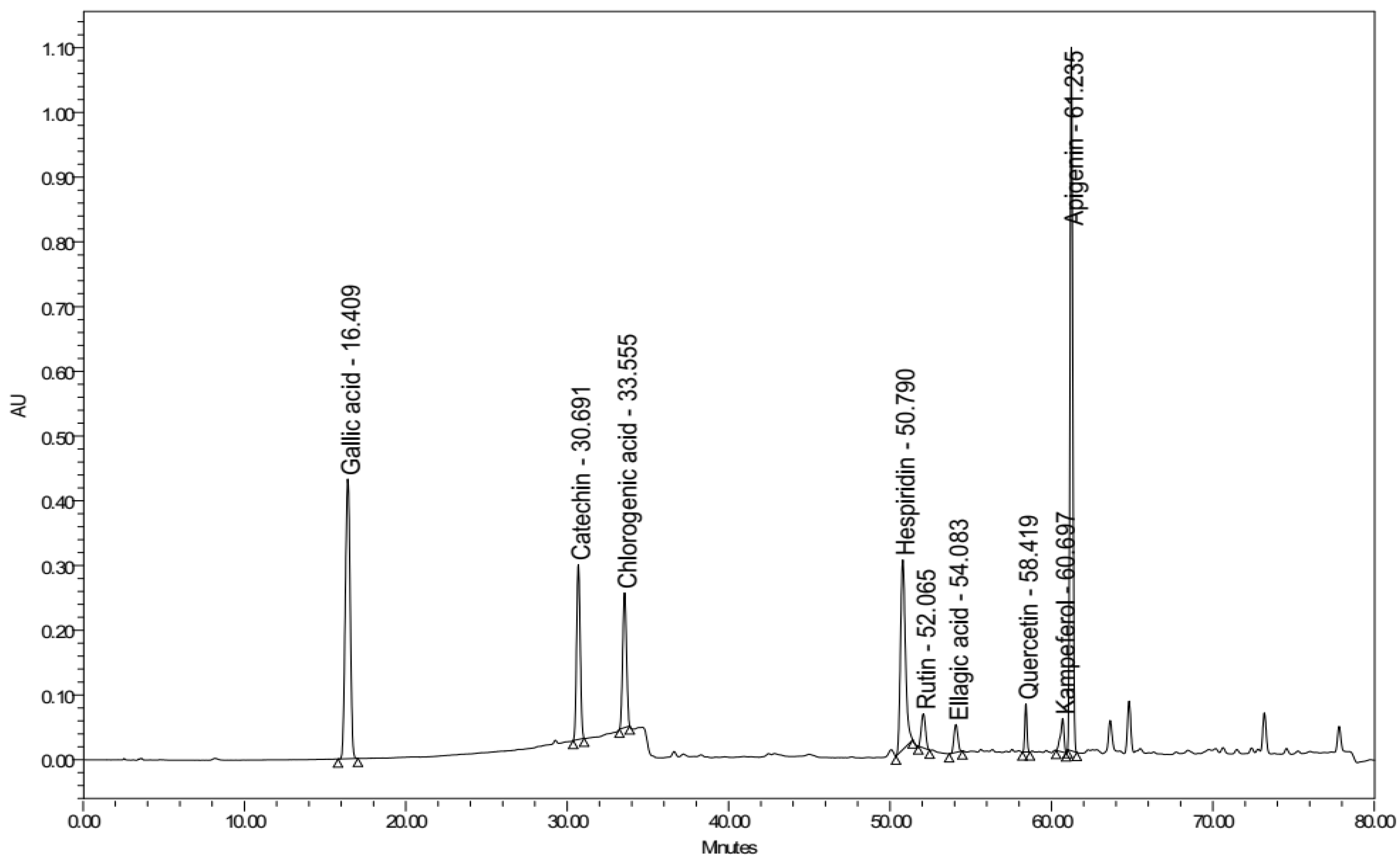
3.5. Identification and Quantification of Flavonoids by Using HPLC Analysis
3.5.1. Qualitative Identification
3.5.2. Quantitative Estimation
Linearity
System Precision
Method Precision
Limits of Detection and Quantification
Analytical Solution Stability
Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lemjabbar-Alaoui, H.; Hassan, O.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Sholl, L.M.; Aisner, D.L.; Allen, T.C.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Dacic, S.; Hariri, L.P.; Kerr, K.M.; Lantuejoul, S.; et al. Liquid biopsy in lung cancer: A perspective from members of the pulmonary pathology society. Arch. Pathol. Lab. Med. 2016, 140, 825–829. [Google Scholar] [CrossRef]
- Mokhtar, A.B.; Ahmed, S.A.; Eltamany, E.E.; Karanis, P. Anti-Blastocystis activity in vitro of Egyptian herbal extracts (Family: Asteraceae) with emphasis on Artemisia judaica. Int. J. Environ. Res. Public Health 2019, 16, 1555. [Google Scholar] [CrossRef]
- El-Massry, K.F.; EL-chorab, A.H.; Farouh, A. Antioxidant activity and volatile components of Egyptian Artemisia judaica. Food Chem. 2002, 79, 331–336. [Google Scholar] [CrossRef]
- Abd-Elhady, H.K. Insecticidal activity and chemical composition of essential oil from Artemisia judaica L. against Callosobruchus maculates (F.) (Coleoptera: Bruchidae). J. Plant Protein Res. 2012, 52, 347–352. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Shiboob, M.H. Antioxidant Activity of phenolic and alkaloid fractions accumulated in Artemisia judaica and Artemisia herbaalba. J. Nat. Rem. 2017, 17, 154–164. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.N.; Mahmood, A.; Khan, M.; Alkhathlan, H.Z. Comparative study on the essential oils of Artemisia judaica and A. herbaalba from Saudi Arabia. Arab. J. Chem. 2020, 13, 2053–2065. [Google Scholar] [CrossRef]
- Moharram, F.A.; Nagy, M.M.; El Dib, R.A.; el-Tantawy, M.M.; El Hossary, G.G.; El-Hosari, D.G. Pharmacological activity and flavonoids constituents of Artemisia judaica L. aerial parts. J. Ethnopharmacol. 2021, 270, 113777. [Google Scholar] [CrossRef] [PubMed]
- Albasher, G.; Alwahaibi, M.; Abdel-Daim, M.M.; Alkahtani, S.; Almeer, A. Protective effects of Artemisia judaica extract compared to metformin against hepatorenal injury in high-fat diet/streptozotocine-induced diabetic rats. Environ. Sci. Pollut Res. 2020, 27, 40525–40536. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.O. Microscopical and phytochemical investigation of Egyptian Artemisia judaica L. Var. Sinaitica tackholm and its free radical scavenging activity. Int. J. Pharmacog. PhytoChem. Res. 2014, 6, 698–709. [Google Scholar]
- Bhat, S.H.; Ullah, M.F.; Abu-Duhier, F.M. Bioactive extract of Artemisia judaica causes in vitro inhibition of dipeptidyl peptidase IV and pancreatic/intestinal enzymes of the carbohydrate absorption cascade: Implication for anti-diabetic new molecular entities (NMEs). Orient Pharm. Exp. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef]
- Zihlif, M.; Afifi, F.; Muhtaseb, R.; Al-Khatib, S.; Abaza, I.; Naffa, R. Screening the antiangiogenic activity of medicinal plants grown and sold in Jordan. Planta Med. 2012, 78, 297–301. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Mabrouk, D.M.; Hassanane, M.M.; Khalil, W.K.B. Protective Effect of Artemisia judaica against doxorubicin-induced toxicity in mice. Annu. Res. Rev. Biol. 2017, 18, 1–10. [Google Scholar] [CrossRef][Green Version]
- Al-Trad, B.; Al Zoubi, M.; Migdady, M.; Lahham, J.; Aljabali, A.A.A.; Shehab, M.; Alomari, S.; Al-Qudah, M.A.; Qar, J.; Muhaidat, R.; et al. Effects of Artemisia judaica essential oil and ethanolic extract on experimentally induced benign prostatic hyperplasia. Pharmacogn. Mag. 2020, 16, 569. [Google Scholar] [CrossRef]
- Nasr, F.A.; Noman, O.M.; Mothana, R.A.; Alqahtani, A.S.; Al-Mishari, A.A. Cytotoxic, antimicrobial and antioxidant activities and phytochemical analysis of Artemisia judaica and A. sieberi in Saudi Arabia. Afr. J. Pharm. Pharmacol. 2020, 14, 278–284. [Google Scholar] [CrossRef]
- Al-Senosy, N.K.; Ebeed, N.M.; Salem, L.M.; Girgis, S.M.; Ahmad, E.S. The anticancer activity of Artemisia judaica crude extract in human hepatocellular carcinoma HepG2 cells by induction of apoptosis and cell cycle arrest. Int. J. Cur. Res. Rev. 2021, 13, 209–215. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Aly, H.F.; Shalaby, N.M.; Albalawy, M.A.; Aboutabl, E.A. Hunting for renal protective phytoconstituents in Artemisia judaica L. and Chrysanthemum coronarium L. (Asteraceae). Egypt Pharm. J. 2014, 13, 46–57. [Google Scholar] [CrossRef]
- Janaćković, P.; Novaković, J.; Soković, M.; Vujisić, L.; Giweli, A.A.; Dajić Stevanović, Z.; Marin, P.D. Composition, and antimicrobial activity of essential oils of Artemisia judaica, A. herbaalba, and A. arborescens from Libya. Arch. Biol. Sci. 2015, 67, 455–466. [Google Scholar] [CrossRef]
- Abdelgaleil, A.M.; Abbassy, M.A.; Belal, A.H.; Abdel Rasoul, M.A. Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica L. BioRes. Technol. 2008, 99, 5947–5950. [Google Scholar] [CrossRef]
- Galal, E.E.; Kandil, A.; Abdel Latif, M.; Khedr, T.; Khafagy, S.M. Cardiac pharmaco-toxicological studies of judaicin, isolated from Artemisia judaica. Planta Med. 1974, 25, 88–91. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, H.Y.; Shibamoto, T.; Jang, T.S.; Lee, S.C.; Shim, J.S.; Hahm, D.H.; Lee, H.J.; Lee, S.; Kang, K.S. Beneficial effects of a medicinal herb, Cirsium japonicum var. maackii, extract and its major component, cirsimaritin on breast cancer metastasis in MDAMB-231 breast cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3968–3973. [Google Scholar] [CrossRef]
- Mahmood, A.; Alkhathlan, H.Z. Isolation, synthesis, and pharmacological applications of cirsimaritin—A short review. J. Med. Plants Res. 2019, 7, 252–260. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a lung squamous carcinoma cells (NCIH-520) proliferation inhibitor. J. Biomol. Struct. Dyn. 2020, 39, 3312–3323. [Google Scholar] [CrossRef]
- Raja, S.B.; Rajendiran, V.; Kasinathan, N.K.; Amrithalakshmi, P.; Venkatabalasubramanian, S.; Murali, M.R.; Devaraj, H.; Devaraj, S.N. Differential cytotoxic activity of quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem. Toxicol. 2017, 106, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell BioSci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nafie, M.S.; Amer, A.M.; Mohamed, A.K.; Tantawy, E.S. Discovery of novel pyrazolo [3,4-b]pyridine scaffold-based derivatives as potential PIM-1 kinase inhibitors in breast cancer MCF-7 cells. Bioorg. Med. Chem. 2020, 28, 115828. [Google Scholar] [CrossRef]
- Tantawy, E.S.; Amer, A.M.; Mohamed, E.K.; Abd Alla, M.M.; Nafie, M.S. Synthesis, characterization of some pyrazine derivatives as anti-cancer agents: In vitro and in silico approaches. J. Mol. Struct. 2020, 1210, 128013. [Google Scholar] [CrossRef]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.S.A.G.; Barakat, A.; Boraei, A.T.A. Discovery of new apoptosis-inducing agents for breast cancer based on ethyl 2-amino-4,5,6,7-tetra hydrobenzo[b]thiophene-3-carboxylate: Synthesis, In vitro, and in vivo activity evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Arafa, K.; Sedky, N.K.; Alakhdar, A.A.; Arafa, R.K. Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chem. Biol. Interact. 2020, 324, 109087. [Google Scholar] [CrossRef]
- Sarhan, A.A.M.; Boraei, A.T.A.; Barakat, A.; Nafie, M.S. Discovery of hydrazide-based pyridazino[4,5-b]indole scaffold as a new phosphoinositide 3-kinase (PI3K) inhibitor for breast cancer therapy. RSC Adv. 2020, 10, 19534–19541. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Ibrahim, A.K.; Yamada, K.; Abdel-Kader, M.S.; Ibrahim, A.K.; Ahmed, S.A.; Badr, J.M.; Nafie, M.S. Chemical profiling, cytotoxic activities through apoptosis induction in MCF-7 cells and molecular docking of Phyllostachys heterocycla bark nonpolar extract. J. Biomol. Struct. Dyn. 2021, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Huang, J.-H.; Cho, E.-C.; Lee, K.-C. Intercalating pyrene with polypeptide as a novel self-assembly nano-carrier for colon cancer suppression in vitro and in vivo. Mater. Sci. Eng. C 2020, 109, 110593. [Google Scholar] [CrossRef]
- Abdel-Hamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC-MS/MS metabolic profiling. Antioxidants 2021, 10, 1701. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some African plants belonging to Kalanchoe species: Antitrypanosomal, cytotoxic, antitopoisomerase I activities and chemical profiling using ultra-performance liquid chromatography/ quadrupole-time of flight. Pharmacogn. Mag. 2021, 17, 6. [Google Scholar]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical profiling, in vitro and in silico anti-microbial and anti-cancer activity evaluations and Staph GyraseB and h-TOP-IIβ receptor-docking studies of major constituents of Zygophyllum coccineum L. Aqueous-ethanolic extract and its subsequent fractions: An approach to validate traditional phytomedicinal knowledge. Molecules 2021, 26, 577. [Google Scholar] [CrossRef]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Stepanova, A.V.; Kudrinskaya, V.A.; Apyari, V.V. Specifics of separation of flavonoids by reverse phase high performance liquid chromatography on the Luna 5u C18(2) column. Mosc. Univ. Chem. Bull. 2012, 67, 254–258. [Google Scholar] [CrossRef]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical profiling, antioxidant, cytotoxic activities, and molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.F.A.; Habib, E.S.; Ibrahim, A.K.; Yamada, K.; Abdel-Kader, M.S.; Ahmed, S.A.; Ibrahim, A.K.; Badr, J.M.; Nafie, M.S. Chemical constituent profiling of Phyllostachys heterocycla var. Pubescens with selective cytotoxic polar fraction through EGFR inhibition in HepG2 cells. Molecules 2021, 26, 940. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A.; Eltamany, E.H.; Ali, I.A.I.; Gebriel, S.M.; Nafie, M.S. Synthesis of new substituted pyridine derivatives as potent anti-liver cancer agents through apoptosis induction: In vitro, in vivo, and in silico integrated approaches. Bioorg. Chem. 2021, 111, 104877. [Google Scholar] [CrossRef] [PubMed]
- ElZahabi, H.S.A.; Nafie, M.S.; Osman, D.; Elghazawy, N.H.; Soliman, D.H.; EL-Helby, A.A.H.; Arafa, R.K. Design, synthesis and evaluation of new quinazolin-4-one derivatives as apoptotic enhancers and autophagy inhibitors with potent antitumor activity. Eur J. Med. Chem. 2021, 222, 113609. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Goda, M.S.; Fahim, J.R.; Hassanean, H.A.; Eltamany, E.E.; Ibrahim, A.K.; AboulMagd, A.M.; Fayez, S.; El-kader, A.M.A.; et al. Thalassosterol, a new cytotoxic aromatase inhibitor ergosterol derivative from the Red Sea seagrass Thalassodendron ciliatum. Mar. Drugs 2020, 18, 354. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Liu, C.; Huang, X.; Zheng, X.; Li, N.; Xu, M.; Mi, S.; Wang, N. Simultaneous determination of esculin and its metabolite esculetin in rat plasma by LC–ESI-MS/MS and its application in pharmacokinetic study. J. Chromatogr. B 2012, 907, 27–33. [Google Scholar] [CrossRef]
- Šibul, F.; Orčić, D.; Berežni, S.; Anačkov, G.; Mimica-Dukić, N. HPLC–MS/MS profiling of wild-growing scentless chamomile. Acta Chromatogr. 2019, 32, 86–94. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Wang, J.; Geng, Y.; Zhou, Y.; Sun, C.; Wang, G. Development of an LC-MS/MS method for quantification of two pairs of isomeric flavonoid glycosides and other ones in rat plasma: Application to pharmacokinetic studies. Biomed. Chromatogr. 2017, 31, e3972. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.C.; Krishna, B.G.; Viswanatha, G.L. Simultaneous determination of quercetin, rutin and kaempferol in the leaf extracts of Moringa oleifera Lam. and Raphinus sativus Linn. by liquid chromatography-tandem mass spectrometry. Chin. J. Integr. Med. 2011, 9, 1022–1030. [Google Scholar] [CrossRef]
- Scigelova, M.; Hornshaw, M.; Giannakopulos, A.; Makarov, A. Fourier Transform Mass Spectrometry. Mol. Cell Proteomics 2011, 10, M111.009431. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, C.; Wang, W.; Zhao, Y. Development, and validation of an LC-ESI-MS/MS method for simultaneous determination of ligustroflavone and rhoifolin in rat plasma and its application to a pharmacokinetic study. J. Chromatogr. Sci. 2017, 55, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Cheruvu, H.S.; Yadav, N.K.; Valicherla, G.R.; Arya, R.K.; Hussain, Z.; Sharma, C.; Arya, K.R.; Singh, R.K.; Datta, D.; Gayen, J.R. LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: Application to pharmacokinetics of Eclipta alba chloroform fraction. J. Chromatogr. B 2018, 1081–1082, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Yuan, Z.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.; Zhang, L.; Wang, N. LC–MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, L.; Tarcomnicu, I.; Dulea, C.; Attili, N.R.B.N.; Ciuca, V.; Peru, D.; Rizea-Savu, S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 8295–8310. [Google Scholar] [CrossRef]
- Kim, S.-B.; Lee, T.; Lee, H.S.; Lee, H.S.; Song, C.K.; Cho, H.-J.; Kim, D.-D.; Maeng, H.-G.; Yoon, I.-S. Development, and validation of a highly sensitive LC–MS/MS method for the determination of acacetin in human plasma and its application to a protein binding study. Arch. Pharm. Res. 2015, 39, 213–220. [Google Scholar] [CrossRef]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Bouhafsoun, A.; Yilmaz, M.A.; Boukeloua, A.; Temel, H.; Harche, M.K. Simultaneous quantification of phenolic acids and flavonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Sci. Technol. 2018, 38, 242–247. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Ling, X.; Lou, H. The combination effects of phenolic compounds and fluconazole on the formation of ergosterol in Candida albicans determined by high-performance liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2005, 336, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shin, Y.G.; Levine, B.S.; Smith, A.C.; Tomaszewski, J.E.; van Breemen, R.B. Quantitative analysis of betulinic acid in mouse, rat and dog plasma using electrospray liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2089–2092. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.B.; Choi, W.K.; Lee, S.J.; Lim, I.; Bae, S.K. Simultaneous determination of β-sitosterol, campesterol, and stigmasterol in rat plasma by using LC-APCI-MS/MS: Application in a pharmacokinetic study of a titrated extract of the unsaponifiable fraction of Zea mays L. J. Sep. Sci. 2016, 39, 4060–4070. [Google Scholar] [CrossRef]
- Fujita, K.; Bunyu, Y.; Kuroda, K.; Ashitani, T.; Shigeto, J.; Tsutsumi, Y. A novel synthetic pathway for tropolone ring formation via the olefin monoterpene intermediate terpinolene in cultured Cupressus lusitanica cells. J. Plant Physiol. 2014, 171, 610–614. [Google Scholar] [CrossRef]
- Huang, M.; Hu, C.; Guo, X.; Gu, X.; Zhao, W.; Wang, Z.; Fang, L.; Zhang, W. Chemical composition of gas and particle–phase products of OH–initiated oxidation of 1,3,5–trimethylbenzene. Atmos. Pollut. Res. 2014, 5, 73–78. [Google Scholar] [CrossRef]
- Takada, D.; Ehara, K.; Saka, S. Gas chromatographic and mass spectrometric (GC-MS) analysis of lignin-derived products from Cryptomeria japonica treated in supercritical water. J. Wood Sci. 2004, 50, 253–259. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Chen, Y.; Zhou, G.; Cao, S.; Peng, X.-D.; Wang, Y.-C.; Peng, X.-J.; Zhang, W.; Zhou, H.-H. LC–MS–MS Quantitative determination of ursolic acid in human plasma and its application to pharmacokinetic studies. Chromatographia 2010, 72, 1107–1113. [Google Scholar] [CrossRef]
- Vandercruyssen, K.; D’Hondt, M.; Vergote, V.; Jansen, H.; Burvenich, C.; De Spiegeleer, B. LC–UV/MS quality analytics of pediatric artemether formulations. J. Pharm. Anal. 2014, 4, 37–52. [Google Scholar] [CrossRef]
- Inamadugu, J.K.; Damaramadugu, R.; Mullangi, R.; Ponneri, V. Simultaneous determination of niacin and its metabolites--nicotinamide, nicotinuric acid and N-methyl-2-pyridone-5-carboxamide--in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Biomed. Chromatogr. 2010, 24, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Chithra, S.; Jasim, B.; Anisha, C.; Mathew, J.; Radhakrishnan, E.K. LC-MS/MS Based identification of piperine production by endophytic Mycosphaerella sp. PF13 from Piper nigrum. Appl. Biochem. Biotechnol. 2014, 173, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Napiroon, T.; Bacher, M.; Balslev, H.; Tawaitakham, K.; Santimaleeworagun, W.; Vajrodaya, S. Scopoletin from Lasianthus lucidus blume (Rubiaceae): A potential antimicrobial against multidrug-resistant Pseudomonas aeruginosa. J. Appl. Pharm. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Solís-Salas, L.M.; Sierra-Rivera, C.A.; Cobos-Puc, L.E.; Ascacio-Valdés, J.A.; Silva-Belmares, S.Y. Antibacterial potential by rupture membrane and antioxidant capacity of purified phenolic fractions of Persea americana leaf extract. Antibiotics 2021, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Dewangan, P.; Kesharwani, D.; Kela, S.P. Hypoglycemic and hypolipidemic activity of scopoletin (coumarin derivative) in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 79–83. [Google Scholar]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Bedell, S.; Wells, J.; Liu, Q.; Breivogel, C. Vitexin as an active ingredient in passionflower with potential as an agent for nicotine cessation: Vitexin antagonism of the expression of nicotine locomotor sensitization in rats. Pharm. Biol. 2019, 57, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3,7,3′,4′-tetrahydroxyflavone inhibits the PI3K/Akt/mTOR and MAPK pathways and ameliorates psoriasis pathology in 2D and 3D organotypic human inflammatory skin models. Cells 2019, 8, 1089. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. ameliorative effects of rhoifolin in scopolamine-induced amnesic zebrafish (Danio rerio) model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Kondža, M.; Bojić, M.; Tomić, I.; Maleš, Ž.; Rezić, V.; Ćavar, I. Characterization of the CYP3A4 enzyme inhibition potential of selected flavonoids. Molecules 2021, 26, 3018. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohammed, S.N.A.; Khatib, A.; Latip, J. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship-based study. BioMed Res. Int. 2017, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid In vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Tanwer, B.S.; Singh, T.; Vijayvergia, R. Total phenolic, total flavonoid content and the DPPH free radical scavenging activity of Melothria maderaspatana (Linn.) Cogn. Int. J. Pharm. Pharm. Sci. 2013, 5, 296–298. [Google Scholar]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties, and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650. [Google Scholar]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, Y.J.; Chung, S.O.; Park, S.U. Recent studies on ursolic acid and its biological and pharmacological activity. EXCLI J. 2016, 15, 221–228. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol: A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; EL-Demerdash, M.; Saxena, P.K. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al Zoubi, M.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 173–184. [Google Scholar] [CrossRef]
- Hoai, T.T.; Yen, P.T.; Dao, T.T.B.; Long, L.H.; Anh, D.X.; Minh, L.H.; Anh, B.Q.; Thuong, N.T. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. J. Nanomater. 2020, 2020, 8867669. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.F. Anticancer effect, and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S. Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp. Ther. Med. 2018, 15, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, K.-C. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013, 29, 229–234. [Google Scholar] [CrossRef]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef]
- Li, Q.; Ren, F.Q.; Yang, C.L.; Zhou, L.M.; Liu, Y.Y.; Xiao, J.; Zhu, L.; Wang, Z.G. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2015, 16, 3035–3042. [Google Scholar] [CrossRef]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression In vitro. J. Cell Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, M.; Liu, Y.; Zhang, Z.; Lu, R.; Lu, J. Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anticancer Drugs 2017, 28, 446–456. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Chen, Y.; Zhang, J.; Li, H.; Yang, Z.; Yang, Y.; Deng, Y.; Zhang, L.; Liu, B. Diosmetin induces apoptosis and enhances the chemotherapeutic efficacy of paclitaxel in non-small cell lung cancer cells via Nrf2 inhibition. Br. J. Pharmacol. 2019, 176, 2079–2094. [Google Scholar] [CrossRef]
- Chien, S.T.; Lin, S.S.; Wang, C.K.; Lee, Y.B.; Chen, K.S.; Fong, Y.; Shih, Y.W. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38α MAPK signaling pathway. Mol. Cell. Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef]
- Fong, Y.; Tang, C.-C.; Hu, H.-T.; Fang, H.-Y.; Chen, B.-H.; Wu, C.-Y.; Yuan, S.-S.; Wang, H.-M.D.; Chen, Y.-C.; Teng, Y.-N.; et al. Inhibitory effect of trans-ferulic acid on proliferation and migration of human lung cancer cells accompanied with increased endogenous reactive oxygen species and β-catenin instability. Chin. Med. 2016, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Sannino, F.; Sansone, C.; Galasso, C.; Kildgaard, S.; Tedesco, P.; Fani, R.; Marino, G.; de Pascale, D.; Lanora, A.; Parilli, E.; et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Sci. Rep. 2018, 8, 1190. [Google Scholar] [CrossRef]
- Tsao, S.M.; Hsia, T.C.; Yin, M.C. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-κB pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.N. Synergistic anticancer activity of combined use of caffeic acid with paclitaxel enhances apoptosis of non-small-cell lung cancer H1299 cells in vivo and in vitro. Cell Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef]
- Wu, M.; Huang, T.; Wang, J.; Chen, P.; Mi, W.; Ying, Y.; Wang, H.; Zhao, D.; Huang, S. Antilung cancer effect of ergosterol and cisplatin-loaded liposomes modified with cyclic arginine-glycine-aspartic acid and octa-arginine peptides. Medicine 2018, 97, e11916. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Liu, C.-H.; Wu, G.-Y.; Lee, T.-Y.; Manubolu, M.; Hsieh, C.-Y.; Yang, C.H.; Sheu, J.-R. Hinokitiol inhibits migration of A549 lung cancer cells via suppression of MMPs and induction of antioxidant enzymes and apoptosis. Int. J. Mol. Sci. 2018, 19, 939. [Google Scholar] [CrossRef]
- Yan, X.; Xu, B. Review Article Ursolic acid induces apoptosis of lung cancer cells by regulating miR-21/KLF6 axis. Int. J. Clin. Exp. Med. 2020, 13, 6306–6315. [Google Scholar]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-T.; Tsai, Y.-C.; Wu, H.-C.; Ho, Y.-J.; Chen, Y.-S.; Yao, C.-H.; Yao, C.-H. Radiosensitization of non-small cell lung cancer by kaempferol. Oncol. Rep. 2015, 34, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, J.; Fan, L.M.; Liu, K.; Zhang, N.; Li, S.W.; Zhu, H.; Gao, H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Ther. Med. 2017, 14, 127–130. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Yang, L.; Li, P. Quercetin inhibits the proliferation and metastasis of human non-small cell lung cancer cell line: The key role of Src-mediated fibroblast growth factor-inducible 14 (Fn14)/nuclear factor kappa B (NF-κB) pathway. Med. Sci. Monit. 2020, 26, e920537-1. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Br. 2018, 21, 1033–1036. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; Santos de Almeida, T. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward | Reverse |
|---|---|---|
| P53 | 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′ | 5′-GCAGCGCCTCACAACCTCCGTCAT-3′ |
| BAX | 5′-GTTTCATCCAGGATCGAGCAG-3′ | 5′-CATCTTCTTCCAGATGGTGA-3′ |
| CASP-3 | 5′-TGGCCCTGAAATACGAAGTC-3′ | 5′-GGCAGTAGTCGACTCTGAAG-3′ |
| CASP-8 | 5′-AATGTTGGAGGAAAGCAAT-3′ | 5′-CATAGTCGTTGATTATCTTCAGC-3′ |
| CASP-9 | 5′-CGAACTAACAGGCAAGCAGC-3′ | 5′-ACCTCACCAAATCCTCCAGAAC-3′ |
| BCL2 | 5′-CCTGTGGATGACTGAGTACC-3′ | 5′-GAGACAGCCAGGAGAAATCA-3′ |
| β-actin | 5′-GTGACATCCACACCCAGAGG-3′ | 5′-ACAGGATGTCAAAACTGCCC-3′ |
| Sample | IC50 (μg/mL) * | |||
|---|---|---|---|---|
| Prostate PC-3 | Breast MDA-MB-231 | Ovarian A2780 | Lung A549 | |
| A. judaica L. crude extract | 59.8 ± 3.25 | 98.6 ± 4.65 | NA | 14.2 ± 0.84 |
| Doxorubicin | 9.36 ± 1.52 | 7.26 ± 0.98 | 2.36 ± 0.65 | 9.98 ± 0.97 |
| Polarity Mode | MZmine ID | Ret. Time (min) | Measured m/z | Calculated m/z | Mass Error (ppm) | Adduct | Molecular Formula | MS/MS Spectrum | Deduced Compound | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coumarins and their glycosides | |||||||||||
| 1 | Negative | 801 | 4.07 | 339.0659 | 339.0716 | −16.81 | [M − H]− | C15H16O9 | 339.1 > 177 | Esculin | [48] |
| 2 | Positive | 1717 | 7.45 | 193.0517 | 193.0501 | 8.29 | [M + H]+ | C10H8O4 | 193 > 178 > 133 > 122 | Scopoletin | [49] |
| Flavonoids and their glycosides | |||||||||||
| 3 | Negative | 1480 | 6.59 | 431.0977 | 431.0978 | −0.23 | [M − H]− | C21H20O10 | 431.1 > 311.1 | Vitexin | [50] |
| 4 | Positive | 1434 | 6.73 | 611.1583 | 611.1612 | −4.75 | [M + H]+ | C27H30O16 | 611.1 > 303.1 | Rutin | [51] |
| 5 | Negative | 1631 | 6.92 | 447.0922 | 447.0927 | −1.12 | [M − H]− | C21H20O11 | 447.1 > 327.1 | Orientin | [50] |
| 6 | Positive | 1566 | 7.13 | 303.0507 | 303.0505 | 0.66 | [M + H]+ | C15H10O7 | 303 > 257 > 229 > 183 > 165 > 153 > 137 | Quercetin | [52] |
| 7 | Positive | 1579 | 7.16 | 287.0554 | 287.0556 | −0.7 | [M + H]+ | C15H10O6 | 287 > 269 > 241 > 213 >149 >137 | Fisetin | [53] |
| 8 | Negative | 1793 | 7.42 | 577.1612 | 577.1557 | 9.53 | [M − H]− | C27H30O14 | 577.1 > 269.1 | Rhoifolin | [54] |
| 9 | Positive | 1869 | 7.76 | 287.0525 | 287.0536 | −3.83 | [M + H]+ | C15H10O6 | 287.2 > 231 > 165.1 > 121.0 | Kaempferol | [53] |
| 10 | Negative | 2065 | 8.59 | 285.0405 | 285.0399 | 2.10 | [M − H]− | C15H10O6 | 285.2 > 133.0 | Luteolin | [55] |
| 11 | Negative | 2068 | 8.65 | 315.0505 | 315.0505 | zero | [M − H]− | C16H12O7 | 315.1 > 300.1 | Isorhamnetin | [56] |
| 12 | Negative | 2171 | 9.23 | 271.0601 | 271.0606 | −1.84 | [M − H]− | C15H12O5 | 271 > 177 > 151 | Naringenin | [49] |
| 13 | Negative | 2324 | 10.08 | 269.0453 | 269.0450 | 1.12 | [M − H]− | C15H10O5 | 269.2 > 116.8 | Apigenin | [55] |
| 14 | Positive | 2739 | 10.34 | 301.0680 | 301.0701 | −6.98 | [M + H]+ | C16H12O6 | 301.1 > 286 > 258.0 | Diosmetin | [57] |
| 15 | Positive | 3059 | 11.52 | 285.0761 | 285.0763 | −0.7 | [M + H]+ | C16H12O5 | 285.0 > 267.9 > 242.1 | Acacetin | [58] |
| Phenolic acids | |||||||||||
| 16 | Negative | 590 | 1.62 | 163.0394 | 163.0395 | −0.61 | [M − H]− | C9H8O4 | 163 > 119 | p-Coumaric acid | [59] |
| 17 | Negative | 725 | 2.43 | 193.0514 | 193.0501 | 6.73 | [M − H]− | C10H10O4 | 193 > 178> 149 > 134 | Ferulic acid | [59] |
| 18 | Negative | 751 | 3.30 | 137.0222 | 137.0231 | −6.6 | [M − H]− | C7H6O3 | 137 > 93 > 65 | p-Hydroxy benzoic acid | [60] |
| 19 | Negative | 991 | 4.97 | 153.0166 | 153.0178 | −7.8 | [M − H]− | C7H6O4 | 153 > 109 | Protocatechuic acid | [60] |
| 20 | Negative | 1163 | 5.55 | 179.0336 | 179.0344 | −4.47 | [M − H]− | C9H8O4 | 179 > 135 > 134 | Caffeic acid | [60] |
| Sterols | |||||||||||
| 21 | Positive | 5376 | 21.9 | 380.3320 | 380.3343 | −6.05 | [M + H − H20]+ | C28H44O | 380 > 69 | Ergosterol | [61] |
| 22 | Negative | 3245 | 22.84 | 455.3567 | 455.3525 | 9.22 | [M − H]− | C30H48O3 | 455 | Betulinic acid | [62] |
| 23 | Positive | 5641 | 23.74 | 413.3615 | 413.3633 | −4.35 | [M + H]+ | C29H48O | 413 > 395.3 > 81.1 | Stigmasterol | [63] |
| Terpenes | |||||||||||
| 24 | Negative | 1071 | 5.29 | 163.0754 | 163.0759 | −3.07 | [M − H]− | C10H12O2 | 163 > 146 > 119 | Hinokitiol/β-thujaplicin | [64] |
| 25 | Positive | 2327 | 8.83 | 121.1004 | 121.1017 | −10.73 | [M + H]+ | C9H12 | 121 > 119 > 105 > 91 > 77 | Mesitylene | [65] |
| 26 | Positive | 3393 | 12.85 | 165.0897 | 165.0916 | −11.5 | [M + H]+ | C10H12O2 | 165 > 149 > 103 | Eugenol | [66] |
| 27 | Negative | 3245 | 22.84 | 455.3567 | 455.3525 | 9.22 | [M − H]− | C30H48O3 | 455 | Ursolic acid | [67] |
| Terpenoid bitter principles | |||||||||||
| 28 | Positive | 5246 | 21.18 | 283.1499 | 283.1545 | −16.25 | [M + H]+ | C15H22O5 | 283 > 265 > 247 > 237 > 209 | Artemisinin | [68] |
| Other classes | |||||||||||
| 29 | Positive | 64 | 1.22 | 123.0553 | 123.0558 | −4.06 | [M + H]+ | C6H6N2O | 123 > 80 | Nicotinamide | [69] |
| 30 | Positive | 3829 | 15.21 | 286.1450 | 286.1443 | 2.45 | [M + H]+ | C17H19NO3 | 286.1 > 201 > 171 > 143 > 135 | Piperine | [70] |
| No. | Detected Metabolite | Type of Study | Mechanism of Action | Ref. |
|---|---|---|---|---|
| 1 | Vitexin | -Animal study with acute lung injury | Vitexin upregulated nuclear factor erythroid-2-related factor2 (Nrf2) and activated heme oxygenase (HO)-1. | [81] |
| 2 | Rutin | -Human lung cancer cell line, A549 | Rutin downregulated the expression of anti-apoptotic gene (Bcl-2) and decreased the levels of tumor necrosis factor (TNF-α). | [97] |
| 3 | Quercetin | -Human lung cancer cell line, A549 -Xenograft animal model | Quercetin downregulated the expression of anti-apoptotic gene (Bcl-2) and upregulated the expression of the proapoptotic gene (Bax). | [98] |
| 4 | Fisetin | -Human lung cancer cell line, A549 | Fisetin induced cell cycle arrest at G2 phase and upregulated the expression of the apoptosis-regulating gene (Caspases 3 and 9). | [99] |
| 5 | Kaempeferol | -Human lung cancer cell line, A549 | Kaempeferol upregulated the expression of proapoptotic gene (Bax) and downregulated the expression of anti-apoptotic genes (Bcl-2 and Bcl-xL). | [100] |
| 6 | Luteolin | -Human lung cancer cell line, A549 | Luteolin suppressed migration and invasion of lung cancer cells. | [101] |
| 7 | Isorhamnetin | -Human lung cancer cell line, A549 -Animal model with Lewis lung cancer cells | Isorhamnetin upregulated the expression of proapoptotic genes (Bax, P53 and Caspase-3) and downregulated the expression of anti-apoptotic genes (Bcl-2, cyclinD1). | [102] |
| 8 | Naringenin | -Human lung cancer cell line, A549 | Naringenin suppressed migration of lung cancer cells, upregulated the expression of proapoptotic genes (Bax and Caspase-3) and downregulated the expression of matrixmetallo proteinases (MMP-2 and MMP-9). | [103] |
| 9 | Apigenin | -Human lung cancer cell line, A549 | Apigenin suppressed migration of lung cancer cells and downregulated the expression of MMP-9. | [104] |
| 10 | Diosmetin | -Human lung cancer cell lines, A549, H1299, H460, SPC-A1, H441, H1650 and Calu-3. -Xenograft animal model | Diosmetin induced apoptosis and enhanced the efficacy of paclitaxel, a chemotherapeutic agent. | [105] |
| 11 | Acacetin | -Human lung cancer cell line, A549 | Acacetin suppressed migration of lung cancer cells and downregulated the expression of MMP-2 and 9. | [106] |
| 12 | Ferulic acid | -Human lung cancer cell line, H1299 | Ferulic acid suppressed migration of lung cancer cells and downregulated the expression of MMP-2 and 9. | [107] |
| 13 | p-Hydroxy benzoic acid | -Human lung cancer cell line, A549 | p-Hydroxy benzoic acid upregulated the expression of proapoptotic gene (Caspase-1) and interleukines (IL1β, and IL18). | [108] |
| 14 | Protocatechuic acid | -Human lung cancer cell lines, A549, H3255, and Calu-6 | Protocatechuic acid upregulated the expression of proapoptotic genes (Bax and Caspase-3) and downregulated the expression of anti-apoptotic genes (Bcl-2) and matrixmetallo proteinases. | [109] |
| 15 | Caffeic acid | -Human lung cancer cell line, H1299 -Xenograft animal model | Caffeic acid enhanced the efficacy of paclitaxel and upregulated the expression of Caspases-3 and 9. | [110] |
| 16 | Ergosterol | -Human lung cancer cell line, A549 | Ergosterol suppressed the proliferation of lung cancer cells. | [111] |
| 17 | Eugenol | -Human lung cancer cell line, A549 -Human embryonic lung fibroblast, MRC-5 | Eugenol suppressed migration of lung cancer cells and downregulated the expression of MMP-2. | [112] |
| 18 | Hinokitiol | -Human lung cancer cell line, A549 | Hinokitiol suppressed migration of lung cancer cells, downregulated the expression of MMP-2 and upregulated the expression of proapoptotic genes (Bax, P53 and Caspase-3). | [113] |
| 19 | Ursolic acid | -Human lung cancer cell line, A549 | Ursolic acid suppressed migration of lung cancer cells and downregulated miR-21 that is correlated with a tumor growth. | [114] |
| 20 | Piperine | -Human fibrosarcoma cell, HT-1080 -B16F10 melanoma animal model | Piperine suppressed metastasise and migration of lung cancer cells. | [115] |
| Co-Crystallized Ligands (Key Interactions) | Ligand-Receptor Interactions towards CDK-2 (PD’a4l) * | Ligand-Receptor Interactions towards EGFR (PDB = 1M17) # |
|---|---|---|
| 2 HB with Leu 83 + 1 Arene-Cation Interaction with Lys 89 | 1 HB with Met 769 | |
| Metabolite 1 | 1 HB with Lys 89 | 1 HB with 769 |
| Metabolite 2 | - | - |
| Metabolite 3 | 1 HB with Lys 89 | - |
| Metabolite 4 | 1 HB with Lys 89 | - |
| Metabolite 5 | 1 HB with Lys 89 + 1 arene-cation interaction with Lys 89 | 1 HB with 769 |
| Metabolite 6 | 1 HB with Leu 83 | 1 HB with 769 |
| Metabolite 7 | 1 HB with Lys 89 + 1 arene-cation interaction with Lys 89 | 1 HB with 769 |
| Metabolite 8 | 1 arene-cation interaction with Lys 89 | - |
| Metabolite 9 | 1 HB with Leu 83 + 1 arene-cation interaction with Lys 89 | - |
| Metabolite 10 | 1 HB with Leu 83 | 1 HB with 769 |
| Metabolite 11 | 1 HB with Leu 83 | - |
| Metabolite 12 | 1 HB with Lys 89 | 1 HB with 769 |
| Metabolite 13 | - | - |
| Metabolite 14 | 2 HB with Leu 83 and Lys 89 | 1 HB with Met 769 |
| Metabolite 15 | 1 arene-cation interaction with Lys 89 | - |
| Metabolite 16 | - | - |
| Metabolite 17 | - | - |
| Metabolite 18 | - | - |
| Metabolite 19 | - | |
| Metabolite 20 | 1 arene-cation interaction with Lys 89 | 1 HB with 769 |
| Metabolite 21 | ||
| Metabolite 22 | 1 HB with Lys 89 | - |
| Metabolite 23 | 1 HB with Leu 83 | |
| Metabolite 24 | - | - |
| Metabolite 25 | - | - |
| Metabolite 26 | - | - |
| Metabolite 27 | 1 HB with Leu 83 + 1 arene-cation interaction with Lys 89 | 1 HB with Met 769 |
| Metabolite 28 | - | - |
| Metabolite 29 | - | - |
| Metabolite 30 | 1 HB with Leu 83 | - |
| Validation Parameters | Rutin | Quercetin | Kaempferol | Apigenin |
|---|---|---|---|---|
| Regression equation | y = 13,199x − 787,148 | y = 15,764.37x − 40,216.27 | y = 71,227x − 46,571 | y = 8938.8x + 357,854 |
| Correlation coefficient (R2) | 0.997 | 0.999 | 0.998 | 0.993 |
| Linearity range (µg/mL) | 10–200 | 5–100 | 10–150 | 5–100 |
| Limit of detection (µg/mL) | 0.7 | 0.5 | 0.4 | 0.5 |
| Limit of quantification (µg/mL) | 2.5 | 1.61 | 1.4 | 1.53 |
| System precision (%RSD) | 3.16 | 2.25 | 1.59 | 2.26 |
| Method precision (%RSD) | 1.93 | 2.78 | 2.19 | 1.31 |
| Concentration (mg/gm) | 6 ±0.019 | 0.413 ± 0.00007 | 0.3603 ± 0.0033 | 3.9 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, M.S.; Nafie, M.S.; Awad, B.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Eltamany, E.E. In Vitro and In Vivo Studies of Anti-Lung Cancer Activity of Artemesia judaica L. Crude Extract Combined with LC-MS/MS Metabolic Profiling, Docking Simulation and HPLC-DAD Quantification. Antioxidants 2022, 11, 17. https://doi.org/10.3390/antiox11010017
Goda MS, Nafie MS, Awad BM, Abdel-Kader MS, Ibrahim AK, Badr JM, Eltamany EE. In Vitro and In Vivo Studies of Anti-Lung Cancer Activity of Artemesia judaica L. Crude Extract Combined with LC-MS/MS Metabolic Profiling, Docking Simulation and HPLC-DAD Quantification. Antioxidants. 2022; 11(1):17. https://doi.org/10.3390/antiox11010017
Chicago/Turabian StyleGoda, Marwa S., Mohamed S. Nafie, Basma M. Awad, Maged S. Abdel-Kader, Amany K. Ibrahim, Jihan M. Badr, and Enas E. Eltamany. 2022. "In Vitro and In Vivo Studies of Anti-Lung Cancer Activity of Artemesia judaica L. Crude Extract Combined with LC-MS/MS Metabolic Profiling, Docking Simulation and HPLC-DAD Quantification" Antioxidants 11, no. 1: 17. https://doi.org/10.3390/antiox11010017
APA StyleGoda, M. S., Nafie, M. S., Awad, B. M., Abdel-Kader, M. S., Ibrahim, A. K., Badr, J. M., & Eltamany, E. E. (2022). In Vitro and In Vivo Studies of Anti-Lung Cancer Activity of Artemesia judaica L. Crude Extract Combined with LC-MS/MS Metabolic Profiling, Docking Simulation and HPLC-DAD Quantification. Antioxidants, 11(1), 17. https://doi.org/10.3390/antiox11010017








