Multitarget Antioxidant NO-Donor Organic Nitrates: A Novel Approach to Overcome Nitrates Tolerance, an Ex Vivo Study
Abstract
1. Introduction
2. Materials and Methods
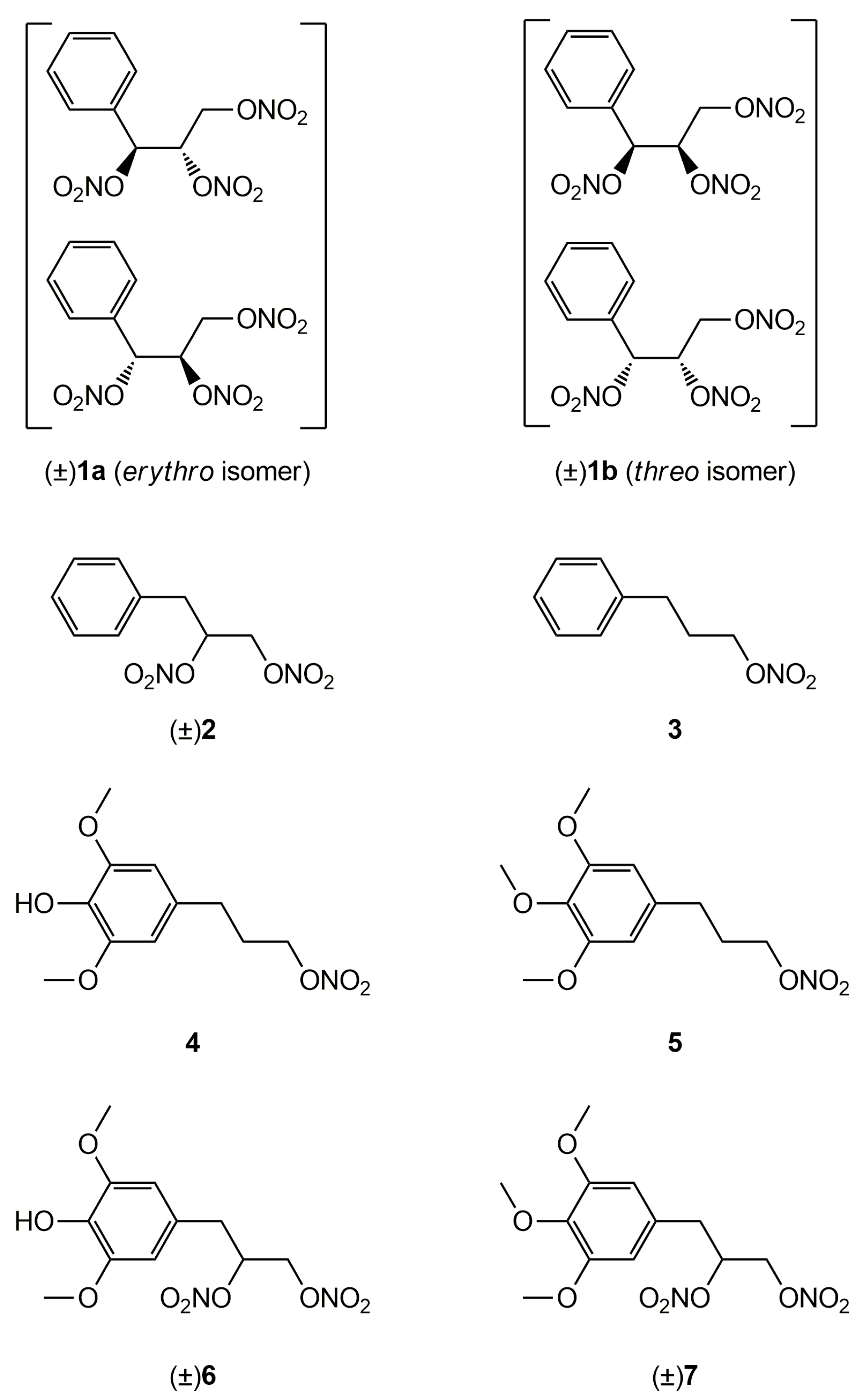
2.1. Synthesis
2.2. Vasodilating Activity
2.2.1. In Vitro Experiments
2.2.2. Ex Vivo Experiments
2.2.3. Statistical Analysis
2.3. Metabolism
2.3.1. Preparation of Liver Microsomes
2.3.2. Incubation Conditions
2.3.3. Liver Microsomes Stability
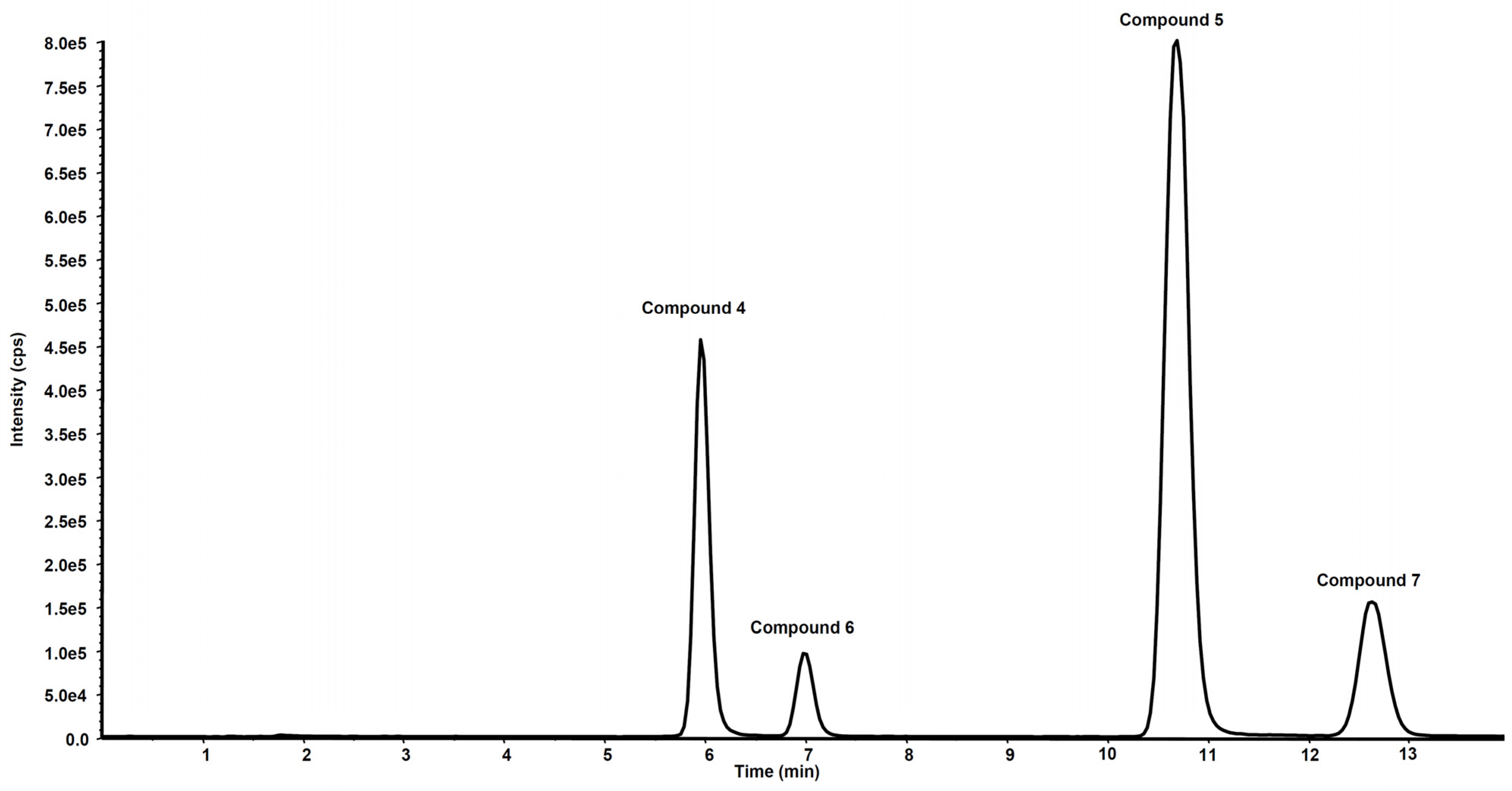
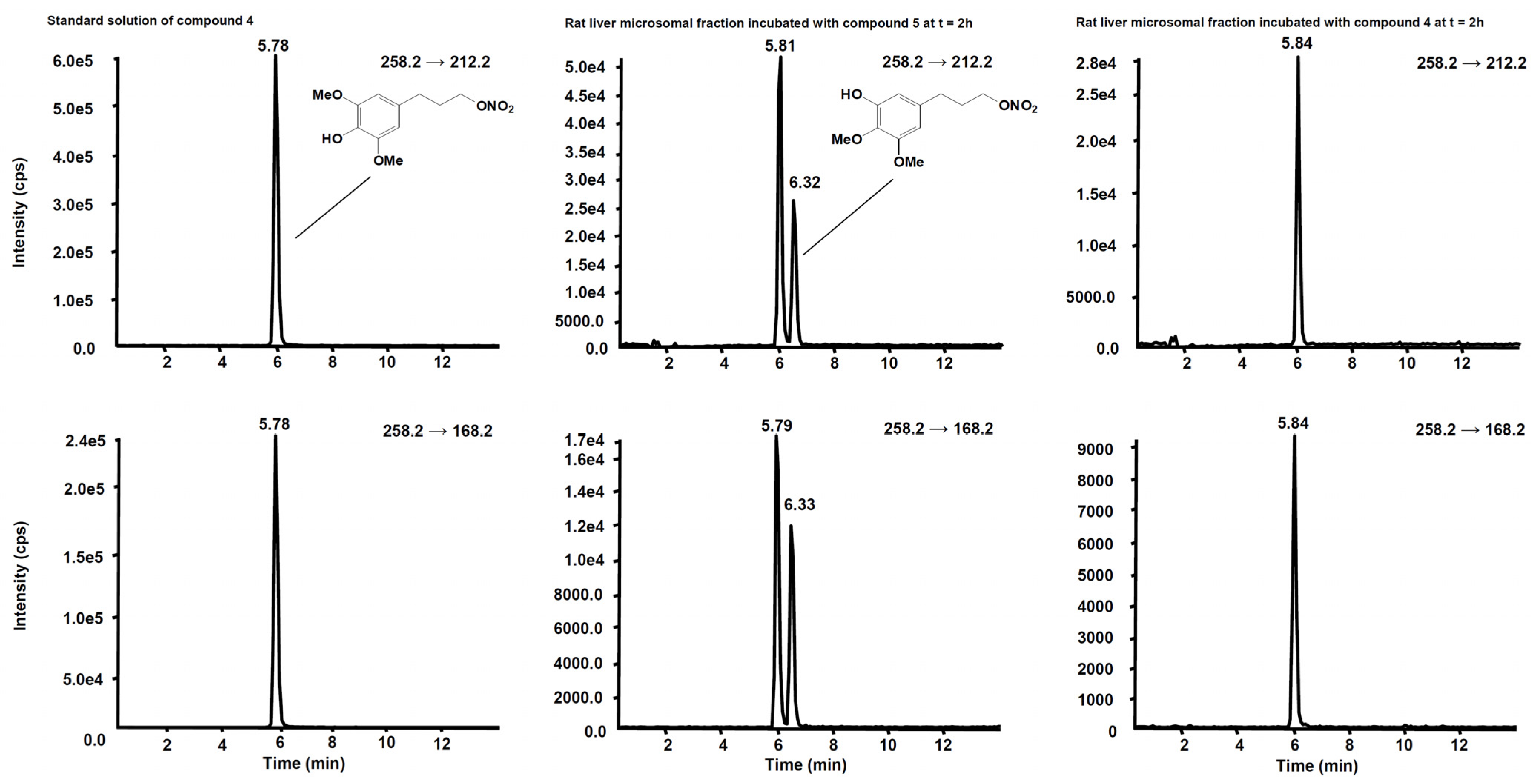
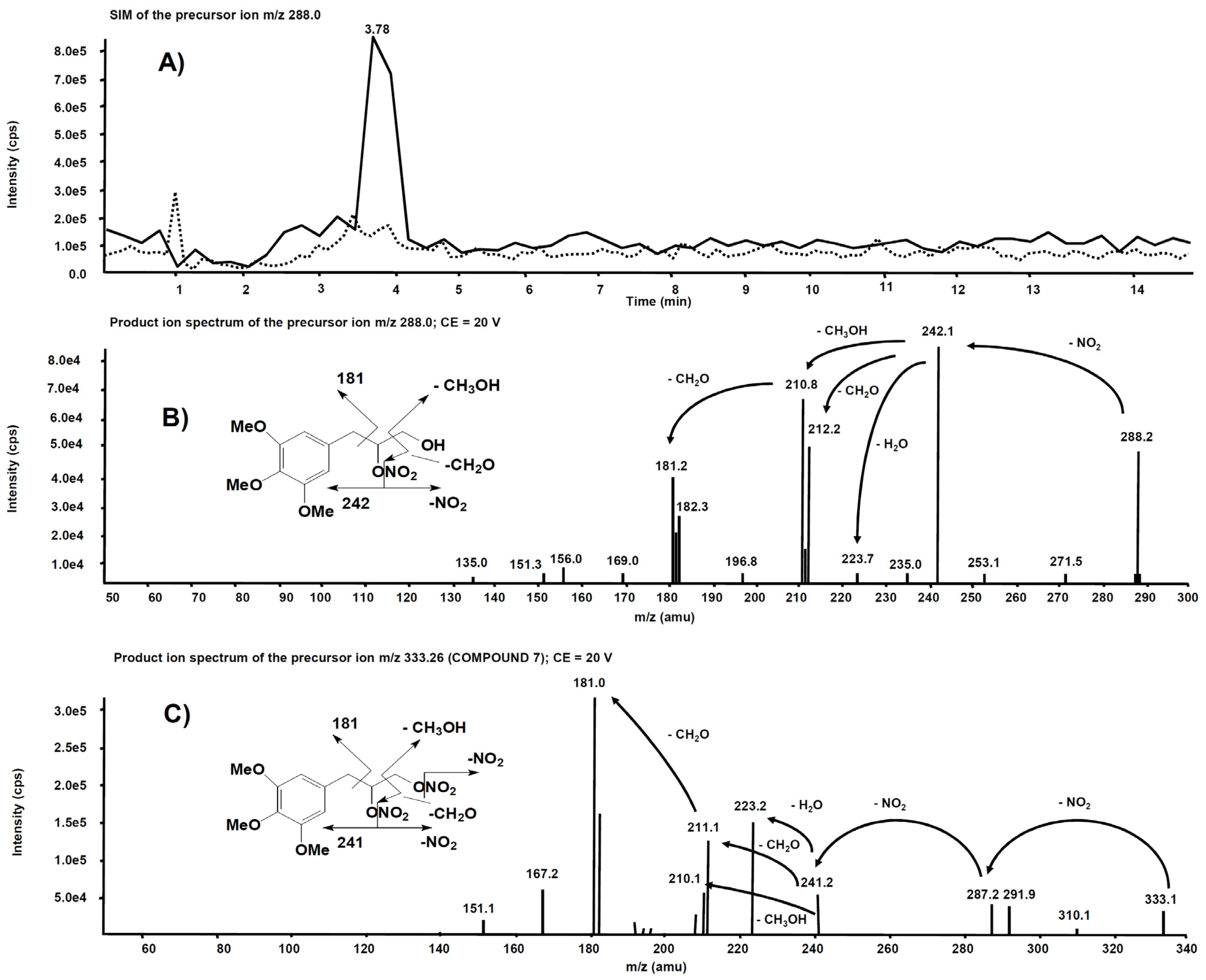
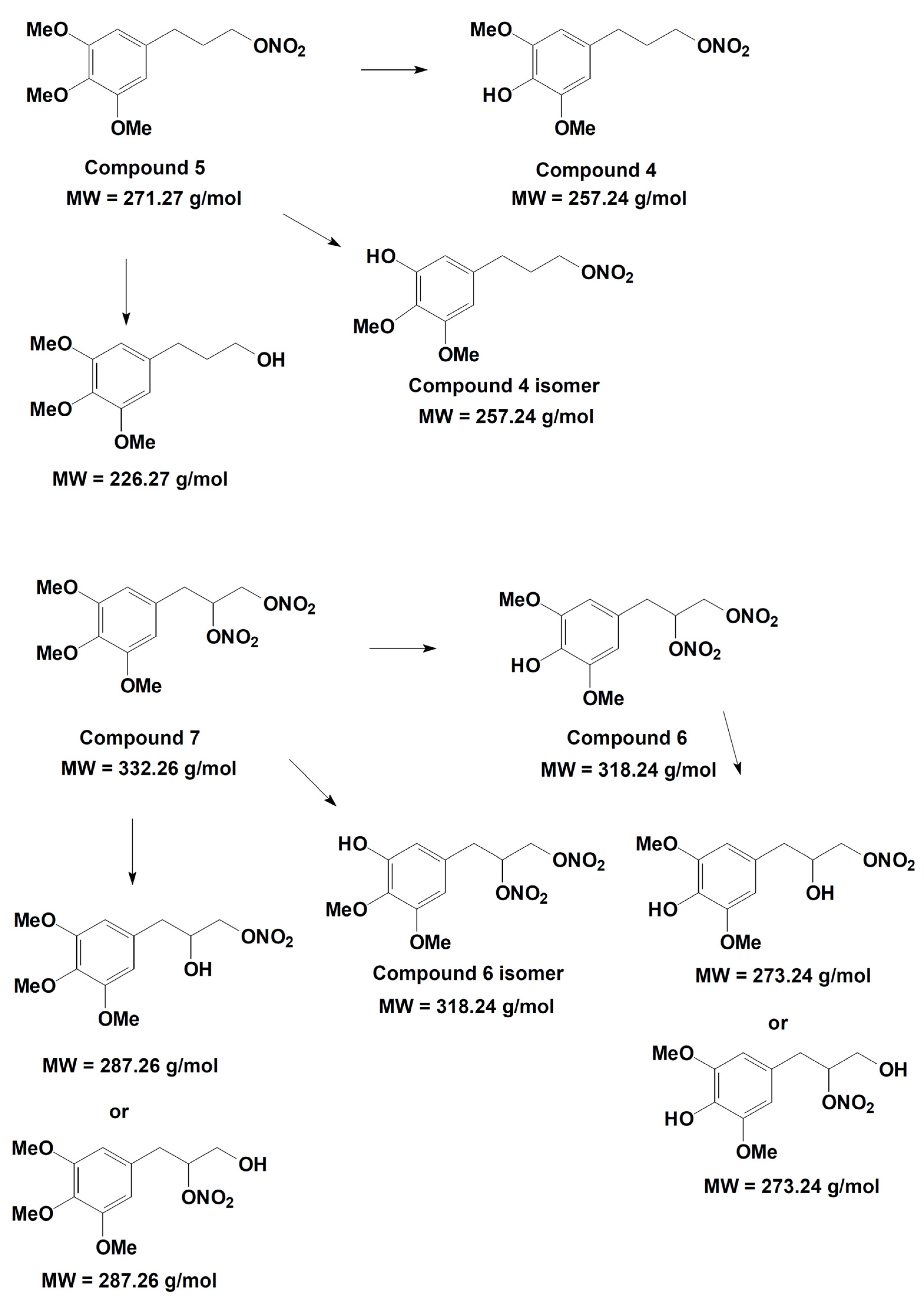
2.3.4. Metabolites Qualitative Search
3. Results
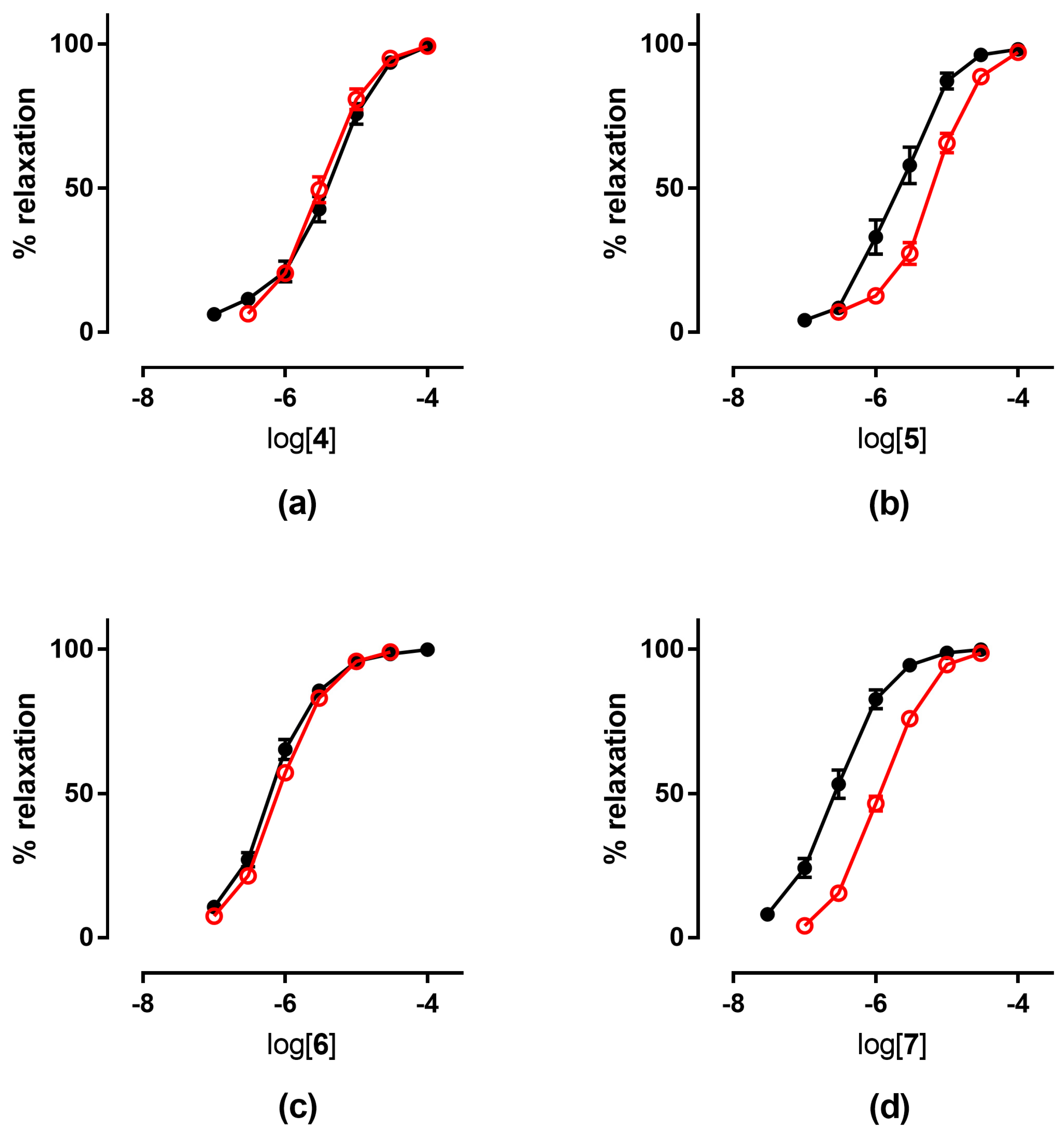
3.1. Vasodilating Activity
3.1.1. In Vitro Experiments
3.1.2. Ex Vivo Experiments
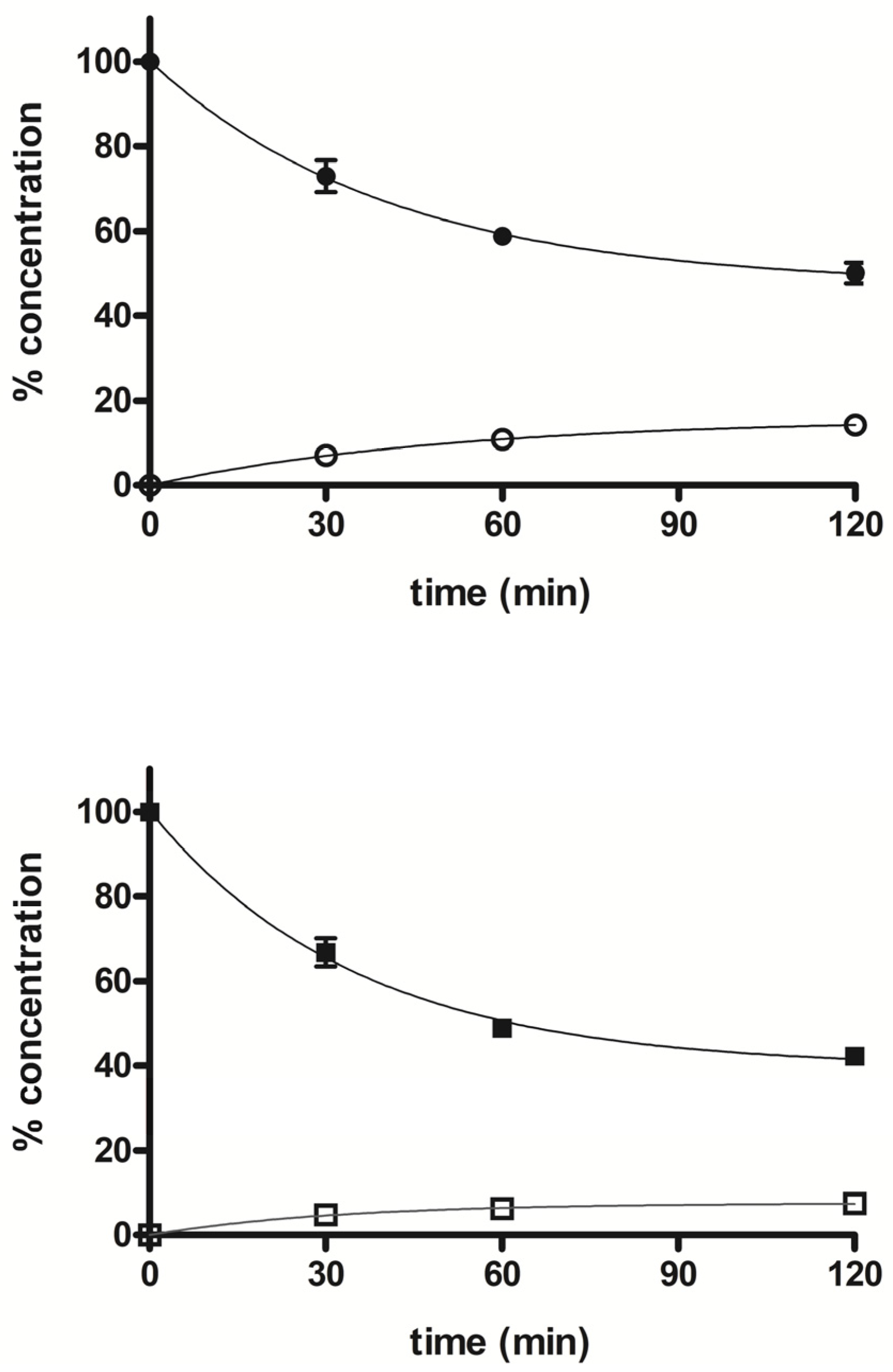
3.2. Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fung, H.L. Biochemical mechanism of nitroglycerin action and tolerance: Is this old mistery solved? Annu. Rev. Pharmacol. Toxicol. 2004, 44, 67–85. [Google Scholar] [CrossRef]
- Daiber, A.; Münzel, T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: Emphasis on redox biology and oxidative stress. Antioxid. Redox Signal. 2015, 23, 899–946. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Malacarne, P.F.; Gajos-Draus, A.; Ding, X.; Daiber, A.; Lundberg, J.O.; Offermanns, S.; Brandes, R.P.; Rezende, F. Vacular biotransformation of organic nitrates is independent of cytochrome P450 monooxygenases. Br. J. Pharmacol. 2021, 178, 1495–1506. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Stamler, J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 8306–8311. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Surdacki, A. Biotransformation of organic nitrates by glutathione S-transferases and other enzymes: An appraisal of the pioneering work by William B. Jakoby. Anal. Biochem. 2020, 113993. [Google Scholar] [CrossRef] [PubMed]
- Opelt, M.; Eroglu, E.; Waldeck-Weiermair, M.; Russwurm, M.; Koesling, D.; Malli, R.; Graier, W.F.; Fassett, J.T.; Schrammel, A.; Mayer, B. Formation of nitric oxide by aldehyde dehydrogenase-2 is necessary and sufficient for vascular bioactivation of nitroglycerin. J. Biol. Chem. 2016, 291, 24076–24084. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Bednarski, M.; Bilska, A.; Iciek, M.; Sokolowska-Jezewicz, M.; Filipek, B.; Wlodek, L. The role of lipoic acid in prevention of nitroglicerin tolerance. Eur. J. Pharmacol. 2008, 591, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Exogenous NO therapy for the treatment and prevention of atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703. [Google Scholar] [CrossRef]
- Daiber, A.; Oelze, M.; Wenzel, P.; Bollmann, F.; Pautz, A.; Kleinert, H. Heme oxygenase-1 induction and organic nitrate therapy: Beneficial effects on endothelial dysfunction, nitrate tolerance, and vascular oxidative stress. Int. J. Hypertens. 2012, 2012, 842632. [Google Scholar] [CrossRef]
- Mizuno, Y.; Harada, E.; Kugimiya, F.; Shono, M.; Kusumegi, I.; Yoshimura, M.; Kinoshita, K.; Yasue, H. East Asian variant mitochondrial aldehyde dehydrogenase-2 genotype exacerbates nitrate tolerance in patients with coronary spastic angina. Circ. J. 2020, 84, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A.; Mülsch, A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005, 97, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, S.F.; Razavi, B.M.; Imenshahidi, M.; Rameshrad, M.; Hosseinzadeh, H. Evaluation of possible effects of crocin against nitrate tolerance and endothelial dysfunction. Iran. J. Basic Med. Sci. 2020, 23, 303–310. [Google Scholar] [PubMed]
- Münzel, T.; Sayegh, H.; Freeman, B.A.; Tarpey, M.M.; Harrison, D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Investig. 1995, 95, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Khong, S.M.L.; Andrews, K.L.; Huynh, N.N.; Venardos, K.; Aprico, A.; Michell, D.L.; Zarei, M.; Moe, K.T.; Dusting, G.J.; Kaye, D.M.; et al. Arginase II inhibition prevents nitrate tolerance. Br. J. Pharmacol. 2012, 166, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.R.; de la Lande, I.S.; Stafford, I.; Bennett, C.L.; Phillipov, G.; Stubberfield, J.; Horowitz, J.D. Nitroglycerin tolerance in human vessels: Evidence of impaired nitroglycerin bioconversion. Circulation 2000, 102, 2810–2815. [Google Scholar] [CrossRef]
- Gongadze, N.; Kezeli, T.D.; Sukoyan, G.V.; Chapichadze, Z.; Dolidze, N.M.; Mirziashvili, M.; Chipashvili, M. Deterioration in hemodynamics reaction, baroreflex sensitivity, sympathetic nerve activity and redox state of thoracic aorta in the experimental model of nitrate tolerance and its pharmacological correction. Pharmacol. Pharm. 2016, 7, 81–88. [Google Scholar] [CrossRef]
- Mayer, B.; Beretta, M. The enigma of nitroglycerin bioactivation and nitrate tolerance: News, views and troubles. Br. J. Pharmacol. 2008, 155, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Wenzel, P.; Oelze, M.; Münzel, T. New insights into bioactivation of organic nitrates, nitrate tolerance and cross-tolerance. Clin. Res. Cardiol. 2008, 97, 12–20. [Google Scholar] [CrossRef]
- Münzel, T.; Steven, S.; Daiber, A. Organic nitrates: Update on mechanism underlying vasodilation, tolerance and endothelial dysfunction. Vasc. Pharmacol. 2014, 63, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Esplugues, J.V.; Rocha, M.; Nuñez, C.; Bosca, I.; Ibiza, S.; Herance, J.R.; Ortega, A.; Serrador, J.M.; D’Ocon, P.; Victor, V.M. Complex I dysfunction and tolerance to nitroglycerin—An approach based on mithocondrial-targeted antioxidants. Circ. Res. 2006, 99, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Opelt, M.; Wölkart, G.; Eroglu, E.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F.; Kollau, A.; Fassett, J.T.; Schrammel, A.; Mayer, B.; et al. Sustained formation of nitroglycerin-derived nitric oxide by aldehyde dehydrogenase-2 in vascular smooth muscle without added reductants: Implications for the development of nitrate tolerance. Mol. Pharmacol. 2018, 93, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Lazzarato, L.; Marcarino, P.; Di Stilo, A.; Fruttero, R.; Vanthuyne, N.; Roussel, C.; Gasco, A. Synthesis of some novel organic nitrates and comparative in vitro study of their vasodilator profile. J. Med. Chem. 2009, 52, 4020–4025. [Google Scholar] [CrossRef] [PubMed]
- Boschi, D.; Tron, G.C.; Lazzarato, L.; Chegaev, K.; Cena, C.; Di Stilo, A.; Giorgis, M.; Bertinaria, M.; Fruttero, R.; Gasco, A. NO-Donor phenols: A new class of products endowed with antioxidant and vasodilator properties. J. Med. Chem. 2006, 49, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Tosco, P.; Marini, E.; Rolando, B.; Lazzarato, L.; Cena, C.; Bertinaria, M.; Fruttero, R.; Reist, M.; Carrupt, P.A.; Gasco, A. Structure-antioxidant activity relationships in a series of NO-donor phenols. ChemMedChem 2008, 3, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- De Candia, M.; Marini, E.; Zaetta, G.; Cellamare, S.; Di Stilo, A.; Altomare, C.D. New organic nitrate-containing benzyloxy isonipecotanilide derivatives with vasodilatory and anti-platelet activity. Eur. J. Pharm. Sci. 2015, 72, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Daiber, A.; Oelze, M.; Chen, Z.; August, M.; Wendt, M.; Ullrich, V.; Mülsch, A.; Schultz, E.; Keany, J.F., Jr.; et al. Central role of mithocondrial aldheyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J. Clin. Investig. 2004, 113, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Schacterle, G.R.; Pollack, R.L. A simple method for the quantitative assay of small amounts of protein in biologic material. Anal. Biochem. 1973, 51, 654–655. [Google Scholar] [CrossRef]
- Aprile, S.; Del Grosso, E.; Tron, G.C.; Grosa, G. In Vitro metabolism study of Combretastatin A-4 in rat and human liver microsomes. Drug Metab. Dispos. 2007, 35, 2252–2261. [Google Scholar] [CrossRef]
- Limbu, R.; Cottrell, G.S.; McNeish, A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE 2018, 13, e0192484. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Godo, S. Diverse Functions of Endothelial NO Synthases System: NO and EDH. J. Cardiovasc. Pharmacol. 2016, 67, 361–366. [Google Scholar] [CrossRef]
- Fontaine, D.; Otto, A.; Fontaine, J.; Berkenboom, G. Prevention of nitrate tolerance by long-term treatment with statins. Cardiovasc. Drugs Ther. 2003, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jabs, A.; Oelze, M.; Mikhed, Y.; Stamm, P.; Kröller-Schön, S.; Welschof, P.; Jansen, T.; Hausding, M.; Kopp, M.; Steven, S.; et al. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vasc. Pharmacol. 2015, 71, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Mikhed, Y.; Fahrer, J.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Welschof, P.; Zinbius, E.; Stamm, P.; Kashani, F.; Roohani, S.; et al. Nitroglycerin induces DNA damage and vascular cell death in the setting of nitrate tolerance. Basic Res. Cardiol. 2016, 11, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.F.; Gozdzik, W.; Frostell, C.; Zielinski, S.; Zielinska, M.; Ratajczak, K.; Skrzypczak, P.; Rodziewicz, S.; Albert, J.; Gustafsson, L.E. Organic mononitrites of 1,2-propanediol act as an effective NO-releasing vasodilator in pulmonary hypertension and exhibit no cross-tolerance with nitroglycerin in anesthetized pigs. Drug Des. Dev. Ther. 2018, 12, 685–694. [Google Scholar] [CrossRef]
- Zhou, S.N.; Lu, J.-X.; Wang, X.-Q.; Shan, M.-R.; Miao, Z.; Pan, G.-P.; Jian, X.; Li, P.; Ping, S.; Pang, X.-Y.; et al. S-nitrosylation of prostacyclin synthase instigates nitrate cross-tolerance in vivo. Clin. Pharmacol. Ther. 2019, 105, 201–209. [Google Scholar] [CrossRef]
- Hink, U.; Daiber, A.; Kayhan, N.; Trischler, J.; Kraatz, C.; Oelze, M.; Mollnau, H.; Wenzel, P.; Vahl, C.F.; Ho, K.K.; et al. Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human blood vessels. J. Am. Coll. Cardiol. 2007, 50, 2226–2232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Münzel, T. Nitric Oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Sign. 2008, 10, 1115–1126. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Gori, T. Nitrate therapy: New aspects concerning molecular action and tolerance. Circulation 2011, 123, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Chlopicki, S. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radic. Biol. Med. 2020, 157, 15–37. [Google Scholar] [CrossRef]
- Smith, R.A.; Murphy, M.P. Mitochondria-targeted antioxidants as therapies. Discov. Med. 2011, 11, 106–114. [Google Scholar] [PubMed]
- Durante, M.; Sgaragli, G.; Biasutto, L.; Mattarei, A.; Fusi, F. Quercetin mitochondriotropic derivatives antagonize nitrate tolerance and endothelial dysfunction of isolated rat aorta rings. Planta Med. 2013, 79, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Muscoli, C.; Dagostino, C.; Giancotti, L.A.; Gliozzi, M.; Sacco, I.; Visalli, V.; Gratteri, S.; Palma, E.; Malara, N.; et al. The effect of peroxynitrite decomposition catalyst MnTBAP on aldehyde dehydrogenase-2 nitration by organic nitrates: Role in nitrate tolerance. Pharmacol. Res. 2014, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]





| Compd | Precurson Ion (m/z) | Declustering Potential (V) | Entrance Potential (V) | Product Ions | Collision Energy (V) | Collision Cell Exit Potential (V) |
|---|---|---|---|---|---|---|
| 4 | 258.2 | 30 | 4 | 258.2 → 212.2 | 12 | 15 |
| 258.2 → 168.2 | 22 | 15 | ||||
| 5 | 272.1 | 29 | 8 | 272.1 → 226.2 | 13 | 18 |
| 272.1 → 182.2 | 20 | 14 | ||||
| 272.1 → 211.1 | 22 | 20 | ||||
| 6 | 319.3 | 63 | 9 | 319.3 → 273.1 | 10 | 18 |
| 319.3 → 167.0 | 18 | 16 | ||||
| 319.3 → 194.8 | 19 | 25 | ||||
| 7 | 333.2 | 40 | 9 | 333.2 → 181.0 | 18 | 15 |
| 333.2 → 167.1 | 34 | 30 | ||||
| 333.2 → 223.2 | 17 | 17 |
| In Vitro Experiments pEC50 ± SE | Ex Vivo Experiments pEC50 ± SE | ||||
|---|---|---|---|---|---|
| Compd | + Benomyl 1 | + Chloral Hydrate 2 | Control | Tolerant Vessels | |
| 1a | 6.68 ± 0.08 3 | 6.49 ± 0.10 3 | 6.92 ± 0.06 | 6.80 ± 0.05 | |
| 1b | 6.66 ± 0.07 3 | 6.46 ± 0.10 3 | 6.57 ± 0.03 3 | 6.70 ± 0.12 | 6.49 ± 0.06 |
| 2 | 7.20 ± 0.15 3 | 6.20 ± 0.12 3 | 5.92 ± 0.3 3 | 7.71 ± 0.11 | 6.67 ± 0.09 4 |
| 3 | 6.80 ± 0.07 3 | 5.82 ± 0.5 3 | 5.52 ± 0.5 3 | 7.36 ± 0.04 | 6.40 ± 0.06 5 |
| 4 | 5.48 ± 0.09 | 4.85 ± 0.07 | 4.79 ± 0.11 | 5.48 ± 0.08 | 5.49 ± 0.05 |
| 5 | 5.52 ± 0.09 | 4.72 ± 0.07 | 4.68 ± 0.03 | 5.64 ± 0.08 | 5.22 ± 0.05 6 |
| 6 | 6.19 ± 0.09 | 5.48 ± 0.08 | 5.59 ± 0.16 | 6.16 ± 0.04 | 6.06 ± 0.03 |
| 7 | 6.62 ± 0.10 | 5.58 ± 0.08 | 5.52 ± 0.06 | 6.55 ± 0.07 | 5.88 ± 0.02 7 |
| GTN | 7.54 ± 0.043 | 6.38 ± 0.07 3 | 6.03 ± 0.05 3 | 7.52 ± 0.04 | 6.10 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, E.; Giorgis, M.; Leporati, M.; Rolando, B.; Chegaev, K.; Lazzarato, L.; Bertinaria, M.; Vincenti, M.; Di Stilo, A. Multitarget Antioxidant NO-Donor Organic Nitrates: A Novel Approach to Overcome Nitrates Tolerance, an Ex Vivo Study. Antioxidants 2022, 11, 166. https://doi.org/10.3390/antiox11010166
Marini E, Giorgis M, Leporati M, Rolando B, Chegaev K, Lazzarato L, Bertinaria M, Vincenti M, Di Stilo A. Multitarget Antioxidant NO-Donor Organic Nitrates: A Novel Approach to Overcome Nitrates Tolerance, an Ex Vivo Study. Antioxidants. 2022; 11(1):166. https://doi.org/10.3390/antiox11010166
Chicago/Turabian StyleMarini, Elisabetta, Marta Giorgis, Marta Leporati, Barbara Rolando, Konstantin Chegaev, Loretta Lazzarato, Massimo Bertinaria, Marco Vincenti, and Antonella Di Stilo. 2022. "Multitarget Antioxidant NO-Donor Organic Nitrates: A Novel Approach to Overcome Nitrates Tolerance, an Ex Vivo Study" Antioxidants 11, no. 1: 166. https://doi.org/10.3390/antiox11010166
APA StyleMarini, E., Giorgis, M., Leporati, M., Rolando, B., Chegaev, K., Lazzarato, L., Bertinaria, M., Vincenti, M., & Di Stilo, A. (2022). Multitarget Antioxidant NO-Donor Organic Nitrates: A Novel Approach to Overcome Nitrates Tolerance, an Ex Vivo Study. Antioxidants, 11(1), 166. https://doi.org/10.3390/antiox11010166







