Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Description of Studies and Quality Assessment
3.1.1. Meta-Analysis
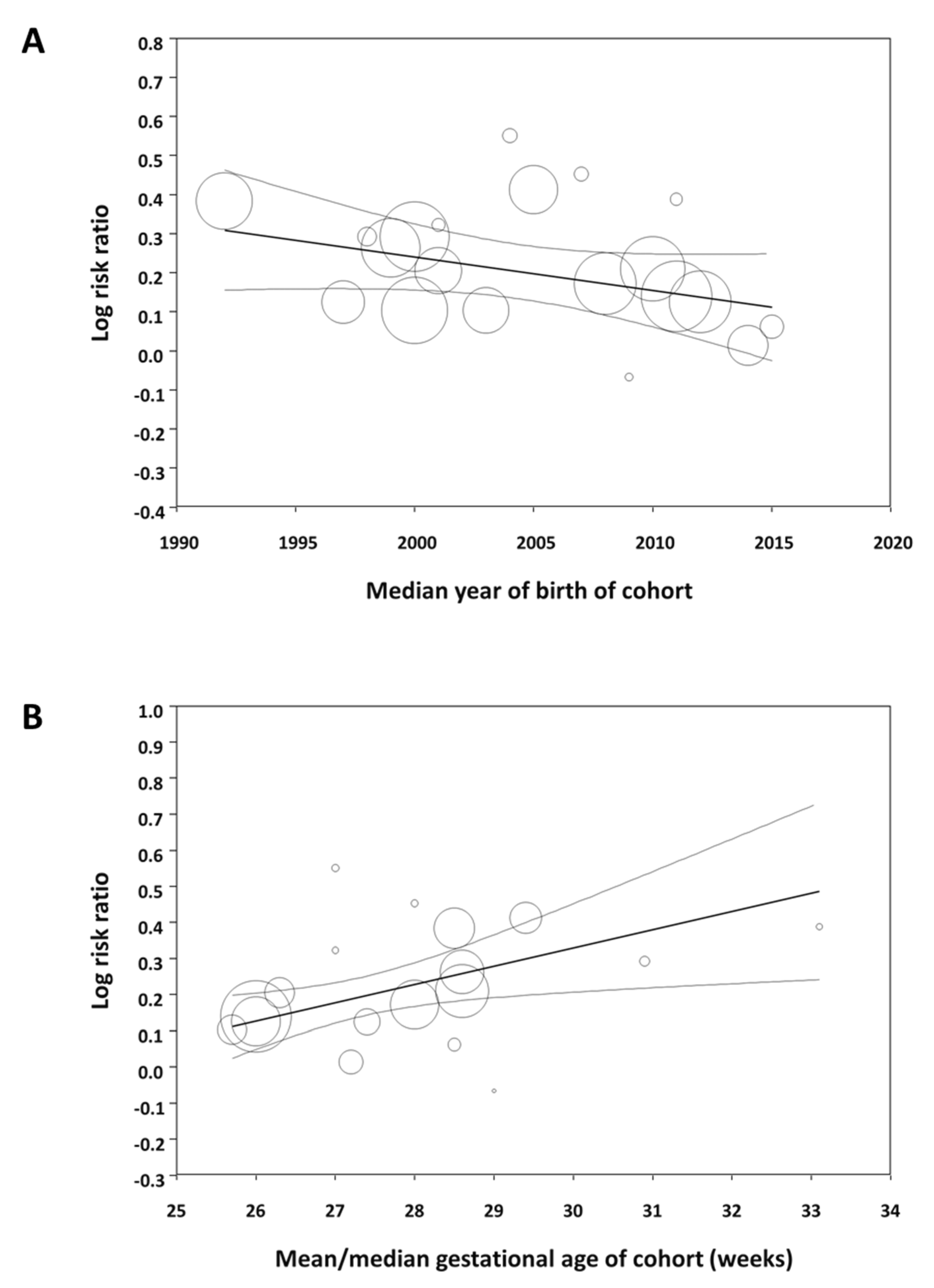
3.1.2. Subgroup Analysis and Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raju, T.N.; Buist, A.S.; Blaisdell, C.J.; Moxey-Mims, M.; Saigal, S. Adults born preterm: A review of general health and system-specific outcomes. Acta Paediatr. 2017, 106, 1409–1437. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Tamura, M.; Namba, F.; Neonatal Research Network of Japan. Role of sex in morbidity and mortality of very premature neonates. Pediatr. Int. 2017, 59, 898–905. [Google Scholar] [CrossRef]
- Boghossian, N.S.; Geraci, M.; Edwards, E.M.; Horbar, J.D. Sex differences in mortality and morbidity of infants born at less than 30 weeks’ gestation. Pediatrics 2018, 142, e20182352. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Cho, S.J.; Kong, K.A.; Park, E.A. Gestational age-specific sex difference in mortality and morbidities of preterm infants: A nationwide study. Sci. Rep. 2017, 7, 6161. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Aly, H. Male gender is associated with intraventricular hemorrhage. Pediatrics 2010, 125, e333–e339. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, D.N.; McGovern, M.; Greene, C.M.; Molloy, E.J. Gender disparities in preterm neonatal outcomes. Acta Paediatr. 2018, 107, 1494–1499. [Google Scholar] [CrossRef]
- McDonald, F.B.; Dempsey, E.M.; O’Halloran, K.D. Caffeine therapy for apnoea of prematurity: Wake up to the fact that sex matters. Exp. Physiol. 2018, 103, 1294–1295. [Google Scholar] [CrossRef]
- Saugstad, O.D. Hypoxanthine as an indicator of hypoxia: Its role in health and disease through free radical production. Pediatr. Res. 1988, 23, 143–150. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Rad. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Vento, M.; Aguar, M.; Escobar, J.; Arduini, A.; Escrig, R.; Brugada, M.; Izquierdo, I.; Asensi, M.A.; Sastre, J.; Saenz, P. Antenatal steroids and antioxidant enzyme activity in preterm infants: Influence of gender and timing. Antioxid. Redox Signal. 2009, 11, 2945–2955. [Google Scholar] [CrossRef]
- Lorente-Pozo, S.; Parra-Llorca, A.; Torres, B.; Torres-Cuevas, I.; Nuñez-Ramiro, A.; Cernada, M.; García-Robles, A.; Vento, M. Influence of sex on gestational complications, fetal-to-neonatal transition, and postnatal adaptation. Front. Pediatr. 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-C.; Tremblay, A. Sex-specificity of oxidative stress in newborns leading to a personalized antioxidant nutritive strategy. Antioxidants 2018, 7, 49. [Google Scholar] [CrossRef]
- Vu, H.D.; Dickinson, C.; Kandasamy, Y. Sex difference in mortality for premature and low birth weight neonates: A systematic review. Am. J. Perinatol. 2018, 35, 707–715. [Google Scholar] [PubMed]
- Garfinkle, J.; Yoon, E.W.; Alvaro, R.; Nwaesei, C.; Claveau, M.; Lee, S.K.; Shah, P.S. Trends in sex-specific differences in outcomes in extreme preterms: Progress or natural barriers? Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Oxford. 2000. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 July 2020).
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2021. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Álvarez-Fuente, M.; Ghazi, A.M.; Degraeuwe, P.; Zimmermann, L.J.; Kramer, B.W.; Villamor, E. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: A systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef]
- Bertino, E.; Coscia, A.; Boni, L.; Rossi, C.; Martano, C.; Giuliani, F.; Fabris, C.; Spada, E.; Zolin, A.; Milani, S. Weight growth velocity of very low birth weight infants: Role of gender, gestational age and major morbidities. Early Hum. Dev. 2009, 85, 339–347. [Google Scholar] [CrossRef]
- Binet, M.-E.; Bujold, E.; Lefebvre, F.; Tremblay, Y.; Piedboeuf, B.; Canadian Neonatal Network. Role of gender in morbidity and mortality of extremely premature neonates. Am. J. Perinatol. 2012, 29, 159–166. [Google Scholar] [CrossRef]
- Chen, C.; Tian, T.; Liu, L.; Zhang, J.; Fu, H. Gender-related efficacy of pulmonary surfactant in infants with respiratory distress syndrome: A STROBE compliant study. Medicine 2018, 97, e0425. [Google Scholar] [CrossRef]
- Derzbach, L.; Treszl, A.; Balogh, Á.; Vásárhelyi, B.; Tulassay, T. Gender dependent association between perinatal morbidity and estrogen receptor-alpha Pvull polymorphism. J. Perinat. Med. 2005, 33, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Deulofeut, R.; Dudell, G.; Sola, A. Treatment-by-gender effect when aiming to avoid hyperoxia in preterm infants in the NICU. Acta Paediatr. 2007, 96, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Elsmén, E.; Pupp, I.H.; Hellström-Westas, L. Preterm male infants need more initial respiratory and circulatory support than female infants. Acta Paediatr. 2004, 93, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Rusconi, F.; Reichman, B.; Adams, M.; Modi, N.; Lehtonen, L.; Kusuda, S.; Vento, M.; Darlow, B.A.; Bassler, D. Neonatal outcomes of extremely preterm twins by sex pairing: An international cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 17–24. [Google Scholar] [CrossRef]
- Griesmaier, E.; Santuari, E.; Edlinger, M.; Neubauer, V.; Waltner-Romen, M.; Kiechl-Kohlendorfer, U. Differences in the maturation of amplitude-integrated EEG signals in male and female preterm infants. Neonatology 2014, 105, 175–181. [Google Scholar] [CrossRef]
- Harris, C.; Zivanovic, S.; Lunt, A.; Calvert, S.; Bisquera, A.; Marlow, N.; Peacock, J.L.; Greenough, A. Lung function and respiratory outcomes in teenage boys and girls born very prematurely. Pediatr. Pulmonol. 2020, 55, 682–689. [Google Scholar] [CrossRef]
- Hintz, S.R.; Kendrick, D.E.; Vohr, B.R.; Poole, W.K.; Higgins, R.D.; NICHD Neonatal Research Network. Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006, 95, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Lin, S.-A.; Chang, Y.-C.; Kuo, H.-K. Correlation between periventricular leukomalacia and retinopathy of prematurity. Eur. J. Ophthalmol. 2012, 22, 980–984. [Google Scholar] [CrossRef]
- Jennische, M.; Sedin, G. Gender differences in outcome after neonatal intensive care: Speech and language skills are less influenced in boys than in girls at 6.5 years. Acta Paediatr. 2003, 92, 364–378. [Google Scholar] [CrossRef]
- Jones, H.P.; Karuri, S.; Cronin, C.M.; Ohlsson, A.; Peliowski, A.; Synnes, A.; Lee, S.K. Actuarial survival of a large Canadian cohort of preterm infants. BMC Pediatr. 2005, 5, 40. [Google Scholar] [CrossRef]
- Kent, A.L.; Wright, I.M.; Abdel-Latif, M.E. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics 2012, 129, 124–131. [Google Scholar] [CrossRef]
- Lauterbach, M.D.; Raz, S.; Sander, C.J. Neonatal hypoxic risk in preterm birth infants: The influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology 2001, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, M.E.; Robaey, P.; Stauder, J.E.; Glorieux, J.; Lefebvre, F. Extreme prematurity in healthy 5-year-old children: A re-analysis of sex effects on event-related brain activity. Psychophysiology 1998, 35, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Månsson, J.; Fellman, V.; Stjernqvist, K.; EXPRESS Study Group. Extremely preterm birth affects boys more and socio-economic and neonatal variables pose sex-specific risks. Acta Paediatr. 2015, 104, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Yogev, Y.; Glezerman, M. Effect of fetal sex on pregnancy outcome in twin pregnancies. Obs. Gynecol. 2009, 114, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, V.; Griesmaier, E.; Ralser, E.; Kiechl-Kohlendorfer, U. The effect of sex on outcome of preterm infants—A population-based survey. Acta Paediatr. 2012, 101, 906–911. [Google Scholar] [CrossRef]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef]
- Ramos-Navarro, C.; Sánchez-Luna, M.; Zeballos-Sarrato, S.; Pescador-Chamorro, I. Antenatal corticosteroids and the influence of sex on morbidity and mortality of preterm infants. J. Matern. Fetal Neonatal Med. 2020, 1–8. [Google Scholar] [CrossRef]
- Shinwell, E.S.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Blickstein, I. “Masculinizing” effect on respiratory morbidity in girls from unlike-sex preterm twins: A possible transchorionic paracrine effect. Pediatrics 2007, 120, e447–e453. [Google Scholar] [CrossRef]
- Skiöld, B.; Alexandrou, G.; Padilla, N.; Blennow, M.; Vollmer, B.; Ådén, U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J. Pediatr. 2014, 164, 1012–1018. [Google Scholar] [CrossRef]
- Spinillo, A.; Montanari, L.; Gardella, B.; Roccio, M.; Stronati, M.; Fazzi, E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev. Med. Child Neurol. 2009, 51, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Hodyl, N.A.; Wright, I.M.; Clifton, V. The influence of sex and antenatal betamethasone exposure on vasoconstrictors and the preterm microvasculature. J. Matern. Fetal Neonatal Med. 2011, 24, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Wright, I.M.; Clifton, V.L. Sex-specific alterations in placental 11β-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am. J. Physiol. Regul. Integrat. Compar. Physiol. 2009, 297, R510–R514. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.E.; Källén, K.; Maršál, K.; Norman, M.; Hellström-Westas, L. Impact of sex on perinatal mortality and morbidity in twins. J. Perinat. Med. 2014, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.K.; Verter, J.; Fanaroff, A.A.; Oh, W.; Ehrenkranz, R.A.; Shankaran, S.; Donovan, E.F.; Wright, L.L.; Lemons, J.A.; Tyson, J.E. Sex differences in outcomes of very low birthweight infants: The newborn male disadvantage. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 83, F182–F185. [Google Scholar] [CrossRef] [PubMed]
- Štimac, T.; Šopić-Rahelić, A.-M.; Ivandić, J.; Ekinja, E.; Blickstein, I. Effect of gender on growth-restricted fetuses born preterm. J. Perinat. Med. 2019, 47, 677–679. [Google Scholar] [CrossRef]
- Tioseco, J.A.; Aly, H.; Essers, J.; Patel, K.; El-Mohandes, A.A. Male sex and intraventricular hemorrhage. Pediatr. Crit. Care Med. 2006, 7, 40–44. [Google Scholar] [CrossRef]
- Tottman, A.C.; Bloomfield, F.H.; Cormack, B.E.; Harding, J.E.; Taylor, J.; Alsweiler, J.M. Sex-specific relationships between early nutrition and neurodevelopment in preterm infants. Pediatr. Res. 2020, 87, 872–878. [Google Scholar] [CrossRef]
- Walker, M.; Fitzgerald, B.; Keating, S.; Ray, J.; Windrim, R.; Kingdom, J. Sex-specific basis of severe placental dysfunction leading to extreme preterm delivery. Placenta 2012, 33, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-W.; Lin, Y.-C.; Wang, S.-T.; Huang, C.-C.; Taiwan Premature Infant Developmental Collaborative Study Group. Identifying risk factors shared by bronchopulmonary dysplasia, severe retinopathy, and cystic periventricular leukomalacia in very preterm infants for targeted intervention. Neonatology 2018, 114, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zou, L.; Lei, X.; Zhang, Y. Gender differences in infant mortality and neonatal morbidity in mixed-gender twins. Sci. Rep. 2017, 7, 8736. [Google Scholar] [CrossRef] [PubMed]
- Zisk, J.L.; Genen, L.H.; Kirkby, S.; Webb, D.; Greenspan, J.; Dysart, K. Do premature female infants really do better than their male counterparts? Am. J. Perinatol. 2011, 28, 241–246. [Google Scholar] [CrossRef]
- Zozaya, C.; Avila-Alvarez, A.; Arruza, L.; Rodrigo, F.G.-M.; Fernandez-Perez, C.; Castro, A.; Cuesta, M.T.; Vacas, B.; Couce, M.L.; Torres, M.V. The effect of morbidity and sex on postnatal growth of very preterm infants: A multicenter cohort study. Neonatology 2019, 115, 348–354. [Google Scholar] [CrossRef]
- Editorial. Putting gender on the agenda. Nature 2010, 465, 665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kardys, I.; Vliegenthart, R.; Oudkerk, M.; Hofman, A.; Witteman, J.C. The female advantage in cardiovascular disease: Do vascular beds contribute equally? Am. J. Epidemiol. 2007, 166, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Van Oyen, H.; Nusselder, W.; Jagger, C.; Kolip, P.; Cambois, E.; Robine, J.-M. Gender differences in healthy life years within the EU: An exploration of the “health–survival” paradox. Int. J. Public Health 2013, 58, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Miller, V.M.; Prakash, Y. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Roseboom, T.; Moore, T.; Moore, L.; Junien, C. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ. 2013, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Clifton, V. Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 2010, 31, S33–S39. [Google Scholar] [CrossRef]
- Clifton, V.; Stark, M.; Osei-Kumah, A.; Hodyl, N. The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta 2012, 33, S37–S41. [Google Scholar] [CrossRef]
- Broere-Brown, Z.A.; Adank, M.C.; Benschop, L.; Tielemans, M.; Muka, T.; Gonçalves, R.; Bramer, W.M.; Schoufour, J.D.; Voortman, T.; Steegers, E.A. Fetal sex and maternal pregnancy outcomes: A systematic review and meta-analysis. Biol. Sex Differ. 2020, 11, 26. [Google Scholar] [CrossRef]
- Schalekamp-Timmermans, S.; Arends, L.R.; Alsaker, E.; Chappell, L.; Hansson, S.; Harsem, N.K.; Jälmby, M.; Jeyabalan, A.; Laivuori, H. Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: A meta-analysis. Int. J. Epidemiol. 2017, 46, 632–642. [Google Scholar]
- James, W.H.; Grech, V. A review of the established and suspected causes of variations in human sex ratio at birth. Early Hum. Dev. 2017, 109, 50–56. [Google Scholar] [CrossRef]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG Int. J. Obs. Gynaecol. 2003, 110, 34–38. [Google Scholar] [CrossRef]
- Cooperstock, M.; Campbell, J. Excess males in preterm birth: Interactions with gestational age, race, and multiple birth. Obs. Gynecol. 1996, 88, 189–193. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Voegtline, K.M. The gestational foundation of sex differences in development and vulnerability. Neuroscience 2017, 342, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K. Births: Final data for 2019. Natl. Vital Stat. Rep. 2021, 70, 1–51. [Google Scholar]
- Peelen, M.J.; Kazemier, B.M.; Ravelli, A.C.; De Groot, C.J.; Van Der Post, J.A.; Mol, B.W.; Hajenius, P.J.; Kok, M. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obs. Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Challis, J.; Newnham, J.; Petraglia, F.; Yeganegi, M.; Bocking, A. Fetal sex and preterm birth. Placenta 2013, 34, 95–99. [Google Scholar] [CrossRef] [PubMed]
- McElrath, T.F.; Hecht, J.L.; Dammann, O.; Boggess, K.; Onderdonk, A.; Markenson, G.; Harper, M.; Delpapa, E.; Allred, E.N.; Leviton, A. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. Am. J. Epidemiol. 2008, 168, 980–989. [Google Scholar] [CrossRef]
- Gagliardi, L. Pregnancy complications and neonatal outcomes: Problems and perspectives. Acta Paediatr. 2014, 103, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Rusconi, F.; Da Fre, M.; Mello, G.; Carnielli, V.; Di Lallo, D.; Macagno, F.; Miniaci, S.; Corchia, C.; Cuttini, M. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: Results of the population-based ACTION cohort study. Pediatr. Res. 2013, 73, 794–801. [Google Scholar] [CrossRef]
- Gagliardi, L.; Rusconi, F.; Bellu, R.; Zanini, R.; Italian Neonatal Network. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics 2014, 134, e154–e161. [Google Scholar] [CrossRef]
- Ghidini, A.; Salafia, C.M. Histologic placental lesions in women with recurrent preterm delivery. Acta Obs. Gynecol. Scand. 2005, 84, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Cataltepe, D. Historical perspectives: Beyond the first breath: Hyaline membrane disease and constructing the neonatal patient, 1959–1975. NeoReviews 2018, 19, e636–e644. [Google Scholar] [CrossRef]
- Farrell, P.M.; Avery, M.E. Hyaline membrane disease. Am. Rev. Respir. Dis. 1975, 111, 657–688. [Google Scholar] [PubMed]
- Raghavan, D.; Jain, R. Increasing awareness of sex differences in airway diseases. Respirology 2016, 21, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.; Simard, M.; Provost, P.R.; Piedboeuf, B.; Tremblay, Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol. Metabol. 2010, 21, 729–738. [Google Scholar] [CrossRef]
- Huizing, M.J.; Cavallaro, G.; Moonen, R.M.; González-Luis, G.E.; Mosca, F.; Vento, M.; Villamor, E. Is the C242T polymorphism of the CYBA gene linked with oxidative stress-associated complications of prematurity? Antioxid. Redox Signal. 2017, 27, 1432–1438. [Google Scholar] [CrossRef]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: A systematic review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef]
- Linsell, L.; Johnson, S.; Wolke, D.; O’Reilly, H.; Morris, J.K.; Kurinczuk, J.J.; Marlow, N. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: A prospective, population-based cohort study. Arch. Dis. Child. 2018, 103, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Martin, J.; Horwood, L.J. Metabolic syndrome in very low birth weight young adults and controls: The New Zealand 1986 VLBW Study. J. Pediatr. 2019, 206, 128–133.e5. [Google Scholar] [CrossRef] [PubMed]

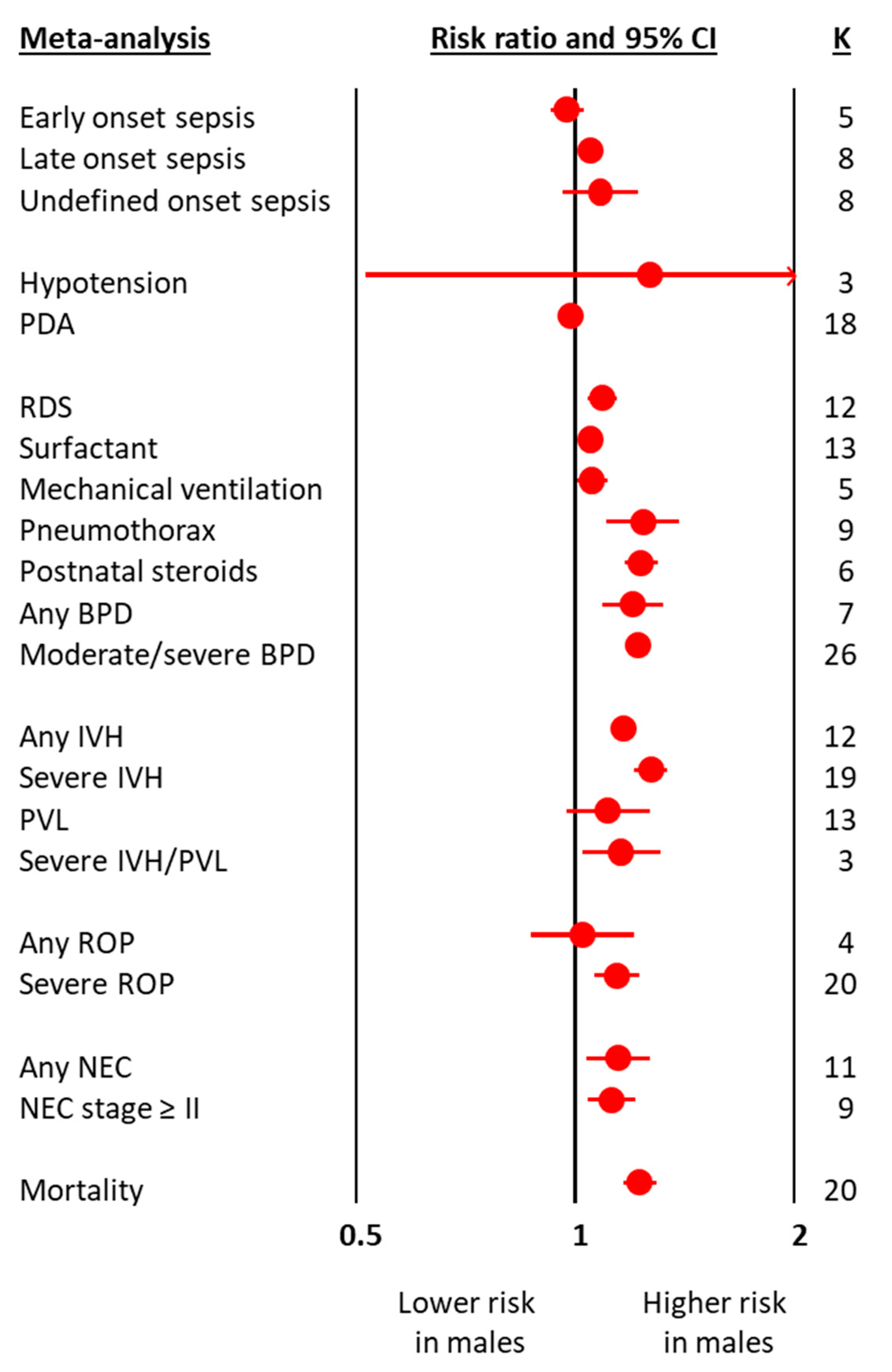
| Meta-Analysis | K | RR | 95% CI | p | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | I2 (%) | p | ||||
| Chorioamnionitis | 6 | 1.001 | 0.953 | 1.052 | 0.969 | 67.3 | 0.009 |
| Hypertensive disorders of pregnancy | 15 | 0.829 | 0.803 | 0.856 | <0.001 | 22.9 | 0.200 |
| Maternal diabetes | 7 | 0.991 | 0.808 | 1.214 | 0.927 | 65.9 | 0.007 |
| Smoking during pregnancy | 3 | 0.987 | 0.800 | 1.218 | 0.901 | 3.9 | <0.001 |
| Prenatal care | 5 | 1.017 | 0.982 | 1.053 | 0.352 | 96.2 | 0.353 |
| Premature rupture of membranes | 6 | 1.006 | 0.947 | 1.068 | 0.852 | 62.8 | 0.020 |
| Prolonged rupture of membranes | 5 | 0.968 | 0.914 | 1.026 | 0.275 | 0.0 | 0.972 |
| Antepartum hemorrhage | 3 | 0.986 | 0.708 | 1.374 | 0.936 | 96.9 | <0.001 |
| Antenatal corticosteroids | 21 | 0.992 | 0.982 | 1.003 | 0.143 | 44.9 | 0.012 |
| Fetal distress | 3 | 0.784 | 0.678 | 0.907 | 0.001 | 0.0 | 0.741 |
| Cesarean-section | 21 | 0.980 | 0.966 | 0.995 | 0.008 | 51.5 | 0.003 |
| Outborn | 9 | 1.077 | 1.027 | 1.128 | 0.002 | 0.0 | 0.682 |
| Apgar 5′ <3 | 3 | 1.269 | 1.132 | 1.422 | <0.001 | 0.0 | 0.726 |
| Apgar 5′ <7 | 3 | 1.010 | 0.946 | 1.077 | 0.772 | 85.2 | 0.001 |
| Intubation at birth | 5 | 1.038 | 1.006 | 1.071 | 0.019 | 66.4 | 0.018 |
| Resuscitation at birth | 3 | 0.990 | 0.609 | 1.609 | 0.968 | 93.2 | <0.001 |
| Birth weight <P10 | 18 | 0.892 | 0.785 | 1.014 | 0.080 | 80.9 | <0.001 |
| Birth weight <P3 | 3 | 1.123 | 0.877 | 1.438 | 0.358 | 51.5 | 0.127 |
| Continuous variables | MD | ||||||
| Gestational age (weeks) | 24 | −0.10 | −0.21 | 0.01 | 0.076 | 87.0 | <0.001 |
| Birth weight (g) | 24 | 47.8 | 34.1 | 61.5 | <0.001 | 91.5 | <0.001 |
| Maternal age (years) | 10 | 0.0 | −0.5 | 0.5 | 0.999 | 92.5 | <0.001 |
| Meta-Analysis | K | RR | 95% CI | p | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | I2 (%) | p | ||||
| Early onset sepsis | 5 | 0.975 | 0.924 | 1.030 | 0.371 | 0.0 | 0.459 |
| Late onset sepsis | 8 | 1.051 | 1.026 | 1.077 | <0.001 | 17.0 | 0.296 |
| Undefined onset sepsis | 8 | 1.083 | 0.962 | 1.218 | 0.186 | 20.6 | 0.266 |
| Hypotension | 3 | 1.270 | 0.514 | 3.140 | 0.605 | 72.7 | 0.026 |
| PDA | 18 | 0.985 | 0.958 | 1.012 | 0.262 | 52.5 | 0.004 |
| RDS | 12 | 1.090 | 1.042 | 1.140 | <0.001 | 96.1 | <0.001 |
| Surfactant | 13 | 1.031 | 1.026 | 1.036 | <0.001 | 41.4 | 0.059 |
| Mechanical ventilation | 5 | 1.054 | 1.003 | 1.108 | 0.038 | 54.7 | 0.066 |
| Pneumothorax | 9 | 1.240 | 1.104 | 1.393 | <0.001 | 42.2 | 0.086 |
| Postnatal steroids | 6 | 1.234 | 1.169 | 1.302 | <0.001 | 37.6 | 0.433 |
| Any BPD | 7 | 1.200 | 1.091 | 1.319 | <0.001 | 66.0 | 0.004 |
| Moderate/severe BPD | 26 | 1.219 | 1.176 | 1.264 | <0.001 | 71.4 | <0.001 |
| Any IVH | 12 | 1.166 | 1.139 | 1.193 | <0.001 | 0.0 | 0.680 |
| Severe IVH | 19 | 1.271 | 1.207 | 1.338 | <0.001 | 40.5 | 0.035 |
| PVL | 13 | 1.110 | 0.971 | 1.269 | 0.128 | 77.3 | <0.001 |
| Severe IVH/PVL | 3 | 1.158 | 1.023 | 1.310 | 0.020 | 80.8 | 0.005 |
| Any ROP | 4 | 1.025 | 0.870 | 1.207 | 0.767 | 66.3 | 0.031 |
| Severe ROP | 20 | 1.143 | 1.065 | 1.226 | <0.001 | 79.2 | <0.001 |
| Any NEC | 11 | 1.145 | 1.036 | 1.266 | 0.008 | 60.1 | 0.003 |
| NEC stage ≥ II | 9 | 1.122 | 1.039 | 1.211 | 0.003 | 32.9 | 0.155 |
| Mortality | 20 | 1.227 | 1.163 | 1.294 | <0.001 | 83.7 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Westering-Kroon, E.; Huizing, M.J.; Villamor-Martínez, E.; Villamor, E. Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression. Antioxidants 2021, 10, 1490. https://doi.org/10.3390/antiox10091490
van Westering-Kroon E, Huizing MJ, Villamor-Martínez E, Villamor E. Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression. Antioxidants. 2021; 10(9):1490. https://doi.org/10.3390/antiox10091490
Chicago/Turabian Stylevan Westering-Kroon, Elke, Maurice J Huizing, Eduardo Villamor-Martínez, and Eduardo Villamor. 2021. "Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression" Antioxidants 10, no. 9: 1490. https://doi.org/10.3390/antiox10091490
APA Stylevan Westering-Kroon, E., Huizing, M. J., Villamor-Martínez, E., & Villamor, E. (2021). Male Disadvantage in Oxidative Stress-Associated Complications of Prematurity: A Systematic Review, Meta-Analysis and Meta-Regression. Antioxidants, 10(9), 1490. https://doi.org/10.3390/antiox10091490







