Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diet
2.3. Leaching Test
2.4. Feeding Trial
2.5. Vibrio Parahaemolyticus Challenge Test
2.6. Sample Collection
2.7. Ascorbic Acid Content
2.8. Immune Parameters in Hemolymph
2.9. Biochemical Parameters in CFH
2.10. Total RNA Extraction and Quantitative Real-Time PCR
2.11. Western Blot
2.12. Calculations and Statistical Analysis
3. Results
3.1. Retention Efficiency of Ascorbic Acid in the Diet
3.2. Growth Performance and Ascorbic Acid Distribution in Tissues
3.3. Hemolymph Immune Parameters
3.4. Anti-Oxidative Parameters in CFH
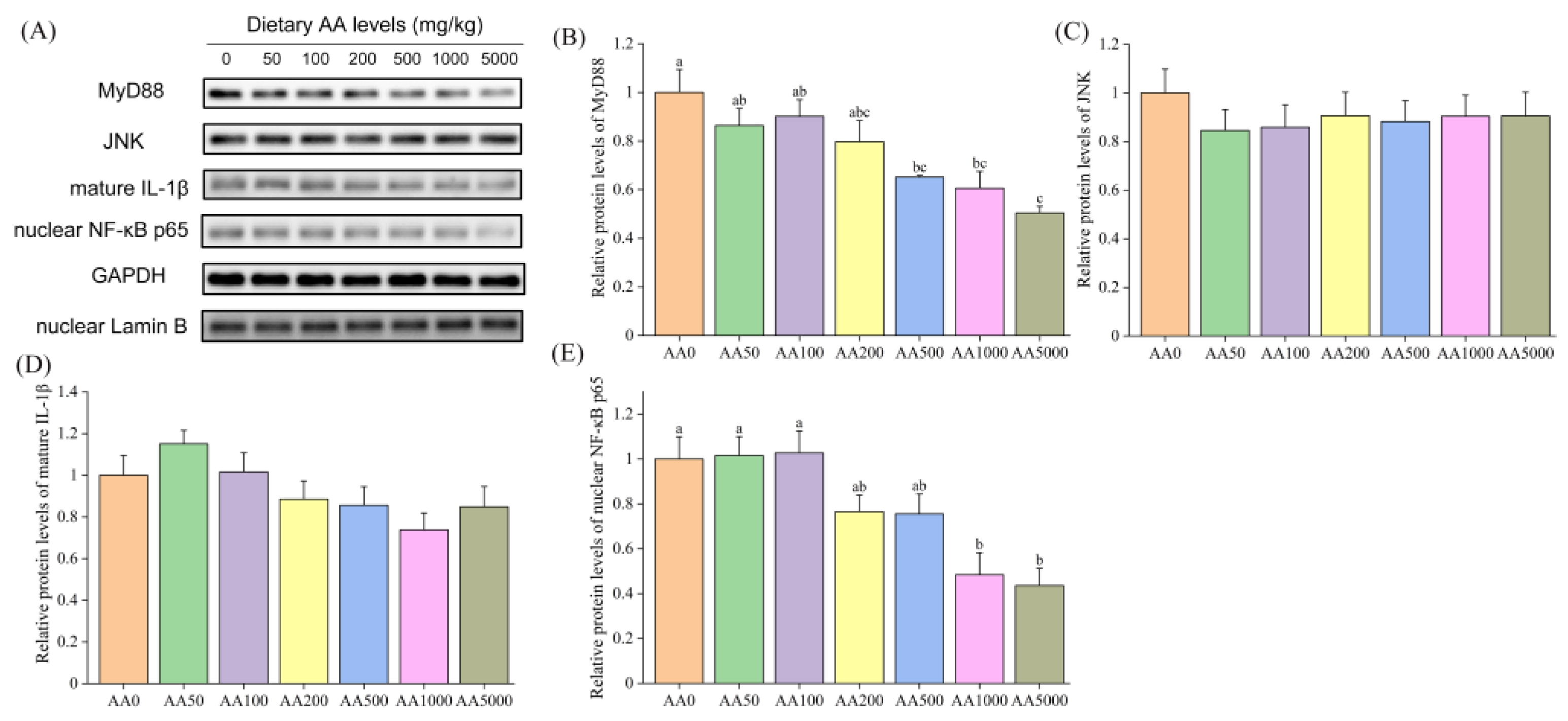
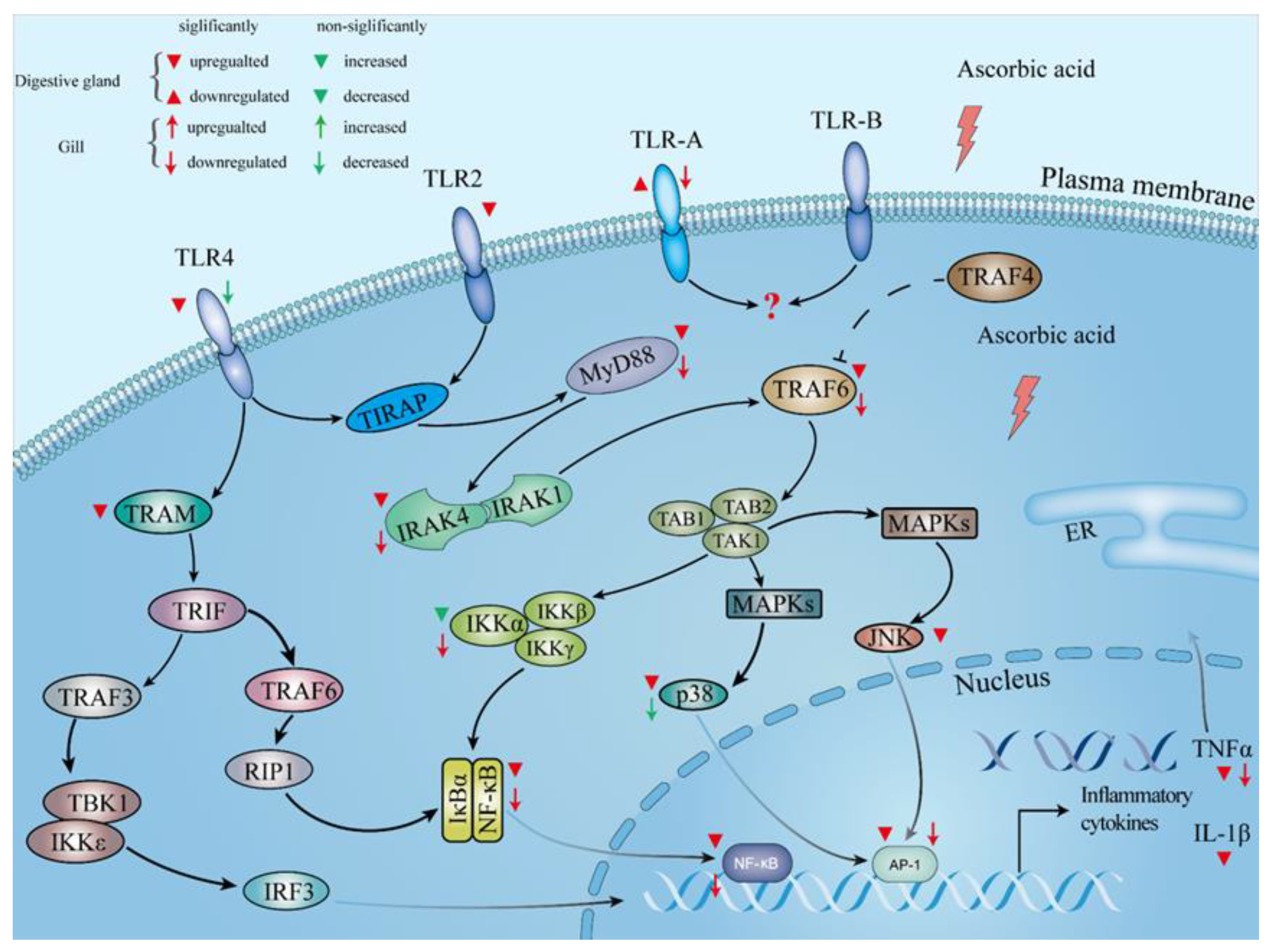
3.5. Expressions of Immune-Related Genes and Proteins in Abalone Digestive Gland and Gill
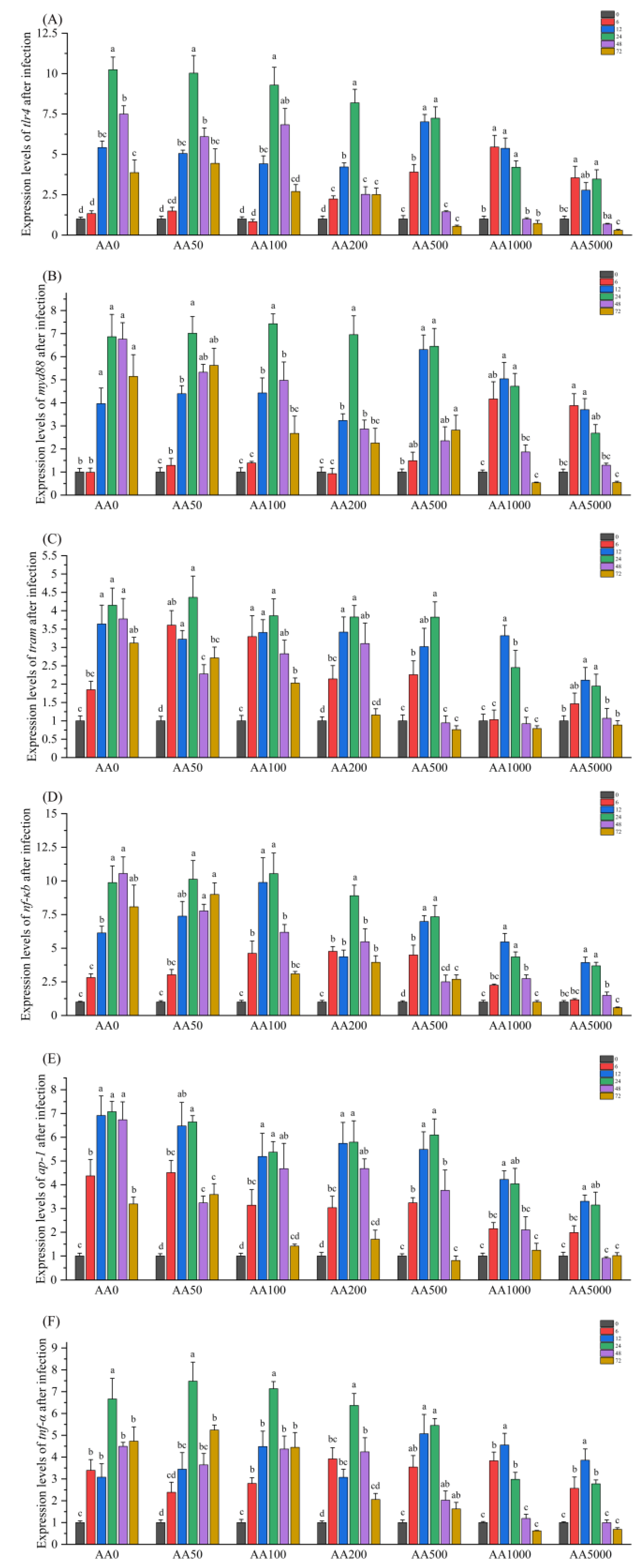
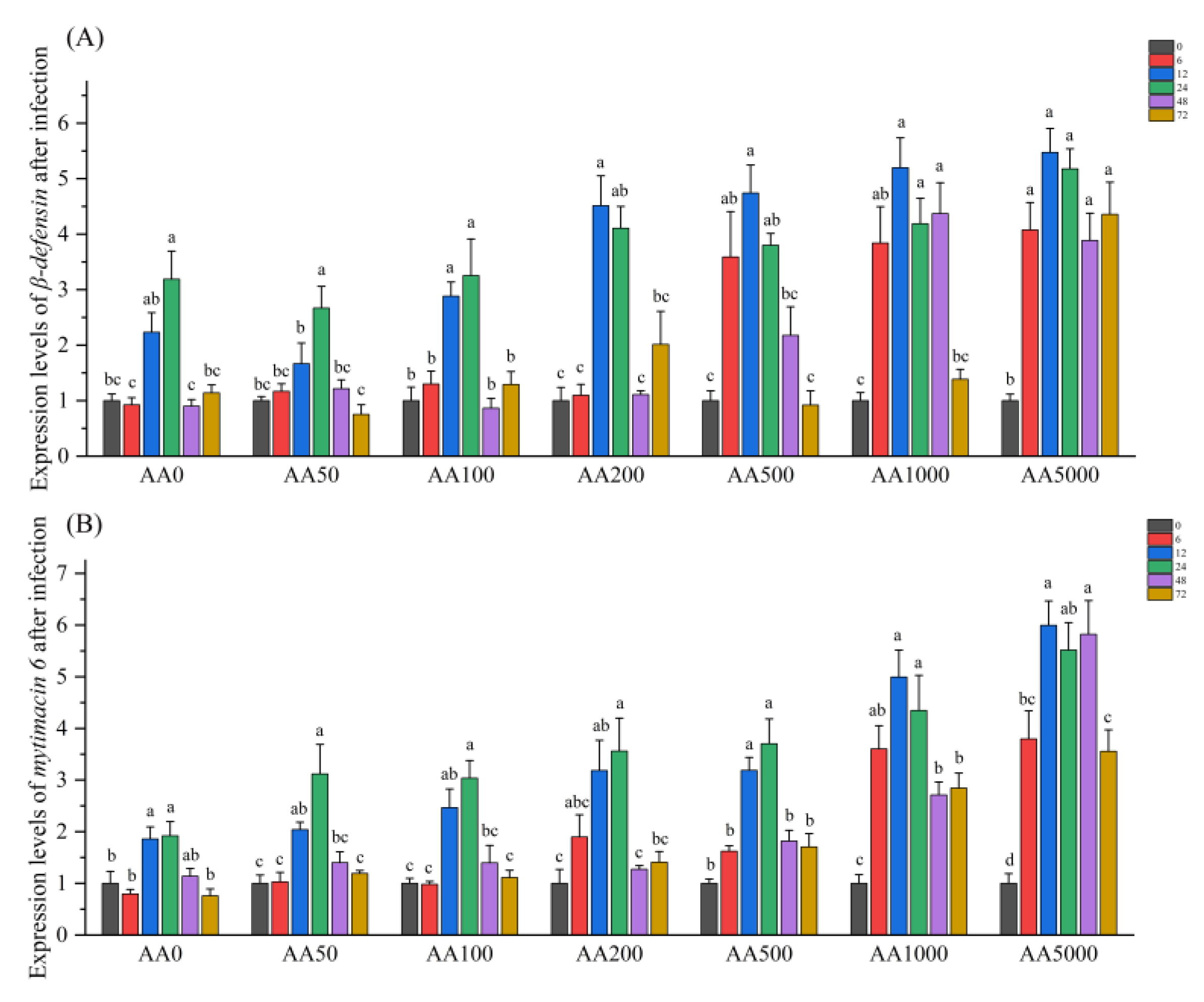
3.6. Expressions of Immune-Related Genes in the Digestive Gland in Response to Vibrio Parahaemolyticus
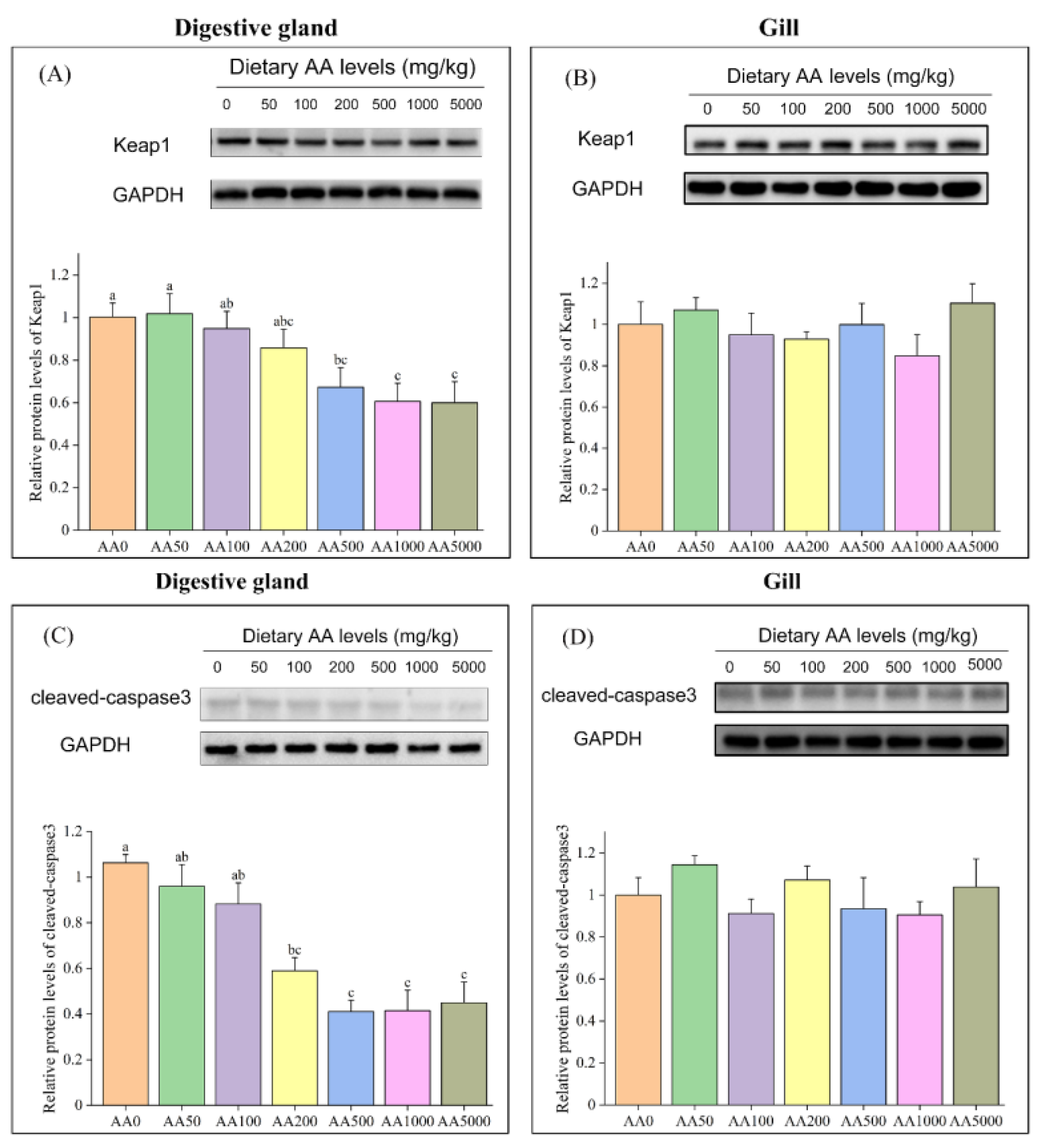
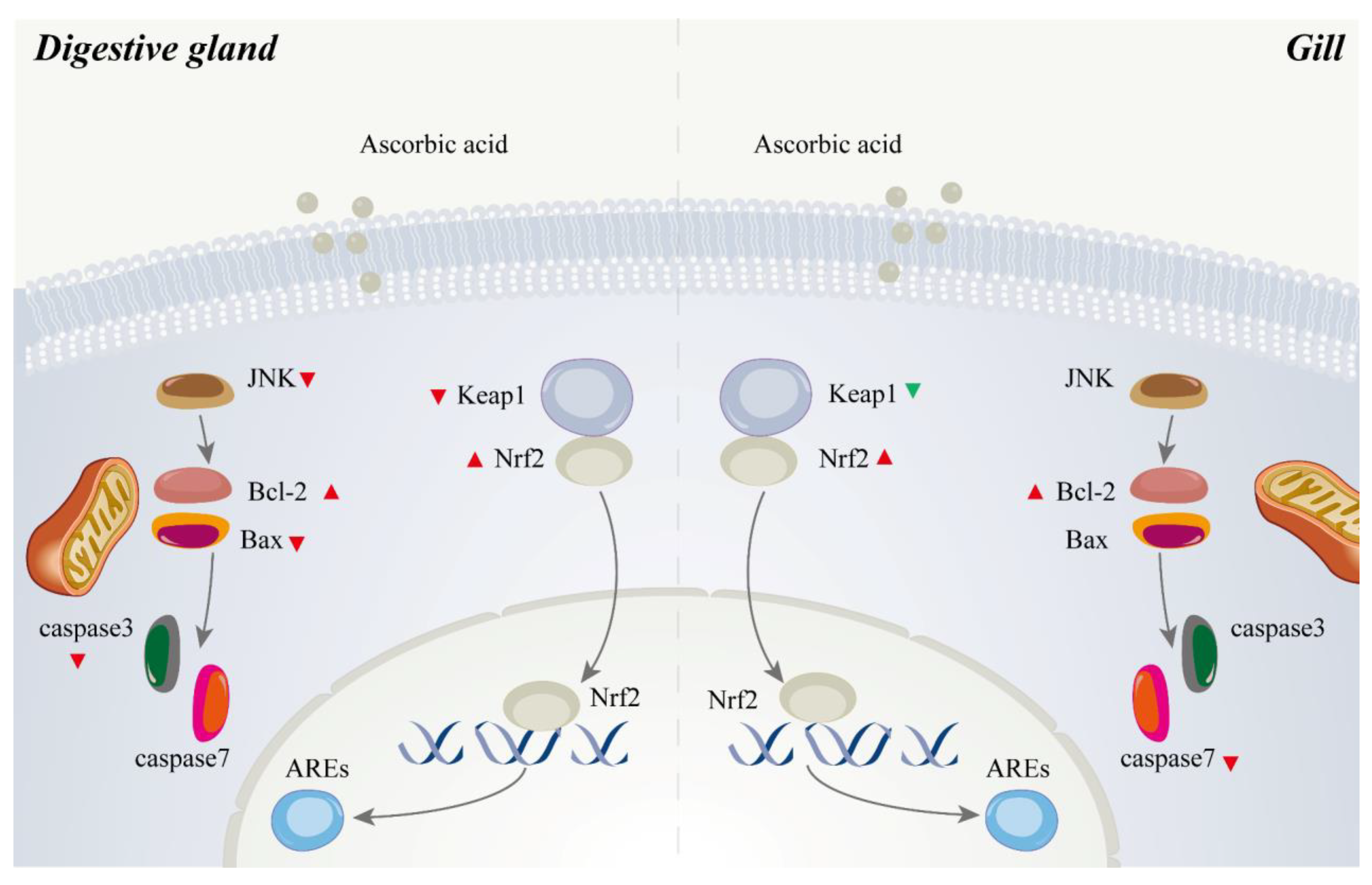
3.7. Expressions of Anti-Oxidation-Related and Apoptosis-Related Genes and Proteins in Digestive Gland and Gill
4. Discussion
4.1. Ascorbic Acid Content in Different Tissues
4.2. Role of Ascorbic Acid in the Regulation of Immunity of Abalone
4.3. Role of Ascorbic Acid in the Regulation of Anti-oxidative Capacity of Abalone
4.4. Role of Ascorbic Acid in the Regulation of Apoptosis of Abalone
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Chapter Seven—Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar]
- Reyes, J.B.D.; Kim, J.H.; Han, G.P.; Won, S.Y.; Kil, D.Y. Effects of dietary supplementation of vitamin C on productive performance, egg quality, tibia characteristics and antioxidant status of laying hens. Livestig. Sci. 2021, 248, 104502. [Google Scholar] [CrossRef]
- Weber, P.; Bendich, A.; Schalch, W. Vitamin C and human health—A review of recent data relevant to human requirements. Int. J. Vitam. Nutr. Res. 1996, 66, 19–30. [Google Scholar] [PubMed]
- Bsoul, S.A.; Terezhalmy, G.T. Vitamin C in health and disease. J. Contemp. Dent. Pract. 2004, 5, 1–13. [Google Scholar] [CrossRef]
- Parker, A.; Cuddihy, S.L.; Son, T.G.; Vissers, M.; Winterbourn, C.C. Roles of superoxide and myeloperoxidase in ascorbate oxidation in stimulated neutrophils and H2O2-treated HL60 cells. Free Radic. Biol. Med. 2011, 51, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R. Ascorbate-mediated stimulation of neutrophil motility and lymphocyte transformation by inhibition of the peroxidase/H2O2/halide system in vitro and in vivo. Am. J. Clin. Nutr. 1981, 34, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Rebora, A.; Crovato, F.; Dallegri, F.; Patrone, F. Repeated staphylococcal pyoderma in two siblings with defective neutrophil bacterial killing. Dermatology 1980, 160, 106–112. [Google Scholar] [CrossRef]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef] [PubMed]
- Washko, P.W.; Wang, Y.H.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar] [CrossRef]
- Levy, R.; Shriker, O.; Porath, A.; Riesenberg, K.; Schlaeffer, F. Vitamin C for the Treatment of Recurrent Furunculosis in Patients with Impaired Neutrophil Functions. J. Infect. Dis. 1996, 173, 1502–1505. [Google Scholar] [CrossRef]
- Chang, H.H.; Chen, C.S.; Lin, J.Y. High Dose Vitamin C Supplementation Increases the Th1/Th2 Cytokine Secretion Ratio, but Decreases Eosinophilic Infiltration in Bronchoalveolar Lavage Fluid of Ovalbumin-Sensitized and Challenged Mice. J. Agric. Food Chem. 2009, 57, 10471–10476. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit Care 2014, 18, 460. [Google Scholar] [CrossRef]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef]
- Yang, M.; Teng, S.; Ma, C.; Yu, Y.; Wang, P.; Yi, C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 2018, 70, 1301–1313. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Ferreira, D.C.M.; Tapsoba, I.; Verchier, Y. Vitamin C stimulates or attenuates reactive oxygen and nitrogen species (ROS, RNS) production depending on cell state: Quantitative amperometric measurements of oxidative bursts at PLB-985 and RAW 264.7 cells at the single cell level. J. Electroanal. Chem. 2008, 615, 34–44. [Google Scholar] [CrossRef]
- Bei, R. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Commentary: Vitamin C: Antioxidant or Pro-Oxidant In Vivo? Free Radic. Res. Commun. 1996, 25, 439–454. [Google Scholar]
- Molina, N.; Morandi, A.C.; Bolin, A.P.; Otton, R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int. Immunopharmacol. 2014, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar] [CrossRef]
- NRC, O.N. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Wu, F.; Huang, F.; Wen, H.; Jiang, M.; Liu, W.; Tian, J.; Yang, C.G. Vitamin C requirement of adult genetically improved farmed tilapia, Oreochromis niloticus. Aquac. Int. 2015, 23, 1203–1215. [Google Scholar] [CrossRef]
- Biswas, B.K.; Biswas, A.; Junichi, I.; Kim, Y.-S.; Takii, K. The optimal dietary level of ascorbic acid for juvenile Pacific bluefin tuna, Thunnus orientalis. Aquac. Int. 2013, 21, 327–336. [Google Scholar] [CrossRef]
- Guary, M.; Kanazawa, A.; Tanaka, N.; Ceccaldi, H.J. Nutritional Requirements of Prawn VI: Requirement for Ascorbic Acid. Mem. Fac. Fish Kagoshima Univ. 1976, 25, 53–57. [Google Scholar]
- Xie, Z.; Niu, C.; Zhang, Z.; Bao, L. Dietary ascorbic acid may be necessary for enhancing the immune response in Siberian sturgeon (Acipenser baerii), a species capable of ascorbic acid biosynthesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 152–157. [Google Scholar] [CrossRef]
- Ren, T.; Koshio, S.; Uyan, O.; Komilus, C.F.; Yokoyama, S.; Ishikawa, M.; Abdul, M.K. Effects of Dietary Vitamin C on Blood Chemistry and Nonspecific Immune Response of Juvenile Red Sea Bream, Pagrus major. J. World Aquac. Soc. 2008, 39, 797–803. [Google Scholar] [CrossRef]
- Shahkar, E.; Yun, H.; Kim, D.-J.; Kim, S.-K.; Lee, B.I.; Bai, S.C. Effects of dietary vitamin C levels on tissue ascorbic acid concentration, hematology, non-specific immune response and gonad histology in broodstock Japanese eel, Anguilla japonica. Aquaculture 2015, 438, 115–121. [Google Scholar] [CrossRef]
- Tewary, A.; Patra, B.C. Use of vitamin C as an immunostimulant. Effect on growth, nutritional quality, and immune response of Labeo rohita (Ham.). Fish Physiol. Biochem. 2008, 34, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Shiau, S.Y. Dietary L-ascorbic acid affects growth, nonspecific immune responses and disease resistance in juvenile grouper, Epinephelus malabaricus. Aquaculture 2005, 244, 215–221. [Google Scholar] [CrossRef]
- Ren, T.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Micheal, F.R.; Uyan, O.; Tung, H.T. Influence of dietary vitamin C and bovine lactoferrin on blood chemistry and non-specific immune responses of Japanese eel, Anguilla japonica. Aquaculture 2007, 267, 31–37. [Google Scholar] [CrossRef]
- Kumari, J.; Sahoo, P. Dietary immunostimulants influence specific immune response and resistance of healthy and immunocompromised Asian catfish Clarias batrachus to Aeromonas hydrophila infection. Dis. Aquat. Org. 2006, 70, 63–70. [Google Scholar] [CrossRef]
- Barros, M.M.; Falcon, D.R.; Oliveira Orsi, R.; Pezzato, L.E.; Fernandes, A.C., Jr.; Guimaraes, I.G.; Fernandes, A., Jr.; Padovani, C.R.; Sartori, M.M.P. Non-specific immune parameters and physiological response of Nile tilapia fed beta-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish Shellfish Immunol. 2014, 39, 188–195. [Google Scholar] [CrossRef]
- Qiao, J.; Du, Z.; Zhang, Y.; Du, H.; Guo, L.; Zhong, M.; Cao, J.; Wang, X. Proteomic identification of the related immune-enhancing proteins in shrimp Litopenaeus vannamei stimulated with vitamin C and Chinese herbs. Fish Shellfish Immunol. 2011, 31, 736–745. [Google Scholar] [CrossRef]
- Trichet, V.V.; Santigosa, E.; Cochin, E.; Gabaudan, J. The Effect of Vitamin C on Fish Health. Diet. Nutr. Addit. Fish Health 2015, 7, 151–171. [Google Scholar]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-kappaB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-kappaB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare. Fish Shellfish Immunol. 2016, 58, 177–192. [Google Scholar] [CrossRef]
- Mau, A.; Jha, R. Aquaculture of two commercially important molluscs (abalone and limpet): Existing knowledge and future prospects. Rev. Aquac. 2018, 10, 611–625. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Chen, Z.-S.; Ke, C.-H.; Zhao, J.; You, W.-W.; Zhang, J.; Dong, W.-T.; Chen, J. Pyrosequencing of Haliotis diversicolor Transcriptomes: Insights into Early Developmental Molluscan Gene Expression. PLoS ONE 2012, 7, e51279. [Google Scholar] [CrossRef] [PubMed]
- Mai, K. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino.: VII. Effects of dietary vitamin C on survival, growth and tissue concentration of ascorbic acid. Aquaculture 1998, 161, 383–392. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Xu, W.; Zhang, W.; Mai, K. Dietary ascorbic acid modulates the expression profile of stress protein genes in hepatopancreas of adult Pacific abalone Haliotis discus hannai Ino. Fish Shellfish Immunol. 2014, 41, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Han, Y.; Wang, Z. Isolation of Vibrio parahaemolyticus from abalone (Haliotis diversicolor supertexta L.) postlarvae associated with mass mortalities. Aquaculture 2006, 257, 161–166. [Google Scholar] [CrossRef]
- Travers, M.A.; Le Goic, N.; Huchette, S.; Koken, M.; Paillard, C. Summer immune depression associated with increased susceptibility of the European abalone, Haliotis tuberculata to Vibrio harveyi infection. Fish Shellfish Immunol. 2008, 25, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems–not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef]
- Mai, K.; Mercer, J.P.; Donlon, J. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. III. Response of abalone to various levels of dietary lipid. Aquaculture 1995, 134, 65–80. [Google Scholar]
- Mai, K.; Mercer, J.P.; Donlon, J. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. IV. Optimum dietary protein level for growth. Aquaculture 1995, 136, 165–180. [Google Scholar]
- Wu, C.; Zhang, W.; Mai, K.; Xu, W.; Zhong, X. Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comp. Biochem. Physiol. C. Toxicol. Pharm. 2011, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC International Publishers: Arlington, VA, USA, 1995. [Google Scholar]
- Anderson, R.S.; Brubacher, L.L.; Calvo, L.R.; Unger, M.A.; Burreson, E.M. Effects of tributyltin and hypoxia on the progression of Perkinsus marinus infections and host defence mechanisms in oyster, Crassostrea virginica (Gmelin). J. Fish Dis. 2010, 21, 371–380. [Google Scholar] [CrossRef]
- Xue, J.; Xu, Y.; Jin, L.; Liu, G.; Sun, Y.; Li, S.; Zhang, J. Effects of traditional Chinese medicine on immune responses in abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol. 2008, 24, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Schmedes, A.; Hlmer, G. A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J. Am. Oil Chem. Soc. 1989, 66, 813–817. [Google Scholar] [CrossRef]
- Currie, K. GENORM: A generalized norm calculation. Comput. Geosci. 1991, 17, 77–89. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2018, 64, 5245–5250. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Peng, H.; Chen, X.; Han, X.; Yu, J.; Wang, W.; Liang, L.; Liu, Z.; Zheng, Y.; et al. Epigenetically upregulated oncoprotein PLCE1 drives esophageal carcinoma angiogenesis and proliferation via activating the PI-PLCε-NF-κB signaling pathway and VEGF-C/Bcl-2 expression. Mol. Cancer 2019, 18, 1–19. [Google Scholar] [CrossRef]
- Ibiyo, L.M.O.; Atteh, J.O.; Omotosho, J.S.; Madu, C.T. Vitamin C (ascorbic acid) requirements of Heterobranchus longifilis fingerlings. Afr. J. Biotechnol. 2007, 6, 1559–1567. [Google Scholar]
- Zou, W.; Lin, Z.; Huang, Y.; Limbu, S.M.; Rong, H.; Yu, C.; Lin, F.; Wen, X. Effect of dietary vitamin C on growth performance, body composition and biochemical parameters of juvenile Chu’s croaker (Nibea coibor). Aquac. Nutr. 2019, 26, 60–73. [Google Scholar] [CrossRef]
- Huang, F.; Wu, F.; Zhang, S.; Jiang, M.; Liu, W.; Tian, J.; Yang, C.; Wen, H. Dietary vitamin C requirement of juvenile Chinese sucker (Myxocyprinus asiaticus). Aquac. Res. 2017, 48, 37–46. [Google Scholar] [CrossRef]
- Xu, Q.; Luo, K.; Zhang, S.; Gao, W.; Zhang, W.; Wei, Q. Sequence analysis and characterization of type I interferon and type II interferon from the critically endangered sturgeon species, A. dabryanus and A. sinensis. Fish Shellfish Immunol. 2019, 84, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Di, J.; Han, P.; Zhang, S.; Xia, L.; Tian, G.; Zhanga, W.; Dun, D.; Xu, Q.; Wei, Q. Transcriptome analysis of the critically endangered Dabry’s sturgeon (Acipenser dabryanus) head kidney response to Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 83, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Machałowski, T.; Jesionowski, T. Hemolymph of molluscan origin: From biochemistry to modern biomaterials science. Appl. Phys. A 2020, 127, 3. [Google Scholar] [CrossRef]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Lee, M.-H.; Shiau, S.-Y. Dietary vitamin C and its derivatives affect immune responses in grass shrimp, Penaeus monodon. Fish Shellfish Immunol. 2002, 12, 119–129. [Google Scholar] [CrossRef]
- OrtuÑO, J.; Esteban, M.A.; Meseguer, J. Effect of high dietary intake of vitamin C on non-specific immune response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 1999, 9, 429–443. [Google Scholar] [CrossRef]
- Abdo, S.E.; Gewaily, M.S.; Abo-Al-Ela, H.G.; Almeer, R.; Soliman, A.A.; Elkomy, A.H.; Dawood, M.A.O. Vitamin C rescues inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Environ. Sci. Pollut. Res. 2021, 28, 28750–28763. [Google Scholar] [CrossRef]
- Kong, F.; Zhu, Y.; Yu, H.; Wang, X.; Azm, F.R.A.; Yuan, J.; Tan, Q. Effect of dietary vitamin C on the growth performance, nonspecific immunity and antioxidant ability of red swamp crayfish (Procambarus clarkii). Aquaculture 2021, 541, 736785. [Google Scholar] [CrossRef]
- Rodrigues, R.A.; da Silva Nunes, C.; Fantini, L.E.; Kasai, R.Y.D.; Oliveira, C.A.L.; Hisano, H.; de Campos, C.M. Dietary ascorbic acid influences the intestinal morphology and hematology of hybrid sorubim catfish (Pseudoplatystoma reticulatum × P. corruscans). Aquac. Int. 2017, 26, 1–11. [Google Scholar]
- Alexander, J.B.; Ingram, G.A. Noncellular nonspecific defence mechanisms of fish. Annu. Rev. Fish. Dis. 1992, 2, 249–279. [Google Scholar] [CrossRef]
- Holland, M.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Ye, X.; Liu, D.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019, 274, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, L.; Wang, H.; Xie, F.; Wang, T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol. 2012, 32, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-C. Influence of Dietary Ascorbic Acid on the Immune Responses of Juvenile Korean Rockfish Sebastes schlegelii. J. Aquat. Anim. Health 2015, 27, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Huang, X.; Chen, N.; Apraku, A.; Wang, W.; Cornel, A.; Rahman, M.M. Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2020, 17, 100383. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Rauta, P.R.; Samanta, M.; Dash, H.R.; Nayak, B.; Das, S. Toll-like receptors (TLRs) in aquatic animals: Signaling pathways, expressions and immune responses. Immunol. Lett. 2014, 158, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Seki, E.; Brenner, D.A. Toll-like receptor signaling in the liver. Gastroenterology 2006, 130, 1886–1900. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Akira, S. Toll-like Receptor Signaling. J. Biol. Chem. 2003, 4, 38105–38108. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Sasai, M.; Yamamoto, M. Pathogen Recognition Receptors: Ligands and Signaling Pathways by Toll-Like Receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Kho, D.T.; Wiltshire, R.; Nelson, V.; Rotimi, O.; Johnson, R.; Angel, C.E.; Graham, E.S. Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. NeuroInflamm. 2015, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-J.; Xu, S.; He, Z.-H.; Shi, X.-Z.; Zhao, X.-F.; Wang, J.-X. Activation of Toll Pathway Is Different between Kuruma Shrimp and Drosophila. Front. Immunol. 2017, 8, 1151. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Chen, H.; Wei, C.; Chen, N.; Wu, W. Potential protection of vitamin C against liver-lesioned mice. Int. Immunopharmacol. 2014, 22, 492–497. [Google Scholar] [CrossRef]
- Liang, T.; Chen, X.; Su, M.; Chen, H.; Lu, G.; Liang, K. Vitamin C exerts beneficial hepatoprotection against Concanavalin A-induced immunological hepatic injury in mice through inhibition of NF-kappaB signal pathway. Food Funct. 2014, 5, 2175–2182. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Y.; Wang, L.; Wang, Y.; Zhao, X.; He, W.; Zhang, P.; Yang, X.; Liu, X.; Tian, L.; et al. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-kappaB pathway. Int. Immunopharmacol. 2015, 28, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Priyathilakaa, T.T.; Bathigeb, S.D.N.K.; Leea, S.; Namc, B.-H.; Lee, J. Transcriptome-wide identification, functional characterization, and expression analysis of two novel invertebrate-type Toll-like receptors from disk abalone (Haliotis discus discus). Fish Shellfish Immunol. 2019, 84, 802–815. [Google Scholar] [CrossRef]
- Takeshita, F.; Ishii, K.J.; Kobiyama, K.; Kojima, Y.; Coban, C.; Sasaki, S.; Ishii, N.; Klinman, D.M.; Okuda, K.; Akira, S.; et al. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur. J. Immunol. 2005, 35, 2477–2485. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Bollrath, J.; Greten, F.R. IKK/NF-kappaB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals 2014, 7, 265–310. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; De Moro, G.; Manfrin, C.; Venier, P.; Pallavicini, A. Big defensins and mytimacins, new AMP families of the Mediterranean mussel Mytilus galloprovincialis. Dev. Comp. Immunol. 2012, 36, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, N.; Jordan, J.; Paul, S.; Reid, S.; Baenkler, H.-W.; Sonnewald, S.; Bäuerle, T.; Vera, J.; Schett, G.; Bozec, A. The AP-1 Transcription Factor c-Jun Promotes Arthritis by Regulating Cyclooxygenase-2 and Arginase-1 Expression in Macrophages. J. Immunol. 2017, 198, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Buck, M.D.; Flamar, A.L.; Saenz, S.A.; Tait Wojno, E.D.; Yudanin, N.A.; Osborne, L.C.; Hepworth, M.R.; Tran, S.V.; Rodewald, H.R.; et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol. 2016, 17, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wu, B.; Meng, F.; Zhou, Z.; Lu, H.; Zhao, H. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 67, 554–560. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Yuan, R.-M.; Liu, Y.-J.; Yang, H.-J.; Liang, G.-Y.; Tian, L.-X. Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2015, 435, 431–436. [Google Scholar] [CrossRef]
- Liang, X.-P.; Li, Y.; Hou, Y.-M.; Qiu, H.; Zhou, Q.-C. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac. Res. 2017, 48, 149–160. [Google Scholar] [CrossRef]
- Hashimoto, K. Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective. Front. Pharm. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Trenti, A.; Grumati, P.; Cusinato, F.; Orso, G.; Bonaldo, P.; Trevisi, L. Cardiac glycoside ouabain induces autophagic cell death in non-small cell lung cancer cells via a JNK-dependent decrease of Bcl-2. Biochem. Pharmacol. 2014, 89, 197–209. [Google Scholar] [CrossRef]
- Kim, B.J.; Ryu, S.W.; Song, B.J. JNK- and p38 Kinase-mediated Phosphorylation of Bax Leads to Its Activation and Mitochondrial Translocation and to Apoptosis of Human Hepatoma HepG2 Cells. J. Biol. Chem. 2006, 281, 21256–21265. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; Yamaguchi, A.A.; Aikawa, J.; Yamazaki, R.K.; de Brito, G.A.; Fernandes, L.C. Fish oil administration mediates apoptosis of Walker 256 tumor cells by modulation of p53, Bcl-2, caspase-7 and caspase-3 protein expression. Lipids Health Dis. 2015, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; Nardo, D.D.; Gao, W.; Vince, A.J.; Hall, C.; Mcarthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Liang, H.-Y.; Luo, S.-W.; Wang, A.-L.; Ye, C.-X. The protective effects of vitamin C on apoptosis, DNA damage and proteome of pufferfish (Takifugu obscurus) under low temperature stress. J. Biol. 2018, 71, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Feidantsis, K.; Georgoulis, I.; Giantsis, I.A.; Michaelidis, B. Treatment with ascorbic acid normalizes the aerobic capacity, antioxidant defence, and cell death pathways in thermally stressed Mytilus galloprovincialis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 255, 110611. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | Content (g/100 g, Dry Matter) |
|---|---|
| Casein (vitamin-free) a | 25.00 |
| Gelatin b | 6.00 |
| SO/MFO (1:1) c | 3.50 |
| Dextrin b | 33.50 |
| Vitamin mix d | 2.00 |
| Mineral mix e | 4.50 |
| Sodium alginate b | 20.00 |
| Choline chloride b | 0.50 |
| Carboxymethyl cellulose b | 5.00 |
| Proximate analysis (dry matter) | |
| Crude protein (%) | 31.25 |
| Crude lipid (%) | 3.42 |
| Ash (%) | 10.67 |
| Group | Initial Weight (g) | Initial Shell Length (mm) | WGR (%) | DISL (μm/Day) | SR (%) |
|---|---|---|---|---|---|
| AA0 | 12.02 ± 0.003 | 48.40 ± 0.53 | 30.38 ± 0.11 | 18.23 ± 3.05 | 84.44 ± 2.22 |
| AA50 | 12.01 ± 0.004 | 48.40 ± 0.07 | 31.11 ± 0.33 | 18.67 ± 0.96 | 85.19 ± 0.74 |
| AA100 | 12.01 ± 0.003 | 48.52 ± 0.3 | 31.52 ± 2.06 | 18.81 ± 2.07 | 82.96 ± 5.19 |
| AA200 | 12.01 ± 0.002 | 48.66 ± 0.24 | 30.06 ± 0.87 | 19.48 ± 0.24 | 80.74 ± 0.74 |
| AA500 | 12.02 ± 0.007 | 48.43 ± 0.23 | 30.27 ± 1.43 | 17.92 ± 1.61 | 88.15 ± 1.48 |
| AA1000 | 12.01 ± 0.001 | 48.19 ± 0.21 | 29.32 ± 1.73 | 20.01 ± 2.38 | 88.15 ± 1.96 |
| AA5000 | 12.01 ± 0.003 | 48.49 ± 0.33 | 29.48 ± 1.4 | 19.01 ± 2.68 | 82.96 ± 2.67 |
| Group | THC (×107 Cells/mL) | RB (OD630/107 Cells·mL−1) | PA (%) |
|---|---|---|---|
| AA0 | 1.07 ± 0.05 d | 0.17 ± 0.012 c | 27.11 ± 2.91 c |
| AA50 | 1.09 ± 0.06 d | 0.18 ± 0.009 c | 29.96 ± 1.45 c |
| AA100 | 1.11 ± 0.05 d | 0.17 ± 0.007 c | 30.92 ± 3.76 c |
| AA200 | 1.23 ± 0.02 cd | 0.20 ± 0.012 c | 35.87 ± 1.62 bc |
| AA500 | 1.37 ± 0.01 bc | 0.27 ± 0.009 b | 38.43 ± 2.06 abc |
| AA1000 | 1.65 ± 0.06 a | 0.35 ± 0.013 a | 46.42 ± 2.73 ab |
| AA5000 | 1.60 ± 0.08 ab | 0.32 ± 0.010 a | 48.92 ± 2.65 a |
| Group | LZM (U/mL) | C3 (mg/L) | C4 (mg/L) |
|---|---|---|---|
| AA0 | 22.92 ± 2.08 c | 27.56 ± 2.77 | 51.720 ± 3.390 |
| AA50 | 21.67 ± 1.82 c | 35.01 ± 3.40 | 54.960 ± 0.950 |
| AA100 | 20.83 ± 2.08 c | 38.79 ± 3.29 | 54.070 ± 3.600 |
| AA200 | 33.33 ± 2.08 bc | 41.77 ± 7.63 | 56.390 ± 0.480 |
| AA500 | 37.50 ± 3.61 b | 41.65 ± 3.31 | 53.660 ± 4.960 |
| AA1000 | 56.25 ± 3.61 a | 48.46 ± 5.93 | 56.420 ± 2.540 |
| AA5000 | 52.08 ± 4.17 a | 41.82 ± 8.68 | 57.840 ± 1.270 |
| Group | T-AOC (μmol/mL) | SOD (U/mL) | CAT (U/mL) | GSH (μmol/L) | GPX (U/mL) | MDA (nmol/mL) |
|---|---|---|---|---|---|---|
| AA0 | 0.34 ± 0.01 c | 8.89 ± 0.06 c | 1.51 ± 0.20 c | 3.86 ± 0.15 d | 2.14 ± 0.11 c | 3.70 ± 0.33 a |
| AA50 | 0.33 ± 0.00 c | 8.83 ± 0.12 c | 1.37 ± 0.15 c | 4.57 ± 0.22 cd | 1.98 ± 0.07 c | 3.80 ± 0.37 a |
| AA100 | 0.34 ± 0.02 c | 10.64 ± 1.02 bc | 1.75 ± 0.41 bc | 5.28 ± 0.19 cd | 2.02 ± 0.08 c | 3.84 ± 0.20 a |
| AA200 | 0.34 ± 0.01 bc | 11.12 ± 0.89 abc | 2.68 ± 0.10 ab | 5.66 ± 0.40 c | 2.39 ± 0.11 bc | 3.70 ± 0.20 a |
| AA500 | 0.39 ± 0.02 abc | 12.36 ± 0.29 ab | 2.80 ± 0.20 ab | 7.80 ± 0.19 b | 3.09 ± 0.29 b | 3.38 ± 0.09 ab |
| AA1000 | 0.41 ± 0.01 a | 13.38 ± 0.44 ab | 3.68 ± 0.23 a | 10.27 ± 0.59 a | 4.28 ± 0.27 a | 2.36 ± 0.08 b |
| AA5000 | 0.40 ± 0.01 ab | 13.54 ± 0.44 a | 3.74 ± 0.20 a | 9.60 ± 0.54 a | 4.24 ± 0.11 a | 2.41 ± 0.28 b |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 |
|---|---|---|---|---|---|---|---|
| tlr2 | 1.29 ± 0.08 a | 0.91 ± 0.03 ab | 0.78 ± 0.06 abc | 0.70 ± 0.10 bc | 0.66 ± 0.10 bc | 0.53 ± 0.09 c | 0.60 ± 0.02 bc |
| tlr4 | 0.90 ± 0.09 a | 0.71 ± 0.08 ab | 0.71 ± 0.06 ab | 0.69 ± 0.06 b | 0.64 ± 0.03 bc | 0.47 ± 0.03 cd | 0.43 ± 0.06 d |
| tlr-a | 1.22 ± 0.09 b | 1.57 ± 0.24 ab | 1.70 ± 0.29 ab | 1.77 ± 0.10 ab | 2.05 ± 0.34 a | 2.18 ± 0.17 a | 2.20 ± 0.19 a |
| tlr-b | 1.07 ± 0.12 | 1.03 ± 0.09 | 0.98 ± 0.12 | 0.91 ± 0.08 | 0.93 ± 0.10 | 0.94 ± 0.05 | 1.04 ± 0.07 |
| myd88 | 0.99 ± 0.08 a | 0.98 ± 0.06 a | 0.93 ± 0.04 a | 0.90 ± 0.09 a | 0.67 ± 0.08 b | 0.47 ± 0.06 bc | 0.43 ± 0.04 c |
| tram | 1.03 ± 0.10 a | 1.00 ± 0.15 ab | 0.92 ± 0.10 ab | 0.95 ± 0.12 ab | 0.82 ± 0.09 ab | 0.65 ± 0.11 b | 0.78 ± 0.05 ab |
| irak4 | 1.11 ± 0.10 a | 0.90 ± 0.09 ab | 0.88 ± 0.09 ab | 0.88 ± 0.10 ab | 0.67 ± 0.06 bc | 0.65 ± 0.06 bc | 0.61 ± 0.06 c |
| traf6 | 0.97 ± 0.03 ab | 0.98 ± 0.08 a | 0.71 ± 0.07 bc | 0.69 ± 0.06 c | 0.61 ± 0.03 c | 0.52 ± 0.05 c | 0.60 ± 0.04 c |
| traf4 | 1.00 ± 0.10 | 0.97 ± 0.07 | 1.01 ± 0.07 | 1.16 ± 0.13 | 0.96 ± 0.07 | 0.96 ± 0.11 | 0.94 ± 0.10 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 |
|---|---|---|---|---|---|---|---|
| ikkα | 0.88 ± 0.08 | 0.83 ± 0.05 | 0.82 ± 0.08 | 0.84 ± 0.05 | 0.70 ± 0.06 | 0.70 ± 0.08 | 0.73 ± 0.06 |
| iκbα | 1.13 ± 0.06 | 1.07 ± 0.08 | 1.20 ± 0.07 | 1.00 ± 0.09 | 1.06 ± 0.04 | 1.14 ± 0.06 | 0.97 ± 0.07 |
| p38 mapk | 1.05 ± 0.13 a | 0.95 ± 0.07 ab | 0.93 ± 0.01 ab | 0.92 ± 0.09 ab | 0.75 ± 0.08 bc | 0.54 ± 0.05 c | 0.57 ± 0.02 c |
| jnk | 0.98 ± 0.04 a | 0.88 ± 0.06 ab | 0.92 ± 0.05 a | 0.81 ± 0.08 ab | 0.70 ± 0.06 bc | 0.48 ± 0.04 d | 0.59 ± 0.06 cd |
| nf-κb | 0.91 ± 0.07 a | 0.91 ± 0.05 a | 0.92 ± 0.03 a | 0.73 ± 0.05 ab | 0.70 ± 0.04 b | 0.58 ± 0.05 b | 0.70 ± 0.11 b |
| ap-1 | 1.25 ± 0.08 a | 0.95 ± 0.06 b | 0.93 ± 0.02 b | 0.89 ± 0.07 b | 0.91 ± 0.06 b | 0.70 ± 0.05 c | 0.74 ± 0.07 b |
| tnf-α | 1.07 ± 0.06 a | 0.88 ± 0.03 ab | 0.76 ± 0.12 ab | 0.81 ± 0.16 ab | 0.66 ± 0.11 b | 0.60 ± 0.07 b | 0.65 ± 0.07 b |
| il 16 | 0.82 ± 0.02 | 0.88 ± 0.04 | 0.81 ± 0.04 | 0.84 ± 0.06 | 0.78 ± 0.08 | 0.86 ± 0.10 | 0.84 ± 0.04 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 |
|---|---|---|---|---|---|---|---|
| tlr2 | 0.91 ± 0.09 | 0.83 ± 0.08 | 0.99 ± 0.06 | 0.89 ± 0.05 | 0.88 ± 0.07 | 0.96 ± 0.11 | 0.93 ± 0.10 |
| tlr4 | 1.04 ± 0.06 | 0.92 ± 0.09 | 1.05 ± 0.10 | 0.87 ± 0.09 | 0.75 ± 0.08 | 0.76 ± 0.07 | 0.74 ± 0.05 |
| tlr-a | 0.75 ± 0.06 a | 0.66 ± 0.04 ab | 0.52 ± 0.05 c | 0.54 ± 0.02 bc | 0.42 ± 0.02 cd | 0.38 ± 0.03 d | 0.38 ± 0.03 d |
| tlr-b | 1.10 ± 0.08 | 1.02 ± 0.08 | 1.18 ± 0.06 | 1.19 ± 0.10 | 1.08 ± 0.08 | 1.14 ± 0.08 | 1.06 ± 0.10 |
| myd88 | 0.92 ± 0.09 a | 0.91 ± 0.06 ab | 0.90 ± 0.09 ab | 0.85 ± 0.10 ab | 0.63 ± 0.10 ab | 0.57 ± 0.12 b | 0.62 ± 0.09 ab |
| tram | 1.09 ± 0.08 | 1.00 ± 0.06 | 0.99 ± 0.04 | 1.00 ± 0.07 | 1.03 ± 0.07 | 0.99 ± 0.06 | 1.09 ± 0.06 |
| irak4 | 0.97 ± 0.04 a | 0.86 ± 0.08 ab | 0.85 ± 0.04 ab | 0.78 ± 0.03 abc | 0.64 ± 0.09 c | 0.64 ± 0.05 c | 0.75 ± 0.02 bc |
| traf6 | 1.06 ± 0.05 a | 0.92 ± 0.08 a | 0.94 ± 0.06 a | 0.94 ± 0.08 a | 0.84 ± 0.13 ab | 0.65 ± 0.06 b | 0.67 ± 0.06 b |
| traf4 | 1.33 ± 0.09 | 1.25 ± 0.13 | 1.26 ± 0.09 | 1.32 ± 0.09 | 1.37 ± 0.09 | 1.32 ± 0.10 | 1.31 ± 0.12 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 |
|---|---|---|---|---|---|---|---|
| ikkα | 0.99 ± 0.08 a | 0.83 ± 0.08 ab | 0.87 ± 0.08 ab | 0.73 ± 0.07 bc | 0.68 ± 0.05 bcd | 0.49 ± 0.04 d | 0.54 ± 0.05 cd |
| iκbα | 1.07 ± 0.01 | 0.92 ± 0.04 | 0.97 ± 0.07 | 0.95 ± 0.05 | 1.09 ± 0.08 | 0.97 ± 0.09 | 0.99 ± 0.08 |
| p38 mapk | 1.01 ± 0.11 | 0.92 ± 0.08 | 0.91 ± 0.09 | 0.96 ± 0.04 | 0.79 ± 0.08 | 0.80 ± 0.09 | 0.74 ± 0.08 |
| jnk | 1.06 ± 0.06 | 0.95 ± 0.06 | 0.98 ± 0.05 | 0.87 ± 0.05 | 0.89 ± 0.06 | 0.90 ± 0.06 | 0.98 ± 0.08 |
| nf-κb | 1.08 ± 0.05 a | 0.97 ± 0.06 ab | 1.02 ± 0.07 ab | 0.86 ± 0.05 bc | 0.77 ± 0.07 c | 0.71 ± 0.03 c | 0.75 ± 0.09 c |
| ap-1 | 0.98 ± 0.05 a | 0.94 ± 0.03 ab | 0.83 ± 0.07 ab | 0.80 ± 0.05 ab | 0.90 ± 0.03 ab | 0.74 ± 0.05 b | 0.84 ± 0.11 ab |
| tnf-α | 1.00 ± 0.02 a | 0.88 ± 0.07 ab | 0.92 ± 0.06 ab | 0.82 ± 0.07 abc | 0.83 ± 0.12 abc | 0.62 ± 0.03 c | 0.69 ± 0.06 bc |
| il 16 | 1.01 ± 0.06 | 0.94 ± 0.14 | 1.07 ± 0.06 | 0.93 ± 0.08 | 1.06 ± 0.09 | 0.93 ± 0.08 | 0.90 ± 0.08 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 | |
|---|---|---|---|---|---|---|---|---|
| Digestive gland | β-defensin | 1.77 ± 0.09 c | 2.06 ± 0.07 bc | 2.10 ± 0.17 bc | 2.02 ± 0.19 bc | 2.51 ± 0.22 b | 3.28 ± 0.44 a | 3.13 ± 0.10 a |
| mytimacin 6 | 1.09 ± 0.10 | 1.10 ± 0.08 | 1.02 ± 0.10 | 1.14 ± 0.10 | 1.12 ± 0.09 | 1.16 ± 0.16 | 1.01 ± 0.10 | |
| arginase-I | 1.34 ± 0.10 c | 1.99 ± 0.14 bc | 2.15 ± 0.21 b | 2.02 ± 0.18 b | 3.25 ± 0.25 a | 2.67 ± 0.25 ab | 2.60 ± 0.26 ab | |
| Gill | β-defensin | 2.45 ± 0.11 | 2.37 ± 0.25 | 2.94 ± 0.22 | 2.92 ± 0.19 | 3.01 ± 0.12 | 2.97 ± 0.41 | 3.00 ± 0.29 |
| mytimacin 6 | 1.71 ± 0.13 c | 1.94 ± 0.15 c | 1.88 ± 0.19 c | 2.08 ± 0.12 bc | 2.69 ± 0.21 ab | 2.75 ± 0.24 a | 2.83 ± 0.36 a | |
| arginase-I | 1.98 ± 0.19 | 1.89 ± 0.14 | 1.87 ± 0.19 | 1.81 ± 0.16 | 1.85 ± 0.12 | 1.92 ± 0.19 | 1.87 ± 0.14 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 | |
|---|---|---|---|---|---|---|---|---|
| Digestive gland | nrf2 | 0.92 ± 0.09 d | 1.11 ± 0.11 cd | 1.58 ± 0.17 bc | 1.96 ± 0.25 ab | 2.13 ± 0.24 a | 2.21 ± 0.16 a | 2.36 ± 0.20 a |
| keap1 | 1.03 ± 0.09 a | 1.03 ± 0.07 a | 1.06 ± 0.08 a | 0.88 ± 0.11 ab | 0.77 ± 0.05 ab | 0.59 ± 0.08 b | 0.62 ± 0.06 b | |
| gpx | 1.04 ± 0.15 c | 1.07 ± 0.13 c | 1.11 ± 0.09 c | 1.41 ± 0.12 bc | 1.64 ± 0.12 ab | 1.91 ± 0.18 a | 1.99 ± 0.20 a | |
| cat | 1.07 ± 0.11 c | 1.06 ± 0.09 c | 1.29 ± 0.07 c | 1.51 ± 0.19 bc | 1.89 ± 0.17 ab | 2.04 ± 0.20 a | 2.01 ± 0.23 ab | |
| gst | 1.26 ± 0.09 | 1.08 ± 0.02 | 1.08 ± 0.14 | 1.20 ± 0.06 | 1.07 ± 0.12 | 1.15 ± 0.11 | 1.15 ± 0.10 | |
| cuznsod | 1.18 ± 0.05 b | 1.17 ± 0.10 b | 1.14 ± 0.04 b | 1.10 ± 0.02 b | 1.26 ± 0.14 ab | 1.51 ± 0.11 a | 1.37 ± 0.05 ab | |
| Gill | nrf2 | 1.08 ± 0.06 b | 1.00 ± 0.11 b | 1.05 ± 0.10 b | 1.01 ± 0.02 b | 1.25 ± 0.20 ab | 1.54 ± 0.16 a | 1.54 ± 0.17 a |
| keap1 | 1.05 ± 0.04 | 1.08 ± 0.09 | 0.99 ± 0.08 | 0.98 ± 0.08 | 0.93 ± 0.14 | 0.78 ± 0.04 | 0.76 ± 0.06 | |
| gpx | 1.01 ± 0.15 b | 0.99 ± 0.14 b | 1.13 ± 0.10 b | 1.12 ± 0.08 b | 1.44 ± 0.22 ab | 1.85 ± 0.10 a | 1.89 ± 0.19 a | |
| cat | 1.14 ± 0.06 | 1.15 ± 0.17 | 1.15 ± 0.05 | 1.22 ± 0.18 | 1.25 ± 0.08 | 1.49 ± 0.08 | 1.33 ± 0.11 | |
| gst | 1.04 ± 0.15 | 1.04 ± 0.08 | 1.19 ± 0.09 | 1.18 ± 0.07 | 1.16 ± 0.05 | 1.40 ± 0.19 | 1.21 ± 0.04 | |
| cuznsod | 1.07 ± 0.05 | 1.27 ± 0.12 | 1.27 ± 0.06 | 1.24 ± 0.08 | 1.20 ± 0.05 | 1.13 ± 0.09 | 1.16 ± 0.08 |
| Gene | AA0 | AA50 | AA100 | AA200 | AA500 | AA1000 | AA5000 | |
|---|---|---|---|---|---|---|---|---|
| Digestive gland | bax | 1.03 ± 0.06 a | 0.92 ± 0.12 ab | 0.95 ± 0.01 ab | 0.85 ± 0.10 abc | 0.68 ± 0.06 c | 0.66 ± 0.04 c | 0.74 ± 0.04 bc |
| caspase3 | 1.27 ± 0.06 a | 1.06 ± 0.08 ab | 1.17 ± 0.08 a | 1.00 ± 0.05 abc | 0.76 ± 0.14 bc | 0.64 ± 0.09 c | 0.65 ± 0.14 c | |
| caspase7 | 1.23 ± 0.08 | 1.18 ± 0.09 | 1.23 ± 0.11 | 1.17 ± 0.09 | 1.18 ± 0.13 | 1.09 ± 0.10 | 1.24 ± 0.04 | |
| bcl-2 | 0.59 ± 0.09 d | 0.64 ± 0.13 cd | 0.81 ± 0.05 cd | 1.17 ± 0.07 bc | 1.71 ± 0.16 ab | 1.75 ± 0.13 a | 1.50 ± 0.13 ab | |
| Gill | bax | 0.95 ± 0.07 | 0.83 ± 0.05 | 0.90 ± 0.05 | 0.91 ± 0.05 | 0.94 ± 0.09 | 0.73 ± 0.07 | 0.76 ± 0.05 |
| caspase3 | 1.30 ± 0.06 | 1.32 ± 0.03 | 1.32 ± 0.05 | 1.23 ± 0.11 | 1.17 ± 0.09 | 1.08 ± 0.07 | 1.11 ± 0.06 | |
| caspase7 | 1.08 ± 0.11 a | 0.97 ± 0.10 ab | 0.94 ± 0.09 ab | 0.69 ± 0.06 c | 0.74 ± 0.07 bc | 0.59 ± 0.05 c | 0.63 ± 0.05 c | |
| bcl-2 | 1.00 ± 0.10 bc | 0.90 ± 0.09 c | 1.04 ± 0.06 bc | 0.99 ± 0.08 c | 1.06 ± 0.07 bc | 1.44 ± 0.13 ab | 1.51 ± 0.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, K.; Li, X.; Wang, L.; Rao, W.; Wu, Y.; Liu, Y.; Pan, M.; Huang, D.; Zhang, W.; Mai, K. Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino. Antioxidants 2021, 10, 1449. https://doi.org/10.3390/antiox10091449
Luo K, Li X, Wang L, Rao W, Wu Y, Liu Y, Pan M, Huang D, Zhang W, Mai K. Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino. Antioxidants. 2021; 10(9):1449. https://doi.org/10.3390/antiox10091449
Chicago/Turabian StyleLuo, Kai, Xinxin Li, Liu Wang, Wanxiu Rao, Yang Wu, Yue Liu, Mingzhu Pan, Dong Huang, Wenbing Zhang, and Kangsen Mai. 2021. "Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino" Antioxidants 10, no. 9: 1449. https://doi.org/10.3390/antiox10091449
APA StyleLuo, K., Li, X., Wang, L., Rao, W., Wu, Y., Liu, Y., Pan, M., Huang, D., Zhang, W., & Mai, K. (2021). Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino. Antioxidants, 10(9), 1449. https://doi.org/10.3390/antiox10091449






