Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extract Preparation
2.2. Total Phenolic and Flavonoid Content
2.3. Instrumentation
2.4. Determination of Antioxidant and Enzyme Inhibitory Effects
2.5. Artemia salina Lethality Test
2.6. Cell Culture and Viability Test
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Content
3.2. HPLC-ES-MSn Analysis
3.3. Quantification of Phytochemicals
3.4. Antioxidant Properties
3.5. Enzyme Inhibitory Properties
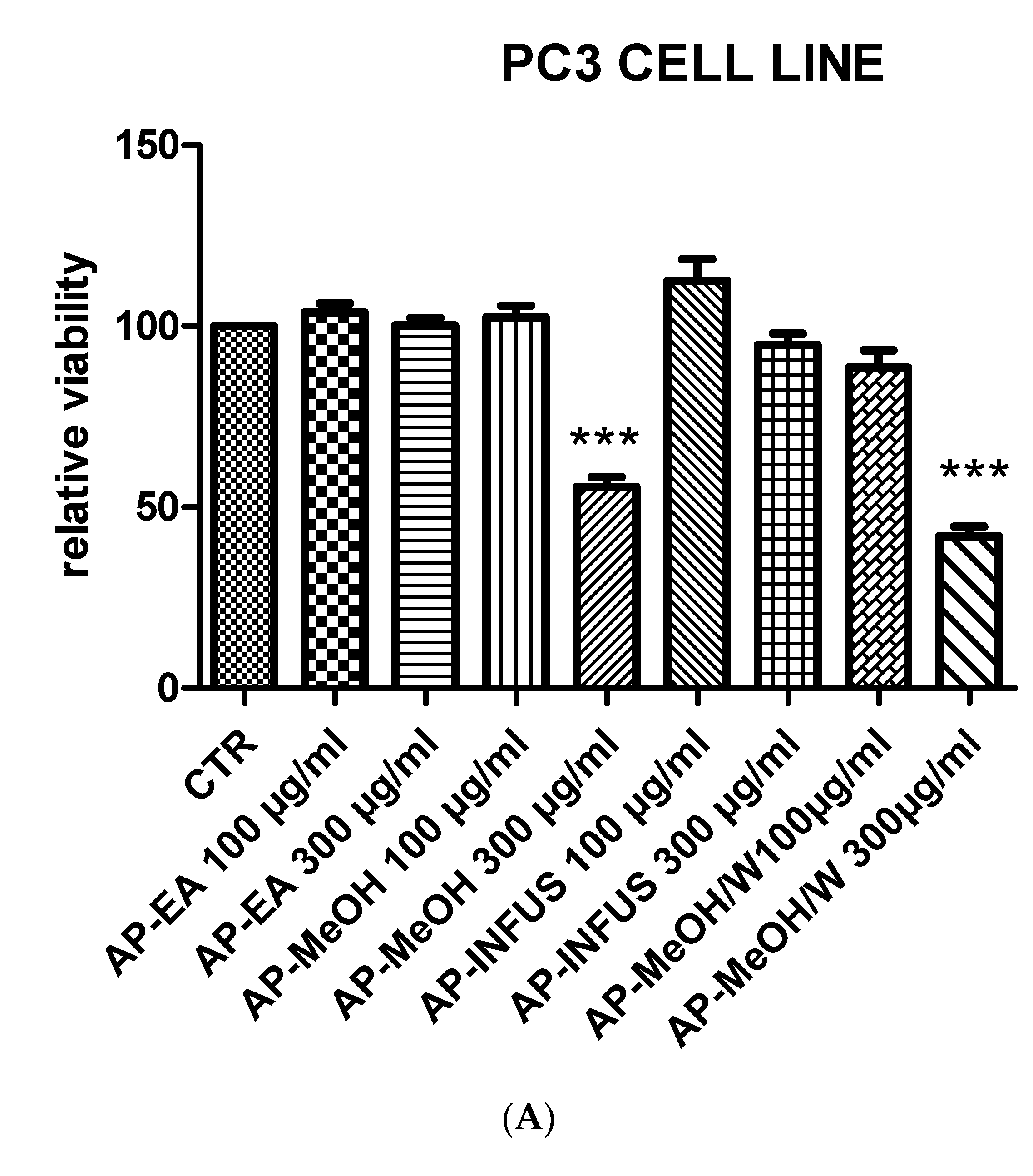
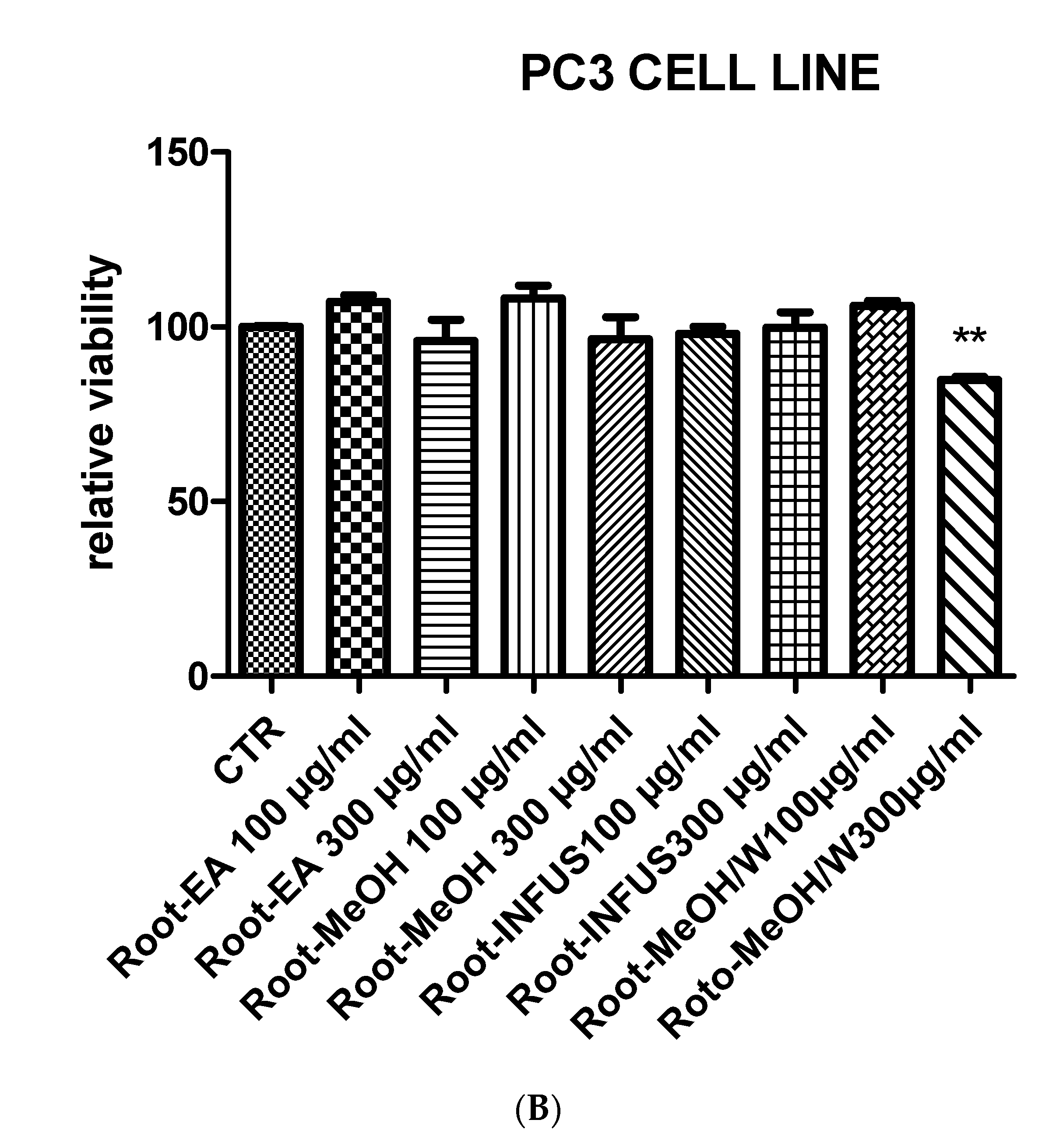
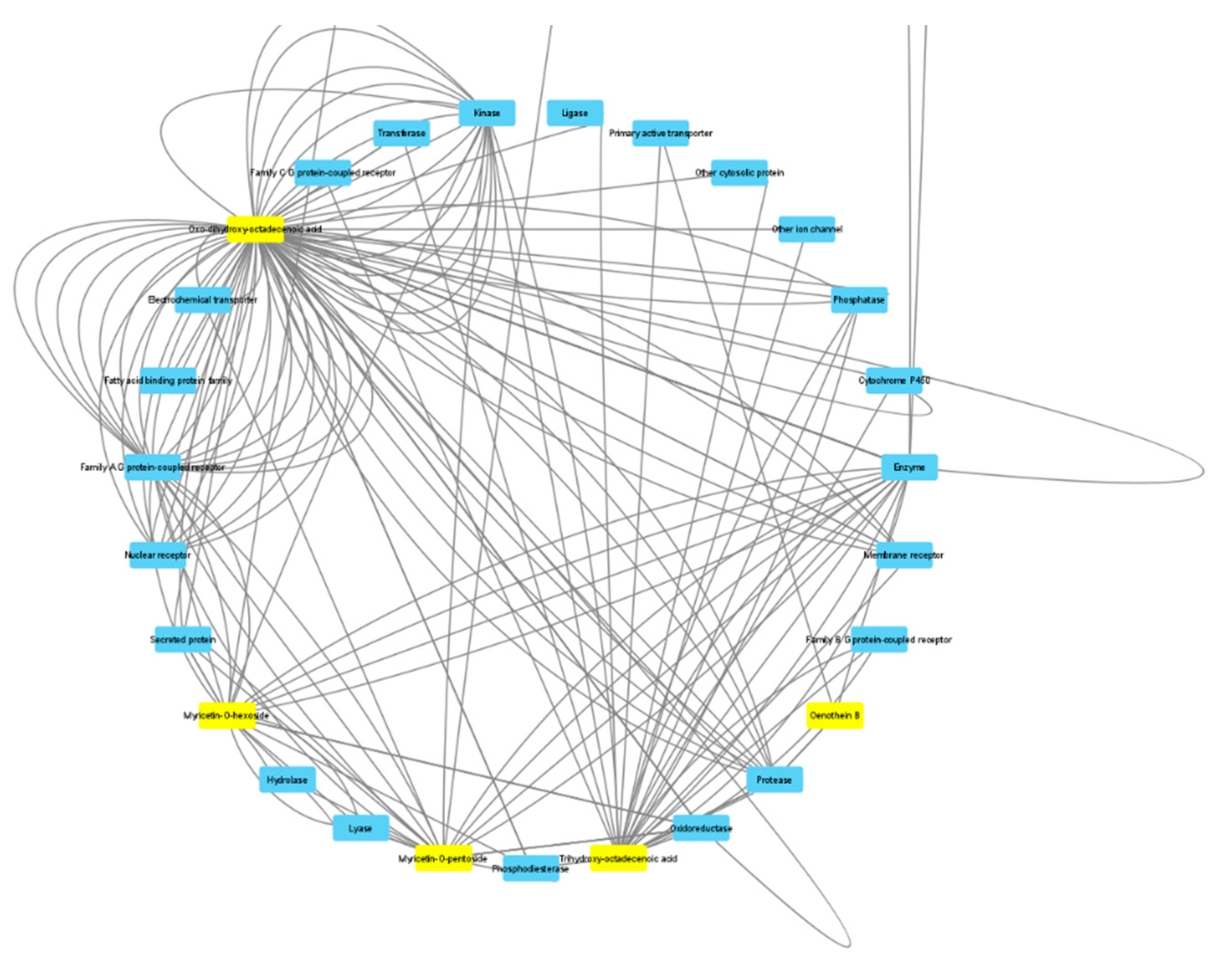
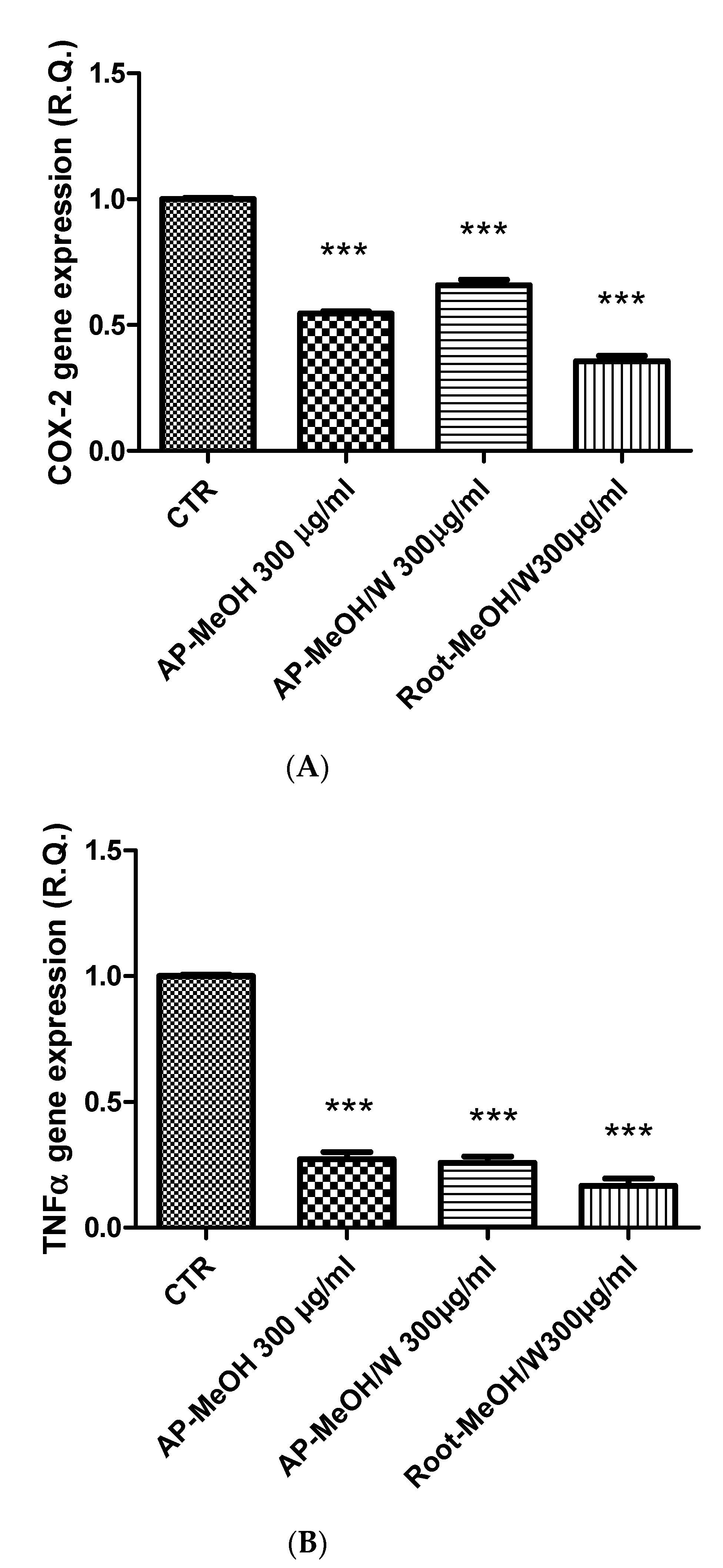
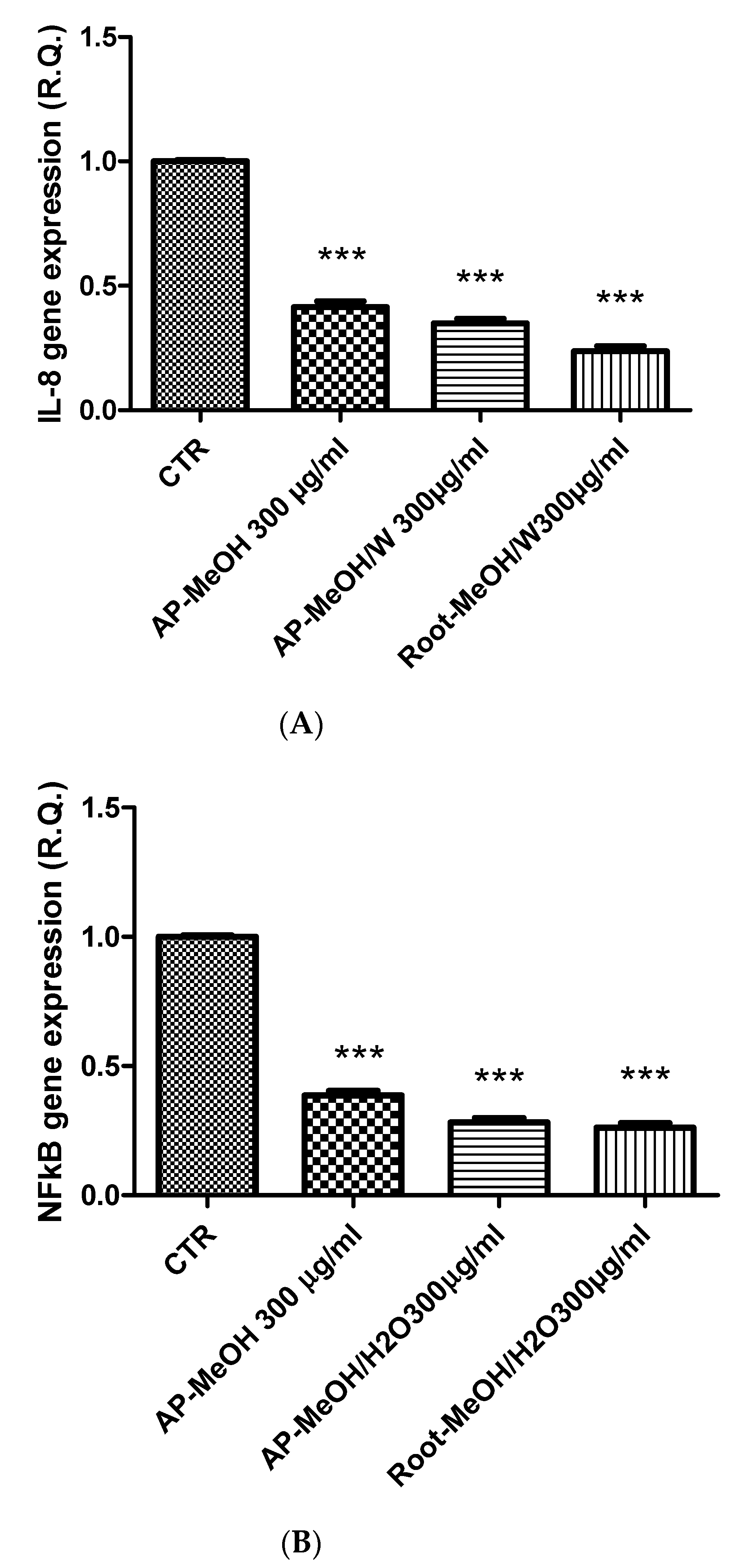
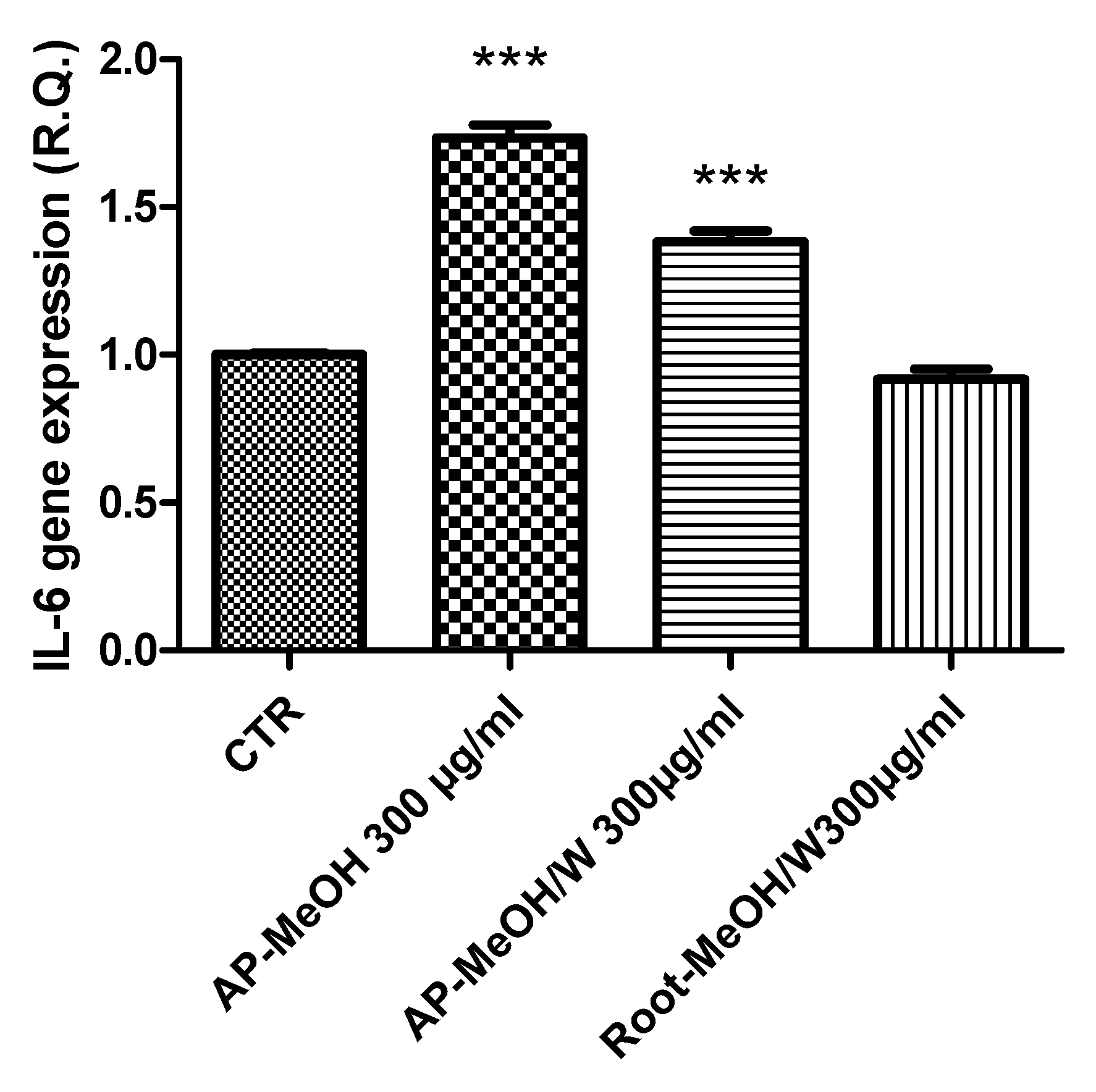
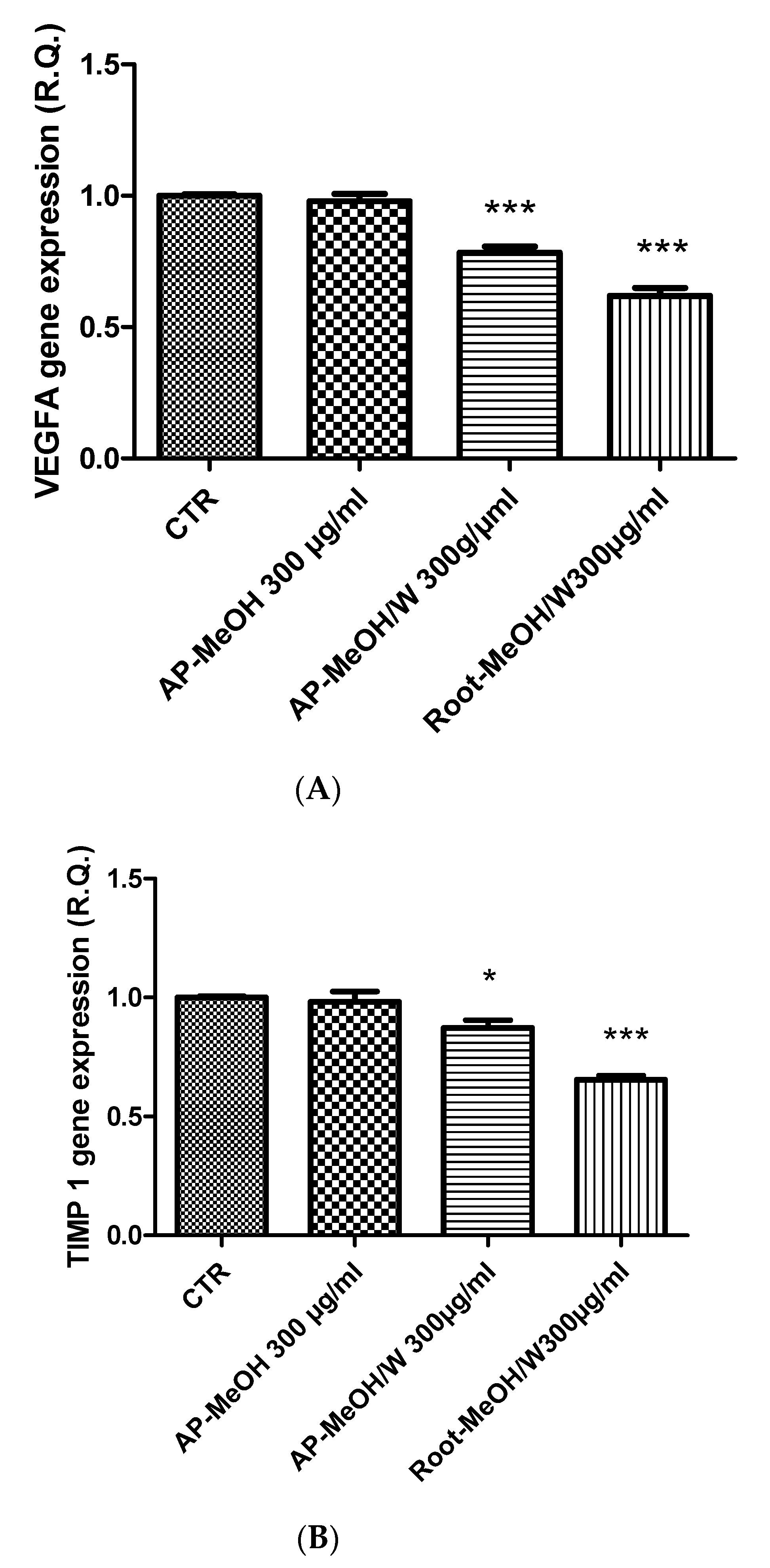
3.6. Toxicological and Pharmacological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkordy, A.A.; Haj-Ahmad, R.R.; Awaad, A.S.; Zaki, R.M. An overview on natural product drug formulations from conventional medicines to nanomedicines: Past, present and future. J. Drug Deliv. Sci. Technol. 2021, 63, 102459. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2020, 209, 112891. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mishra, V.; Hemani, V.; Verma, A.; Jain, A.; Jain, S.; Charyulu, R.N. Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends Food Sci. Technol. 2021, 110, 594–610. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Global documentation of traditionally used medicinal plants in cancer management: A systematic review. S. Afr. J. Bot. 2021, 138, 424–494. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Farzaei, M.H.; Bishayee, A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: A mechanistic review. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Piwowarski, J.; Czerwińska, M.; Kiss, A. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Dzhafar, S.S.; Dalar, A.; Mükemre, M.; Suat, E.; Yildiz, D.; Yunusoğlu, O. Phytochemical Profile and in vitro and in vivo Anticonvulsant and Antioxidant Activities of Epilobium hirsutum. Int. J. Second. Metab. 2020, 7, 63–76. [Google Scholar] [CrossRef]
- Korkmaz, M.; Karakuş, S.; Özçelik, H.; Selvi, S. An ethnobotanical study on medicinal plants in Erzincan, Turkey. Indian J. Tradit. Knowl. 2016, 15, 192–202. [Google Scholar]
- Şahin, Y.E.; Erbay, M.Ş.; Sezin, A.; Kantar, R.; Kültür, Ş.; Melikoğlu, G. Plants used in traditional treatment of prostate diseases in Turkey. İstanbul J. Pharm. 2019, 49, 191–203. [Google Scholar]
- Kawarty, A.M.A.; Behçet, L.; Çakilcioğlu, U. An ethnobotanical survey of medicinal plants in Ballakayati (Erbil, North Iraq). Turk. J. Bot. 2020, 44, 345–357. [Google Scholar] [CrossRef]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207. [Google Scholar] [CrossRef]
- Ege, T.; Gençler Ozkan, A.; Sen, A.; Adalı, O. Effects of folk medicinal plant epilobium hirsutum l. And its ingredient ellagic acid on rat liver bile acid synthesizing cyps in rats. Pharmacol. Online 2018, 3, 200–215. [Google Scholar]
- Sheikh, N.A.; Desai, T.R.; Tirgar, P.R. Evaluation of iron chelating and antioxidant potential of Epilobium hirsutum for the management of iron overload disease. Biomed. Pharmacother. 2017, 89, 1353–1361. [Google Scholar] [CrossRef]
- Sõukand, R.; Mattalia, G.; Kolosova, V.; Stryamets, N.; Prakofjewa, J.; Belichenko, O.; Kuznetsova, N.; Minuzzi, S.; Keedus, L.; Prūse, B.; et al. Inventing a herbal tradition: The complex roots of the current popularity of Epilobium angustifolium in Eastern Europe. J. Ethnopharmacol. 2020, 247, 112254. [Google Scholar] [CrossRef]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B. Gaining momentum: Popularization of Epilobium angustifolium as food and recreational tea on the Eastern edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef] [PubMed]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic profiling, in vitro bioaccessibility and in vivo bioavailability of a commercial bioactive Epilobium angustifolium L. extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Fonte, G.; Saija, A.; Tita, B. Inhibition of intestinal motility and secretion by extracts of Epilobium spp. in mice. J. Ethnopharmacol. 2006, 107, 342–348. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crop. Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Egil, A.C.; Ozdemir, B.; Gok, B.; Kecel-Gunduz, S.; Budama-Kilinc, Y. Synthesis, characterization, biological activities and molecular docking of Epilobium parviflorum aqueous extract loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 161, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukula-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar] [CrossRef]
- Esposito, C.; Santarcangelo, C.; Masselli, R.; Buonomo, G.; Nicotra, G.; Insolia, V.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; et al. Epilobium angustifolium L. extract with high content in oenothein B on benign prostatic hyperplasia: A monocentric, randomized, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111414. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Karonen, M.; Salminen, J.P. Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hevesi Tóth, B.; Blazics, B.; Kéry, A. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, G.; Mariola, D.; Anna, P.; Piotr, K.; Natalia, W.; Aneta, S.; Marcin, O.; Bogna, O.; Zdzisław, Ł.; Aurelia, P.; et al. Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium augustifolium) herb in different harvest times. Ind. Crop. Prod. 2018, 123, 208–220. [Google Scholar] [CrossRef]
- Barakat, H.H.; Hussein, S.A.M.; Marzouk, M.S.; Merfort, I.; Linscheid, M.; Nawwar, M.A.M. Polyphenolic metabolites of Epilobium hirsutum. Phytochemistry 1997, 46, 935–941. [Google Scholar] [CrossRef]
- Bazylko, A.; Kiss, A.K.; Kowalski, J. High-performance thin-layer chromatography method for quantitative determination of oenothein B and quercetin glucuronide in aqueous extract of Epilobii angustifolii herba. J. Chromatogr. A 2007, 1173, 146–150. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic Characterization, Antioxidant Activity, and Enzyme Inhibitory Properties of Berberis thunbergii DC. Leaves: A Valuable Source of Phenolic Acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef] [Green Version]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef] [Green Version]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. A natural formula containing lactoferrin, Equisetum arvensis, soy isoflavones and vitamin D3 modulates bone remodeling and inflammatory markers in young and aged rats. J. Biol. Regul. Homeost. Agents 2016, 30, 985–996. [Google Scholar] [PubMed]
- Pourmorad, F.; Ebrahimzadeh, M.A.; Mahmoudi, M.; Yasini, S. Antinociceptive activity of methanolic extract of Epilobium hirsutum. Pak. J. Biol. Sci. PJBS 2007, 10, 2764–2767. [Google Scholar]
- Herrera Alvarez, L.V.; Zielinski, A.A.F.; Alberti, A.; Nogueira, A. Monitoring of the phenolic compounds and in vitro antioxidant activity of apple beverages according to geographical origin and their type: A chemometric study. LWT 2017, 84, 385–393. [Google Scholar] [CrossRef]
- Dittgen, C.L.; Hoffmann, J.F.; Chaves, F.C.; Rombaldi, C.V.; Filho, J.M.C.; Vanier, N.L. Discrimination of genotype and geographical origin of black rice grown in Brazil by LC-MS analysis of phenolics. Food Chem. 2019, 288, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Beta, T. Discrimination of geographical origin of Napirira bean (Phaseolus vulgaris L.) based on phenolic profiles and antioxidant activity. J. Food Compos. Anal. 2017, 62, 217–222. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Verardo, G.; Duse, I.; Callea, A. Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid. Rapid Commun. Mass Spectrom. 2009, 23, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharm. 2013, 65, 1044–1054. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Myrica faya: A New Source of Antioxidant Phytochemicals. J. Agric. Food Chem. 2014, 62, 9722–9735. [Google Scholar] [CrossRef] [PubMed]
- Gouveia-Figueira, S.C.; Castilho, P.C. Phenolic screening by HPLC–DAD–ESI/MSn and antioxidant capacity of leaves, flowers and berries of Rubus grandifolius Lowe. Ind. Crop. Prod. 2015, 73, 28–40. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In vitro inhibition of the transcription factor NF-κB and cyclooxygenase by bamboo extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Berini, J.L.; Brockman, S.A.; Hegeman, A.D.; Reich, P.B.; Muthukrishnan, R.; Montgomery, R.A.; Forester, J.D. Combinations of abiotic factors differentially alter production of plant secondary metabolites in five woody plant species in the boreal-temperate transition zone. Front. Plant. Sci. 2018, 9, 1257. [Google Scholar] [CrossRef] [Green Version]
- Usenik, V. The influence of the production system on the composition of phytochemicals in Prunus domestica L. fruit. J. Food Compos. Anal. 2021, 95, 103701. [Google Scholar] [CrossRef]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Ghasemi Pirbalouti, A. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crop. Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Ricardez-Miranda, L.E.; Lagunes-Espinoza, L.C.; Hernández-Nataren, E.; Palma-López, D.J.; Conde-Martínez, F.V. Water restriction during the vegetative and reproductive stages of Capsicum annuum var. glabriusculum, and its effect on growth, secondary metabolites and fruit yield. Sci. Hortic. 2021, 285, 110129. [Google Scholar] [CrossRef]
- Lorini, A.; Aranha, B.C.; Antunes, B.d.F.; Otero, D.M.; Jacques, A.C.; Zambiazi, R.C. Metabolic profile of olive leaves of different cultivars and collection times. Food Chem. 2021, 345, 128758. [Google Scholar] [CrossRef]
- Bouterfas, K.; Mehdadi, Z.; Elaoufi, M.M.; Latreche, A.; Benchiha, W. Antioxidant activity and total phenolic and flavonoids content variations of leaves extracts of white Horehound (Marrubium vulgare Linné) from three geographical origins. Ann. Pharm. Françaises 2016, 74, 453–462. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Araújo, T.A.; de Almeida e Castro, V.T.N.; da Silva Solon, L.G.; da Silva, G.A.; das Graças Almeida, M.; da Costa, J.G.M.; de Amorim, E.L.C.; Albuquerque, U.P. Does rainfall affect the antioxidant capacity and production of phenolic compounds of an important medicinal species? Ind. Crop. Prod. 2015, 76, 550–556. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Yeloojeh, K.A.; Saeidi, G.; Sabzalian, M.R. Drought stress improves the composition of secondary metabolites in safflower flower at the expense of reduction in seed yield and oil content. Ind. Crop. Prod. 2020, 154, 112496. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [Green Version]
- WHO. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Jawed, A.; Singh, G.; Kohli, S.; Sumera, A.; Haque, S.; Prasad, R.; Paul, D. Therapeutic role of lipases and lipase inhibitors derived from natural resources for remedies against metabolic disorders and lifestyle diseases. S. Afr. J. Bot. 2019, 120, 25–32. [Google Scholar] [CrossRef]
- Rathod, P.; Yadav, R.P. Anti-diabesity potential of various multifunctional natural molecules. J. Herb. Med. 2021, 27, 100430. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Seetaloo, A.D.; Aumeeruddy, M.Z.; Rengasamy Kannan, R.R.; Mahomoodally, M.F. Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management—A systematic review. S. Afr. J. Bot. 2019, 120, 3–24. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.T.; Zargaham, M.K.; Khan, A.U.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with Reference of Acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Kawakami, K.; Li, P.; Uraji, M.; Hatanaka, T.; Ito, H. Inhibitory Effects of Pomegranate Extracts on Recombinant Human Maltase–Glucoamylase. J. Food Sci. 2014, 79, H1848–H1853. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Potential antihyperglycaemic effect of myricetin derivatives from Syzygium malaccense. J. Funct. Foods 2016, 22, 325–336. [Google Scholar] [CrossRef]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Radulović, N.S.; Mladenović, M.Z.; Randjelovic, P.J.; Stojanović, N.M.; Dekić, M.S.; Blagojević, P.D. Toxic essential oils. Part IV: The essential oil of Achillea falcata L. as a source of biologically/pharmacologically active trans-sabinyl esters. Food Chem. Toxicol. 2015, 80, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.; Jamali, T.; Ardestani, S.K.; Kavoosi, G. Synergistic effect of Zataria Multiflora essential oil on doxorubicin-induced growth inhibition of PC3 cancer cells and apoptosis. Complement. Ther. Clin. Pract. 2021, 42, 101286. [Google Scholar] [CrossRef]
- Eskandari, E.; Heidarian, E.; Amini, S.A.; Saffari-Chaleshtori, J. Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J. Cancer Res. 2016, 12, 1266–1271. [Google Scholar] [CrossRef]
- di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic Profile and Antioxidant/Anti-Inflammatory Effects of Industrial Hemp Water Extract in Fibroblasts, Keratinocytes and Isolated Mouse Skin Specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Verratti, V.; Brunetti, L.; Ferrante, C.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; Wang, R.; Berardinelli, F. Physiological and pathological levels of prostaglandin E2 in renal parenchyma and neoplastic renal tissue. Prostaglandins Other Lipid Mediat. 2019, 141, 11–13. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Hong, S.; Xia, X.; Cao, Y.; McDermott, J.; Mu, Y.; Han, J.J. Immune Cell Types and Secreted Factors Contributing to Inflammation-to-Cancer Transition and Immune Therapy Response. Cell Rep. 2019, 26, 1965–1977. [Google Scholar] [CrossRef] [Green Version]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharm. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell Mol. Life Sci. 2014, 71, 659–672. [Google Scholar] [CrossRef]
- Orlando, G.; Chiavaroli, A.; Ferrante, C.; Recinella, L.; Leone, S.; Brunetti, L.; Di Simone, S.; Menghini, L.; Petrucci, M.; Zengin, G. Protective effects induced by the food supplement Fluxonorm® in the lower urinary tract. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3074–3082. [Google Scholar]


| Parts | Solvents | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|
| Aerial parts | EA | 43.52 ± 1.09 d | 10.49 ± 0.17 d |
| MeOH | 254.55 ± 0.72 a | 87.66 ± 0.38 a | |
| Infusion | 211.37 ± 1.98 b | 27.59 ± 4.17 c | |
| MeOH/Water | 202.97 ± 2.19 c | 41.80 ± 0.66 b | |
| Roots | EA | 32.52 ± 0.40 d | 6.36 ± 0.15 a |
| MeOH | 109.16 ± 0.05 c | 2.54 ± 0.16 c | |
| Infusion | 152.64 ± 1.25 b | 3.71 ± 0.94 b | |
| MeOH/Water | 156.27 ± 0.95 a | 2.41 ± 0.07 c |
| No. | tR (min) | [M-H]− m/z | m/z (% Base Peak) | Assigned Identification | Aerial Parts | Roots |
|---|---|---|---|---|---|---|
| 1 | 1.8 | 377 | MS2 [377]: 341 (100), 179 (55), 161 (23) MS3 [377→341]: 179 (100), 119 (35), 113 (9), 101 (11) | Disaccharide (HCl adduct) | MeOH MEOH:H2O | MeOH MEOH:H2O EA |
| 2 | 1.8 | 195 | MS2 [195]: 177 (41), 159 (15), 129 (100) | Gluconic acid | Inf | Infusion MEOH:H2O |
| 3 | 2.1 | 191 | MS2 [191]: 173 (37), 111 (100) | Isocitric acid | All | -- |
| 4 | 2.3 | 783 [M-2H]2− | MS2 [783]: 935 (26), 765 (100), 698 (6), 633 (3) MS3 [783→765]: 765 (100), 615 (4), 597 (32), 301 (20), 275 (8) | Oenothein B isomer | MeOH MEOH:H2O | MeOH MEOH:H2O EA |
| 5 | 2.6 | 191 | MS2 [191]: 173 (28), 111 (100) | Citric acid | All | İnfusion |
| 6 | 3.1 | 483 | MS2 [483]: 331 (100), 193 (14), 169 (58) MS3 [483→331]: 193 (54), 169 (100) | Digalloylglucose | Inf MEOH:H2O | MeOH İnfusion MEOH:H2O |
| 7 | 3.3 | 169 | MS2 [169]: 125 (100) | Gallic acid | All | All |
| 8 | 3.8 | 483 | MS2 [483]: 331 (100), 169 (52) MS3 [483→331]: 193 (100), 169 (100) | Digalloylglucose | MeOH Inf MEOH:H2O | MeOH İnfusion MEOH:H2O |
| 9 | 5.3 | 299 | MS2 [299]: 179 (100), 161 (38), 143 (44), 119 (98), 113 (44), 101 (56) | Unknown | All | -- |
| 10 | 6.0 | 311 | MS2 [311]: 179 (54), 149 (100), 135 (6) | Caftaric acid | MeOH Inf MEOH:H2O | -- |
| 11 | 7.7 | 783 [M-2H]2− | MS2 [783]: 935 (25), 765 (100), 698 (7) MS3 [783→765]: 765 (100), 597 (14), 301 (16) | Oenothein B isomer | MeOH Inf MEOH:H2O | All |
| 12 | 8.8 | 163 | MS2 [163]: 119 (100) | Coumaric acid | MeOH Inf MEOH:H2O | Inf |
| 13 | 10.3 | 325 | MS2 [325]: 193 (100) MS3 [325→193]: 178 (69), 149 (100), 134 (57) | Ferulic acid pentoside | MeOH Inf MEOH:H2O | MeOH Inf MEOH:H2O |
| 14 | 10.5 | 431 | MS2 [431]: 385 (100), 223 (11) MS3 [431→385]: 223 (82), 205 (45), 179 (16), 161 (36), 153 (100) | Roseoside (formate adduct) | MeOH Inf MEOH:H2O | MeOH EA |
| 15 | 11.7 | 785 | MS2 [785]: 765 (60), 633 (66), 483 (100), 301 (24) | hexahydroxydiphenoyl-digalloyl-glucose | MeOH Inf MEOH:H2O | MeOH Inf MEOH:H2O |
| 16 | 13.0 | 635 | MS2 [635]: 465 (100) MS3 [635→465]: 313 (100), 235 (14), 169 (45) | Trigalloylglucose | MeOH MEOH:H2O | MeOH MEOH:H2O |
| 17 | 17.1 | 479 | MS2 [479]: 317 (100), 316 (95) MS3 [479→317]: 271 (94), 179 (100), 151 (36) | Myricetin-O-hexoside | All | All |
| 18 | 19.7 | 449 | MS2 [449]: 317 (43), 316 (100) MS3 [449→316]: 271 (100), 179 (39), 151 (32) | Myricetin-O-pentoside | All | All |
| 19 | 20.1 | 463 | MS2 [463]: 317 (66), 316 (100) MS3 [463→316]: 271 (100), 179 (34), 151 (26) | Myricetin-O-deoxyhexoside | All | All |
| 20 | 20.9 | 463 | MS2 [463]: 301 (100) MS3 [463→301]: 255 (32), 179 (86), 151 (100) | Quercetin-O-hexoside | All | MeOH EA |
| 21 | 23.5 | 447 | MS2 [447]: 285 (100), 255 (27) MS3 [447→285]: 257 (8), 255 (100), 227 (10) | Kaempferol.-O-hexoside | All | MeOH EA |
| 22 | 23.5 | 433 | MS2 [433]: 301 (100) MS3 [433→301]: 271 (45), 179 (100), 151 (75) | Quercetin-O-pentoside | All | MeOH EA MEOH:H2O |
| 23 | 24.3 | 433 | MS2 [433]: 301 (100) MS3 [433→301]: 271 (26), 255 (19), 179 (100), 151 (40) | Quercetin-O-pentoside | All | EA |
| 24 | 24.8 | 447 | MS2 [447]: 301 (100) MS3 [447→301]: 179 (75), 151 (100) | Quercetin-O-deoxyhexoside | All | All |
| 25 | 26.1 | 417 | MS2 [417]: 285 (70), 284 (100) MS3 [417→284]: 255 (100) | Kaempferol-O-pentoside | All | MeOH EA |
| 26 | 26.9 | 317 | MS2 [317]: 179 (100), 151 (35) | Myricetin | MeOH EA MEOH:H2O | -- |
| 27 | 28.9 | 431 | MS2 [431]: 285 (100) MS3 [431→285]: 255 (100) | Kaempferol-O-deoxyhexoside | All | EA |
| 28 | 29.6 | 711 | MS2 [711]: 665 (62), 503 (100) MS3 [711→503]: 485 (100), 453 (30), 441 (60), 409 (32) | Saponin | All | All |
| 29 | 32.3 | 711 | MS2 [711]: 665 (75), 503 (100) MS3 [711→503]: 485 (100), 441 (15) | Saponin | All | All |
| 30 | 35.5 | 301 | MS2 [301]: 179 (100), 151 (99) | Quercetin | MeOH EA MEOH:H2O | -- |
| 31 | 39.0 | 327 | MS2 [327]: 291 (56), 229 (100), 211 (87), 171 (96) | Oxo-dihydroxy-octadecenoic acid | All | All |
| 32 | 40.2 | 695 | MS2 [695]: 649 (62), 487 (100) | Unknown | MeOH Inf MEOH:H2O | MeOH Inf MEOH:H2O |
| 33 | 40.5 | 329 | MS2 [329]: 311 (23), 293 (31), 229 (100), 211 (98), 171 (78) | Trihydroxy-octadecenoic acid | All | All |
| Peak | Compound | MeOH | Inf | MeOH:H2O | EA |
|---|---|---|---|---|---|
| 1 | Disaccharide | 1.01 | 0.00 | 0.62 | 0.00 |
| 2 | Gluconic acid | 0.00 | 0.55 | 0.00 | 0.00 |
| 3 | Isocitric acid | 0.21 | 0.15 | 0.78 | 0.38 |
| 4 | Oenothein B isomer | 3.45 | 0.00 | 1.07 | 0.00 |
| 5 | Citric acid | 0.16 | 0.05 | 0.37 | 0.29 |
| 6 | Digalloylglucose | 0.00 | 0.51 | 0.08 | 0.00 |
| 7 | Gallic acid | 0.13 | 0.20 | 1.56 | 1.41 |
| 8 | Digalloylglucose | 0.16 | 0.75 | 0.34 | 0.00 |
| 9 | Unknown | 1.08 | 1.86 | 0.90 | 1.15 |
| 10 | Caftaric acid | 0.23 | 1.16 | 0.34 | 0.00 |
| 11 | Oenothein B isomer | 13.45 | 37.99 | 12.68 | 0.00 |
| 12 | Coumaric acid | 0.61 | 2.86 | 1.03 | 0.00 |
| 13 | Ferulic acid pentoside | 0.79 | 3.29 | 0.87 | 0.00 |
| 14 | Roseoside | 1.57 | 0.92 | 1.06 | 0.00 |
| 15 | HHDP-digalloyl-glucose | 0.73 | 0.29 | 0.77 | 0.00 |
| 16 | Trigalloylglucose | 0.72 | 0.00 | 0.60 | 0.00 |
| 17 | Myricetin-O-hexoside | 21.32 | 14.43 | 22.88 | 7.84 |
| 18 | Myricetin-O-pentoside | 15.26 | 8.80 | 17.09 | 18.20 |
| 19 | Myricetin-O-deoxyhexoside | 8.80 | 6.73 | 9.41 | 18.59 |
| 20 | Quercetin-O-hexoside | 2.56 | 1.73 | 2.44 | 2.38 |
| 21 | Kaempferol.-O-hexoside | 1.52 | 1.23 | 1.62 | 1.51 |
| 22 | Quercetin-O-pentoside | 3.21 | 1.80 | 3.53 | 2.08 |
| 23 | Quercetin-O-pentoside | 2.60 | 1.20 | 2.12 | 3.90 |
| 24 | Quercetin-O-deoxyhexoside | 5.82 | 3.61 | 5.26 | 16.55 |
| 25 | Kaempferol-O-pentoside | 1.46 | 0.75 | 1.04 | 1.27 |
| 26 | Myricetin | 2.12 | 0.00 | 1.53 | 1.10 |
| 27 | Kaempferol-O-deoxyhexoside | 0.56 | 0.28 | 0.57 | 1.46 |
| 28 | Saponin | 1.82 | 1.92 | 2.09 | 1.13 |
| 29 | Saponin | 1.21 | 0.91 | 1.08 | 0.91 |
| 30 | Quercetin | 0.68 | 0.00 | 0.52 | 0.74 |
| 31 | Oxo-dihydroxy-octadecenoic acid | 2.59 | 3.22 | 2.68 | 11.80 |
| 32 | Unknown | 2.08 | 1.01 | 1.43 | 0.00 |
| 33 | Trihydroxy-octadecenoic acid | 2.09 | 1.79 | 1.66 | 7.29 |
| Peak | Compound | MeOH | Inf | MeOH:H2O | EA |
|---|---|---|---|---|---|
| 1 | Disaccharide | 7.38 | 0.00 | 1.23 | 0.24 |
| 2 | Gluconic acid | 0.00 | 3.08 | 1.17 | 0.00 |
| 3 | Isocitric acid | 0.00 | 0.41 | 0.00 | 0.00 |
| 4 | Oenothein B isomer | 4.51 | 0.00 | 7.03 | 1.15 |
| 5 | Citric acid | 0.00 | 0.00 | 0.00 | 0.00 |
| 6 | Digalloylglucose | 0.65 | 0.89 | 0.42 | 0.00 |
| 7 | Gallic acid | 1.05 | 0.30 | 3.22 | 0.38 |
| 8 | Digalloylglucose | 0.69 | 1.77 | 1.12 | 0.00 |
| 9 | Unknown | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | Caftaric acid | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | Oenothein B isomer | 43.13 | 79.93 | 46.24 | 2.30 |
| 12 | Coumaric acid | 0.00 | 0.28 | 0.00 | 0.00 |
| 13 | Ferulic acid pentoside | 0.12 | 1.04 | 0.46 | 0.00 |
| 14 | Roseoside | 0.86 | 0.00 | 0.00 | 0.77 |
| 15 | HHDP-digalloyl-glucose | 6.34 | 0.54 | 10.71 | 0.00 |
| 16 | Trigalloylglucose | 3.29 | 0.00 | 2.93 | 0.00 |
| 17 | Myricetin-O-hexoside | 2.52 | 0.45 | 2.45 | 2.35 |
| 18 | Myricetin-O-pentoside | 2.07 | 0.20 | 1.71 | 2.46 |
| 19 | Myricetin-O-deoxyhexoside | 2.26 | 0.51 | 2.00 | 6.39 |
| 20 | Quercetin-O-hexoside | 0.23 | 0.00 | 0.00 | 1.38 |
| 21 | Kaempferol.-O-hexoside | 0.20 | 0.00 | 0.00 | 0.52 |
| 22 | Quercetin-O-pentoside | 0.16 | 0.00 | 0.24 | 0.67 |
| 23 | Quercetin-O-pentoside | 0.00 | 0.00 | 0.00 | 0.82 |
| 24 | Quercetin-O-deoxyhexoside | 0.56 | 0.10 | 0.39 | 3.58 |
| 25 | Kaempferol-O-pentoside | 0.13 | 0.00 | 0.00 | 0.44 |
| 26 | Myricetin | 0.00 | 0.00 | 0.00 | 0.00 |
| 27 | Kaempferol-O-deoxyhexoside | 0.00 | 0.00 | 0.00 | 0.66 |
| 28 | Saponin | 3.52 | 1.45 | 3.26 | 6.14 |
| 29 | Saponin | 1.85 | 0.72 | 1.61 | 3.76 |
| 30 | Quercetin | 0.00 | 0.00 | 0.00 | 0.00 |
| 31 | Oxo-dihydroxy-octadecenoic acid | 7.12 | 4.50 | 5.18 | 27.11 |
| 32 | Unknown | 4.47 | 0.55 | 3.53 | 9.27 |
| 33 | Trihydroxy-octadecenoic acid | 6.89 | 3.28 | 5.11 | 29.62 |
| Parts | Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | PBD (mmol TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| Aerial parts | EA | 74.54 ± 1.02 d | 118.95 ± 0.88 d | 83.28 ± 2.58 d | 37.43 ± 1.53 d | 3.11 ± 0.23 | 43.25 ± 0.97 c |
| MeOH | 798.89 ± 0.71 a | 1382.08 ± 0.81 a | 1642.42 ± 5.42 b | 677.83 ± 6.13 b | 6.80 ± 0.32 | 63.31 ± 0.79 a | |
| Infusion | 751.13 ± 2.73 c | 1254.58 ± 5.10 b | 1819.57 ± 65.86 a | 756.63 ± 8.28 a | 4.72 ± 0.15 | 58.19 ± 0.24 b | |
| MeOH/Water | 778.54 ± 2.74 b | 1078.21 ± 9.41 c | 1270.20 ± 16.43 c | 573.81 ± 20.77 c | 4.25 ± 0.01 | 37.69 ± 3.15 d | |
| Roots | EA | 59.40 ± 0.92 d | 114.75 ± 0.18 c | 105.14 ± 7.37 d | 46.03 ± 1.79 d | 2.08 ± 0.32 | 25.89 ± 4.62 c |
| MeOH | 316.60 ± 0.40 b | 551.11 ± 0.57 b | 635.13 ± 11.15 c | 218.59 ± 9.91 c | 3.81 ± 0.19 | 39.40 ± 1.40 a | |
| Infusion | 312.62 ± 0.37 c | 551.40 ± 0.42 b | 1065.96 ± 19.02 a | 424.87 ± 3.33 a | 3.93 ± 0.01 | 45.22 ± 1.17 a | |
| MeOH/Water | 317.72 ± 0.11 a | 552.33 ± 0.29 a | 845.00 ± 29.67 b | 376.40 ± 8.80 b | 3.76 ± 0.05 | 44.01 ± 1.95 ab |
| Parts | Solvents | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | α-Amylase (mmol ACAE/g) | α-Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Aerial parts | EA | 2.69 ± 0.25 b | 4.72 ± 0.35 a | 90.24 ± 3.48 b | 0.95 ± 0.09 a | 1.57 ± 0.04 b |
| MeOH | 4.48 ± 0.35 a | 2.34 ± 0.46 b | 106.68 ± 2.02 a | 1.02 ± 0.08 a | 1.62 ± 0.03 a | |
| Infusion | na | 1.68 ± 0.10 c | 49.80 ± 1.54 c | 0.17 ± 0.01 c | na | |
| MeOH/Water | 2.76 ± 0.11 c | 1.11 ± 0.18 d | 103.84 ± 1.27 a | 0.73 ± 0.13 b | na | |
| Roots | EA | 4.35 ± 0.36 a | 5.18 ± 0.44 a | 79.30 ± 1.18 b | 0.62 ± 0.12 b | 1.66 ± 0.01 c |
| MeOH | 3.00 ± 0.57 b | 4.94 ± 0.69 ab | 98.31 ± 3.23 a | 0.83 ± 0.06 a | 1.86 ± 0.01 b | |
| Infusion | 1.01 ± 0.06 c | 4.14 ± 0.59 b | 34.47 ± 1.09 c | 0.14 ± 0.01 c | 1.96 ± 0.01 a | |
| MeOH/Water | 2.81 ± 0.15 b | 4.23 ± 0.13 ab | 96.84 ± 0.48 a | 0.61 ± 0.06 b | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ak, G.; Zengin, G.; Mahomoodally, M.F.; Llorent-Martínez, E.; Orlando, G.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; Di Simone, S.C.; et al. Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure? Antioxidants 2021, 10, 1389. https://doi.org/10.3390/antiox10091389
Ak G, Zengin G, Mahomoodally MF, Llorent-Martínez E, Orlando G, Chiavaroli A, Brunetti L, Recinella L, Leone S, Di Simone SC, et al. Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure? Antioxidants. 2021; 10(9):1389. https://doi.org/10.3390/antiox10091389
Chicago/Turabian StyleAk, Gunes, Gokhan Zengin, Mohamad Fawzi Mahomoodally, Eulogio Llorent-Martínez, Giustino Orlando, Annalisa Chiavaroli, Luigi Brunetti, Lucia Recinella, Sheila Leone, Simonetta Cristina Di Simone, and et al. 2021. "Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure?" Antioxidants 10, no. 9: 1389. https://doi.org/10.3390/antiox10091389
APA StyleAk, G., Zengin, G., Mahomoodally, M. F., Llorent-Martínez, E., Orlando, G., Chiavaroli, A., Brunetti, L., Recinella, L., Leone, S., Di Simone, S. C., Menghini, L., & Ferrante, C. (2021). Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure? Antioxidants, 10(9), 1389. https://doi.org/10.3390/antiox10091389















