On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations
Abstract
:1. Introduction
2. Human Skin: Structure and Function
3. The Skin as an Immune Organ
3.1. Non-Immune Cells as Key Immunological Mediators
3.2. Immune Skin Cells
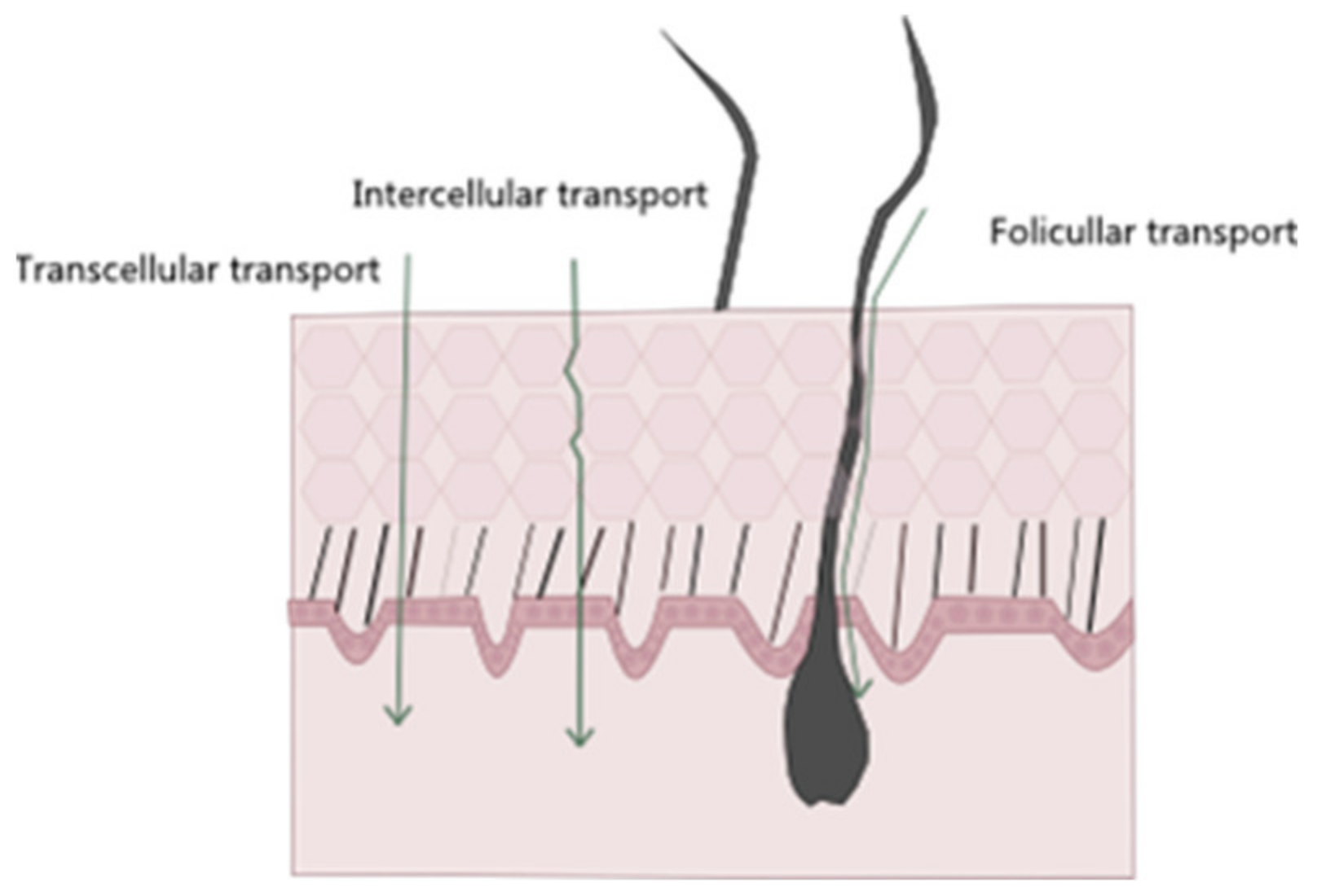
4. The Skin as a Barrier in Cutaneous Delivery
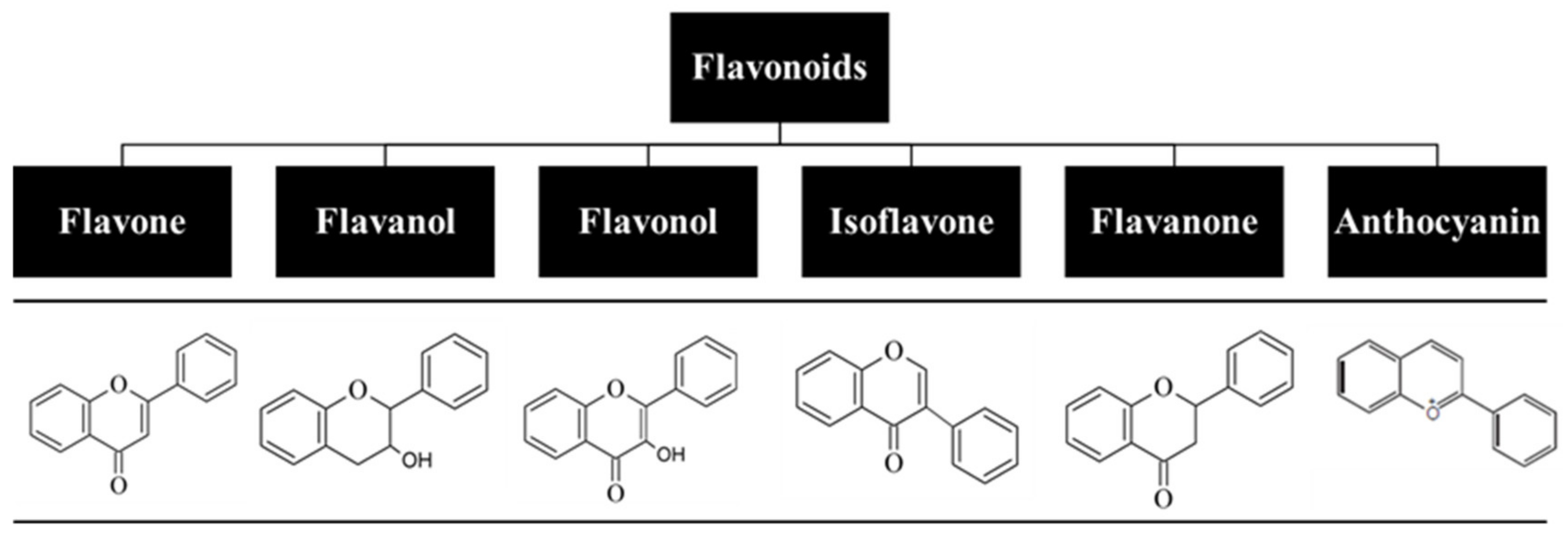
5. Flavonoids: Relevant Biochemical and Biological Properties
5.1. Antioxidant Properties
5.2. Anti-Inflammatory Properties
5.3. Anticancer Properties
5.4. Antibacterial Properties
6. Bioavailability of Flavonoids
7. The Need for Nanocarriers in Cutaneous Flavonoid Delivery
7.1. Nano-Delivery Systems: Advantages and Limitations
7.2. Nano-Delivery Systems Applied for Flavonoid Cutaneous Administration
8. Cutaneous Delivery Systems of Flavonoids for Treatment of Skin Pathologies
8.1. Examples of Nanocarriers Designed for Flavonol Cutaneous Delivery
8.2. Examples of Nanocarriers Designed for Other Flavonoid Classes’ Topical Delivery
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 4, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and Development of Nanovehicle-Based Delivery Systems for Preventive or Therapeutic Supplementation with Flavonoids. Curr. Med. Chem. 2009, 17, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.V.; Casagrande, R. Studies in Natural Products Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36. [Google Scholar]
- Nagula, R.L.; Wairkar, S.J. Recent advances in topical delivery of flavonoids: A review. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Yang Wang, T.; Li, Q.; Shun Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2017, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Khan, H.S.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2018, 194, 107–131. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Shelke, S.J.; Shinkar, D.M.; Saudagar, R.B. Topical gel: A novel approach for development of topical drug delivery system. Int. J. Pharm. Technol. 2013, 5, 2739–2763. [Google Scholar]
- Menaa, F.; Menaa, A.; Menaa, B. Polyphenols Nano-Formulations for Topical Delivery and Skin Tissue Engineering. In Polyphenols in Human Health and Disease; Elsevier Inc: Amsterdam, The Netherlands, 2014; pp. 839–848. [Google Scholar]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An Update of the Defensive Barrier Function of Skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Costa Lima, S.A.; Reis, S. Nanoparticles in Life Sciences and Biomedicine; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery. Dermatol 2012, 3, 2065–2073. [Google Scholar]
- Forster, M.; Bolzinger, M.A.; Fessi, H.; Briancon, S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur. J. Dermatol. 2009, 19, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matejuk, A. Skin Immunity. Arch. Immunol. et Ther. Exp. 2018, 66, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Flacher, V.; Tripp, C.H.; Mairhofer, D.G.; Steinman, R.M.; Stoitzner, P.; Idoyaga, J.; Romani, N. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol. Med. 2014, 6, 1191–1204. [Google Scholar] [CrossRef]
- Shklovskaya, E.; O’Sullivan, B.J.; Ng, L.G.; Roediger, B.; Thomas, R.; Weninger, W.; de St Groth, B.F. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 2011, 108, 18049–18054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashem, S.W.; Hani, M.; Kaplan, D.H. Skin Immunity to Candida albicans. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kitashima, D.Y.; Kobayashi, T.; Woodring, T.; Idouchi, K.; Doebel, T.; Voisin, B.; Adachi, T.; Ouchi, T.; Takahashi, H.; Nishifuji, K. Langerhans Cells Prevent Autoimmunity via Expansion of Keratinocyte Antigen-Specific Regulatory T Cells. eBioMedicine 2018, 27, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.K.; Philips, R.L.; Eriksson, A.U.; Kim, P.J.; Halder, R.C.; Lee, D.J.; Singh, R.R. Langerhans cells maintain local tissue tolerance in a model of systemic autoimmune disease. J. Immunol. 2015, 195, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, E.X.; Fang, C.; Bane, K.L.; Long, A.T.; Naudin, C.; Kucukal, E.; Gandhi, A.; Brett-Morris, A.; Mumaw, M.M.; Izadmehr, S.; et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Invest. 2018, 128, 944–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar] [CrossRef]
- Long, H.; Zhang, G.; Wang, L.; Lu, Q. Eosinophilic skin diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 189–213. [Google Scholar] [CrossRef]
- Souyoul, S.A.; Saussy, K.P.; Lupo, M.P. Nutraceuticals: A review. Dermatol. Ther. 2018, 8, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Marks, J.G.; Miller, J.J. Lookingbill Marks’ Principles of Dermatology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 2–10. [Google Scholar]
- Zaki, N.M. Progress and Problems in Nutraceuticals Delivery. J. Bioequiv. Bioavailab. 2014, 6, 75–77. [Google Scholar]
- Routes for Drug Administration through the Skin. Available online: https://www.msdmanuals.com/home/drugs/administration-and-kinetics-of-drugs/drug-administration (accessed on 17 May 2019).
- Pouillot, A.; Dayan, N.; Polla, A.S.; Polla, L.L.; Polla, B.S. The stratum corneum: A double paradox. J. Cosmet. Dermatol. 2008, 7, 143–148. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.N.; Attama, A.A. Application of Nanotechnology in Drug Delivery; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Van Gele, M.; Geusens, B.; Brochez, L.; Speeckaert, R.; Lambert, J. Three-dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opin Drug Deliv. 2011, 8, 705–720. [Google Scholar] [CrossRef]
- Kulkarni, V.S. Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–36. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sang, S.; Hou, Z.; Lambert, J.D.; Yang, C.S. Redox properties of tea polyphenols and related biological activities. Antioxid. Redox Signal. 2005, 7, 1704–1714. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Robards, K. Bioactivity and structure of biophenols as mediators of chronic diseases. Crit. Rev. Food Sci. Nutr. 2008, 48, 929–966. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.H.; Khera, R.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. Annual burden and costs of hospitalization for high-need, high-cost patients with chronic gastrointestinal and liver diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zheng, Y.; Chen, X. Drugs for autoimmune inflammatory diseases: From small molecule compounds to anti-TNF biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Baker, D.; Marta, M.; Pryce, G.; Giovannoni, G.; Schmierer, K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017, 16, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mak, P.; Leung, Y.K.; Tang, W.Y.; Harwood, C.; Ho, S.M. Apigenin Suppresses Cancer Cell Growth through ERβ1. Neoplasia 2006, 8, 896–904. [Google Scholar]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Yen, G.C.; Lai, H.H. Inhibition of reactive nitrogen species effects in vitro and in vivo by isoflavones and soy-based food extracts. J. Agric. Food Chem. 2003, 51, 7892–7900. [Google Scholar] [CrossRef]
- D’Ischia, M.; Panzella, L.; Manini, P.; Napoletano, A. The chemical basis of the antinitrosating action of polyphenolic cancer chemopreventive agents. Curr. Med. Chem. 2006, 13, 3133–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, T.; Prakosay, I.; Date, R.A.; Sies, H.; Schewe, T. Oxidative modification of low-density lipoprotein: Lipid peroxidation by myeloperoxidase in the presence of nitrite. Biol. Chem. 2004, 385, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; Tromp, M.N.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A.A. Flavonoids as scavengers of nitric oxide radical. Biophys. Res. Commun. 1995, 214, 755–759. [Google Scholar] [CrossRef]
- Trujillo, M.; Ferre-Sueta, G.; Radi, R. Peroxynitrite detoxification and its biologic implications. Antioxid. Redox Signal. 2008, 10, 1607–1619. [Google Scholar] [CrossRef]
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with alpha-linolenic acid. Free Radic. Biol. Med. 1999, 27, 1313–1323. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure–Activity Relationships for Antioxidant Activities of a Series of Flavonoids in a Liposomal Syste. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic Compounds Rutin and o-Coumaric Acid Ameliorate Obesity Induced by High-Fat Diet in Rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef]
- Amália, P.M.; Possa, M.N.; Augusto, M.C.; Francisca, L.S. Quercetin prevents oxidative stress in cirrhotic rats. Dig. Dis. Sci. 2007, 52, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, L.M.; Yang, L.; Liu, Z.L. Evidence for α-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef]
- Frank, J.; Budek, A.; Lundh, T.; Parker, R.S.; Swanson, J.E.; Lourenço, C.F.; Gago, B.; Laranjinha, J.; Vessby, B.; Kamal-Eldin, A. Dietary flavonoids with a catechol structure increase α-tocopherol in rats and protect the vitamin from oxidation in vitro. J. Lipid Res. 2006, 47, 2718–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, S.; Ishihara, M.; Atsumi, T.; Kadoma, Y. A quantitative approach to the free radical interaction between alpha-tocopherol or ascorbate and flavonoids. In Vivo 2006, 20, 445–452. [Google Scholar] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Ristagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filasi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanità. 2007, 43, 394–405. [Google Scholar]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care. 2007, 10, 724–728. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang. S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquay, J.B.; Haenen, G.R.; Stender, G.; Wiseman, S.A.; Tijburg, L.B.; Bast, A.J. Protection against nitric oxide toxicity by tea. Agric. Food Chem. 2000, 48, 5768–5772. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Rahman, R.M.; Appleton, I.J. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef]
- Cíz, M.; Pavelková, M.; Gallová, L.; Králová, J.; Kubala, L.; Lojek, A. The influence of wine polyphenols on reactive oxygen and nitrogen species production by murine macrophages RAW 264.7. Physiol. Res. 2008, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, T.Y.; Kim, Y.K.; Baik, J.H.; So, S.; Hahm, K.B.; Surh, Y.J. Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: Stress-responsive transcription factors and MAP kinases as potential targets. Mutat. Res. 2005, 579, 214–224. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, J.K. (−)-Epigallocatechin-3-gallate Blocks the Induction of Nitric Oxide Synthase by Down-Regulating Lipopolysaccharide-Induced Activity of Transcription Factor Nuclear Factor-κB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jeong, H.J.; Lee, K.M.; Myung, N.Y.; An, N.H.; Yang, W.M.; Park, S.K.; Lee, H.J.; Hong, S.H.; Kim, H.M.; et al. Epigallocatechin-3-gallate suppresses NF-κB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem. 2007, 18, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Epigallocatechin Gallate Inhibits Phorbol Ester-Induced Activation of NF-κB and CREB in Mouse Skin: Role of p38 MAPK. Ann. N. Y. Acad. Sci. 2007, 1095, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Clichici, S.; Daicoviciu, D.; Adriana, M.; Postescu, I.D. Photochemoprevention of cutaneous neoplasia through natural products. Exp. Oncol. 2009, 31, 9–15. [Google Scholar] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 2006, 27, 19–35. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Chen, D.; Milacic, V.; Chen, M.S.; Wan, S.B.; Lam, W.H.; Huo, C.; Landis-Piwowar, K.R.; Cui, Q.C.; Wali, A.; Chan, T.H.; et al. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008, 23, 487–496. [Google Scholar]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green Tea Component, Catechin, Induces Apoptosis of Human Malignant B Cells via Production of Reactive Oxygen Species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S.J. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). Nutrients 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C.K. Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: Mediated by p53 pathway and inhibition in U1B, U4-U6 UsnRNAs expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. Hepatology 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Sah, J.F.; Balasubramanian, S.; Eckert, R.L.; Rorke, E.A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway: Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2004, 279, 12755–12762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.A.; Adhami, V.M.; Afaq, F.; Ahmad, N.; Mukhtar, H.J. Modulation of phosphatidylinositol-3-kinase/protein kinase B-and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. Cell. Biochem. 2004, 91, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell. Biochem. 2006, 287, 109–116. [Google Scholar] [CrossRef]
- Zhen, M.C.; Huang, X.H.; Wang, Q.; Sun, K.; Liu, Y.J.; Li, W.; Zhang, L.J.; Cao, L.Q.; Chen, X.L. Green tea polyphenol epigallocatechin-3-gallate suppresses rat hepatic stellate cell invasion by inhibition of MMP-2 expression and its activation. Acta Pharmacol. Sin. 2006, 27, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of Various Temperatures and pH Values on the Extraction Yield of Phenolics from Litchi Fruit Pericarp Tissue and the Antioxidant Activity of the Extracted Anthocyanins. Int. J. Mol. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lee, M.J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.T.; et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Eriksson, U.; Edgar, B.; Cullberg, G.; Hedner, T. Flavonoids in grapefruit juice inhibit the in vitro hepatic metabolism of 17β-estradiol. Eur. J. Drug Metab. Pharmacok. 1995, 20, 219–224. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 11, 1–34. [Google Scholar]
- Cermak, R.; Wolffram, S. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2008, 4, 17–35. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.K. Nanomedicine: Application of nanobiotechnology in medical practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano. 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Simões, S.; Moreira, J.N.; Fonseca, C.; Düzgüneş, N.; de Lima, M.C. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef]
- Gasco, M.R. Lipid nanoparticles: Perspectives and challenges. Adv. Drug Deliv. Rev. 2007, 59, 377–378. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and transdermal drug delivery: From simple potions to smart technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles. In Encyclopaedia of Pharmaceutical Technology; Marcel Dekker Inc: New York, NY, USA, 1994. [Google Scholar]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Panyam, J.; Sahoo, S.K.; Prabha, S.; Bargar, T.; Labhasetwar, V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly (D, L-lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2003, 262, 1–11. [Google Scholar] [CrossRef]
- Bala, I.; Hariharan, S.; Kumar, M.N. Critical Reviews™ in Therapeutic Drug Carrier Systems. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Serizawa, T.J. Preparation and Characterization of Surfactant-Free Nanoparticles Composed of Stereoregular Poly(methyl methacrylate)s. Nanosci. Nanotechnol. 2009, 9, 591–597. [Google Scholar] [CrossRef]
- Chana, J.; Forbes, B.; Jones, S.A. The synthesis of high molecular weight partially hydrolysed poly (vinyl alcohol) grades suitable for nanoparticle fabrication. J. Nanosci. Nanotechnol. 2008, 8, 5739–5747. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly (alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 2001, 34, 223–227. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Borin, M.F.; Lopez, R.F.; Fonseca, M.J. In vitro evaluation of quercetin cutaneous absorption from topical formulations and its functional stability by antioxidant activity. Int. J. Pharm. 2007, 328, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; Casagrande, R.; Verri, W.A., Jr.; Georgetti, S.R.; Bentley, M.V.; Fonseca, M.J. Quercetin in lyotropic liquid crystalline formulations: Physical, chemical and functional stability. AAPS Pharm. Sci. Technol. 2008, 9, 591–596. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M.J. Incorporation of quercetin in lipid microparticles: Effect on photo-and chemical-stability. Pharm. Biomed. Anal. 2009, 49, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Design of self-assembling peptides and their biomedical applications. Int. J. Nanomed. 2011, 6, 1621. [Google Scholar]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Discovery of the consistently well-performed analysis chain for SWATH-MS based pharmacoproteomic quantification. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar]
- Bose, S.; Michniak-Kohn, B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef]
- Chessa, M.; Caddeo, C.; Valenti, D.; Manconi, M.; Sinico, C.; Fadda, A.M. Effect of penetration enhancer containing vesicles on the percutaneous delivery of quercetin through new born pig skin. Pharmaceutics 2011, 3, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical Anti-Inflammatory Potential of Quercetin in Lipid-Based Nanosystems: In Vivo and In Vitro Evaluation. Pharmaceut. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.; Huang, C.-T.; Fu, L.-T.; Huang, Y.-B.; Tsai, Y.-H.; Wu, P.-C. The Effect of Submicron Emulsion Systems on Transdermal Delivery of Kaempferol. Chem. Pharmaceut. Bull. 2012, 60, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive Antioxidant and Antiinflammatory Effects of Flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-R.; Hung, C.-F.; Lin, Y.-K.; Fang, J.-Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Munyendo, W.L.L.; Zhang, Z.; Abbad, S.; Waddad, A.Y.; Lv, H.; Baraza, L.D.; Zhou, J. Micelles of TPGS modified apigenin phospholipid complex for oral administration: Preparation, in vitro and in vivo evaluation. J. Biomed. Nanotechnol. 2013, 9, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.N.; Zhang, Y.T.; Wang, Q.; Xu, L.; Feng, N.P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Masarudin, M.J.; Umar Kura, A.; Abas, F.; Fakurazi, S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int. J. Nanomed. 2016, 11, 3417–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1069–1074. [Google Scholar] [CrossRef]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. ACS Biomat. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 4, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target 2005, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hwang, T.L.; Huang, Y.L.; Fang, C.L. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int. J. Pharm. 2006, 310, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Al-Karaki, R. Nanotechnological Innovations Enhancing the Topical Therapeutic Efficacy of Quercetin: A Succinct Review. Curr. Drug Deliv. 2020, 17, 270–278. [Google Scholar] [CrossRef]
- Guan, F.; Wang, Q.; Bao, Y.; Chao, Y. Anti-rheumatic effect of quercetin and recent developments in nano formulation. RSC Adv. 2021, 11, 7280–7293. [Google Scholar] [CrossRef]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative Evaluation of the Effect of Permeation Enhancers, Lipid Nanoparticles and Colloidal Silica on in vivo Human Skin Penetration of Quercetin. Skin Pharmacol. Physiol. 2013, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mandal, S.; Sinha, J.; Mukhopadhyay, S.; Das, N.; Basu, M.K. Quercetin: Critical evaluation as an antileishmanial agent in vivo in hamsters using different vesicular delivery modes. J. Drug Target 2002, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Shamseddin, A.; Aubert-Pouëssel, A.; Devoisselle, J.M.; Begu, S. Dermal quercetin lipid nanocapsules: Influence of the formulation on antioxidant activity and cellular protection against hydrogen peroxide. Int. J. Pharm. 2017, 518, 167–176. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, W.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Silva, A.P.; Nunes, B.R.; De Oliveira, M.C.; Koester, L.S.; Mayorga, P.; Bassani, V.L.; Teixeira, H.F. Development of topical nanoemulsions containing the isoflavone genistein. Pharmazie 2009, 64, 32–35. [Google Scholar] [PubMed]
- Carlotti, M.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S.J. Resveratrol in Solid Lipid Nanoparticles. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H.L.; Beck, R.C.; Windbergs, M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B. 2014, 114, 144–149. [Google Scholar] [CrossRef]
- Tsai, Y.; Lee, K.; Huang, Y.; Huang, C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010, 388, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Adelli, G.R.; Hingorani, T.; Punyamurthula, N.; Prachetan, S.; Majumdar, S. Evaluation of topical hesperetin matrix film for back-of-the-eye delivery. Eur. J. Pharm. Biopharm. 2015, 92, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Kilor, V.; Sapkal, N.; Vaidya, G. Design and development of novel microemulsion based topical formulation of Hesperidin. Int. J. Pharm. Pharm. Sci. 2015, 7, 142–148. [Google Scholar]
- Haritima, J. Papr reduction using scs-slm technique in stfbc mimo-ofdm. ARPN J. Eng. Appl. Sci. 2017, 12, 3218–3221. [Google Scholar]
- Telange, D.R.; Patin, A.T. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2016, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Priprem, A.; Limsitthichaikoon, S.; Thappasarapong, S.; Complex, A.A. Preparation, Anti-inflammatory Activity of Topical Anthocyanins by Complexation and Niosomal Encapsulation. Int. J. Chem. Mol. Eng. 2015, 9, 133–137. [Google Scholar]
- Baolin, L.; Weiwei, W.; Ning, T. Topical Application of Luteolin Inhibits Scratching Behavior Associated with Allergic Cutaneous Reaction in Mice. Planta Med. 2005, 71, 424–428. [Google Scholar] [CrossRef] [PubMed]
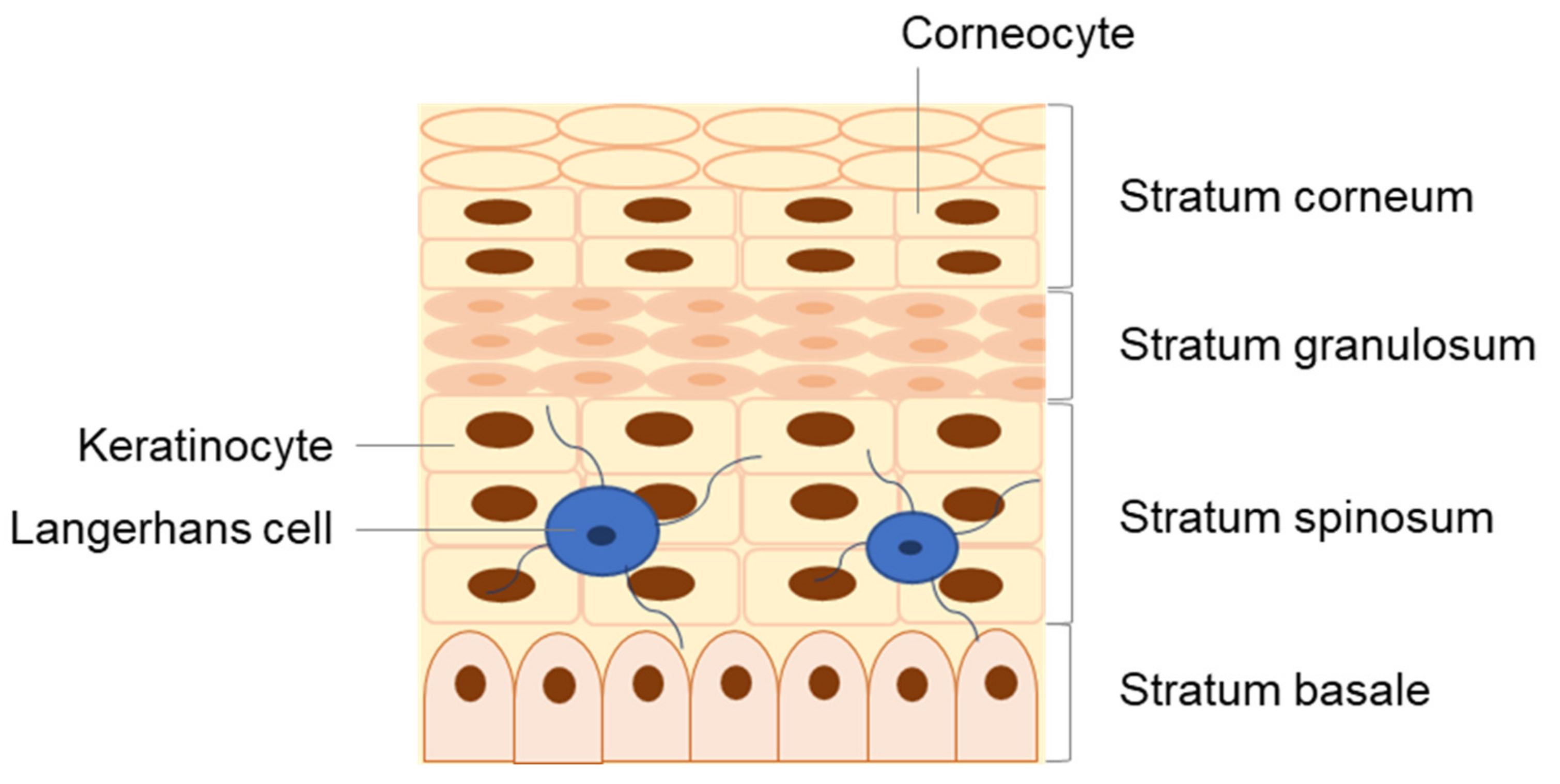


| Cell Type | Location in the Skin | Immunological Role | Ref. |
|---|---|---|---|
| Langerhans cells | Epidermis | Sentinel role | [19,25] |
| Migration to lymph nodes to induce adaptive immune responses | |||
| Induction of tolerance | |||
| Production of pro-inflammatory cytokines and chemokines | |||
| Dermal DCs | Papillary dermis | Antigen presentation | [25] |
| Cytokine and chemokine secretion | |||
| Plasmacytoid DCs | Dermis | Production of IFN-α | [21,25] |
| Macrophages | Papillary and reticular dermis | Antimicrobial activity | [19,25] |
| Production of pro- and anti-inflammatory mediators | |||
| Production of cytokines and chemokines | |||
| Phagocytosis of pathogenic agents and necrotic debris | |||
| Mast cells | Papillary and reticular dermis | Production of inflammatory mediators involved in allergic responses and asthma | [19] |
| Recruitment of immune cells | |||
| Production of inflammatory cytokines | |||
| B lymphocytes | Reticular dermis | Production of autoantibodies | [27,28] |
| specific to components of the skin | |||
| Non-immune cells (keratinocytes and fibroblasts) | Epidermis and reticular dermis | Provide physical barrier and structural integrity | [20,21,22,25] |
| Production of inflammatory cytokines and AMPs in response to injury or pathogen invasion | |||
| Neutrophils | Reticular dermis | Phagocytosis during pathogen invasion | [29,30] |
| Release of chemo-attractants to recruit other neutrophils to the site of inflammation | |||
| Eosinophils | Reticular dermis | Defense against parasites | [31] |
| Flavonoid | Molecular Targets | Biological Role | Mechanisms of Action | Ref. |
|---|---|---|---|---|
| Catechin, Epigallocatechin | ERK, NF-kB, Rac1, AP-1, p38 | Anticarcinogenic | Inhibition of iNOS expression | [46,47,48,49] |
| Reduction of NF-kB and AP-1 activity | ||||
| Apigenin | Akt, ERK, caspase-12, caspase-3, MAPK, ROS, COX-2, IL-6, TNF-α, IL-1β, iNOS, PGE2 | Anti-inflammatory, Anticarcinogenic | Inhibition of intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and E-selectin expression | [9,46,47,50,51] |
| Inhibition of prostaglandin synthesis and IL-6 production | ||||
| Luteolin | Akt, ERK, caspase-12, caspase-3, MAPK, ROS, COX-2, IL-6, TNF-α, IL-1β, iNOS, PGE2 | Anti-inflammatory, anticarcinogenic | Inhibition of the upregulation of monocytes adhesion and VCAM-1 expression and NF-kB activity | [9,46,47,50,51] |
| Quercetin | PKC, AP-1, H2O2, iNOS, MDA, citrate synthase, MMP-9, MMP-2, COX-2, ERK | Antioxidant, anti-inflammatory | Inhibition of NO production and iNOS protein expression | [46,47,52] |
| Inhibition of cyclooxygenase and lipoxygenase activities | ||||
| Hesperetin | GSH reductase, iNOS, 3-nitropropionic acid, COX2, NF-kB, IL-1, TNF-α | Antioxidant | Blood lipid-lowering and cholesterol-lowering agents | [46,47,52] |
| Flavonoid | Nanoformulation | Skin Model | Therapeutic Application | Ref. |
|---|---|---|---|---|
| Quercetin | Solid lipid nanoparticles | Human skin | Delay UVB radiation-mediated cell damage and necrosis | [139] |
| Non-ionic emulsion with high lipid content | Pig ear skin | Inhibition of UVB-induced cutaneous oxidative stress and inflammation | [4] | |
| Anionic emulsion with low lipid content | Pig ear skin | Inhibition of UVB-induced cutaneous oxidative stress and inflammation | [4] | |
| Lecithin-chitosan nanoparticles | Male Kunming mice | Topical delivery system with a wide range of applications | [137] | |
| Lipid microparticles | n.a. | Enhance quercetin stability in topical formulations | [136] | |
| Colloidal silica emulsion | Human skin | Optimization of a formulation with enhance penetration into human SC | [156] | |
| Chitosan nanoparticles | HaCaT cells | Potential therapeutic agent for topical use against UVB radiation | [138] | |
| Penetration Enhancer containing Vesicles (PEVs) | New born pig skin | New formulation for dermal delivery of quercetin, with various therapeutic applications | [140] | |
| Polylactide nanocapsules; Multilamellar liposomes; Niosomes | Subcutaneous injection in amistogote-infected hamsters | Antileishmanial agent | [3,157] | |
| Liposomes with penetration enhancing vesicles (PEV) | Female CD-1 mice | Anti-inflammatory agent | [5,157] | |
| Lipid nanocapsules | Acute monocytic leukemia cell line (THP1–1 cell) | Antioxidant, anti-inflammatory agent | [5,158] | |
| Nanoparticle suspension | Mice | Antioxidant agent | [5,149] | |
| Catechins | Multilamellar phosphatidylcholine-liposomes | Female nude mouse (Balb/c-nu, 6–8 weeks old) | Use of liposomes for the local delivery, including skin and tumor deposition, of polyphenols | [3,151] |
| Ethanol enriched liposomes | Female nude mouse (Balb/c-nu, 6–8 week) | Antioxidant and chemopreventive activity | [152] | |
| Cream | Iranian rabbits | Wound healing effect | [5,159] | |
| Tansfersomes containing EGCG and hyaluronic acid (HA) | HaCaT cells | Synergize the UV radiation-protective ability of EGCG and HA along with imparting antioxidant and antiaging effects | [5,150] | |
| Genistein | Nanoemulsion | Pig ear skin | New formulation for dermal delivery of genistein, with various therapeutic applications | [3,160] |
| Kaempferol | Submicron emulsions | Sprague Dawley rat | Promising vehicle for topical kaempferol application | [142] |
| Resveratrol | Solid lipid Nanoparticles | Porcine skin | Protection from photodegradation | [161] |
| Resveratrol + curcumin | Lipid-core Nanocapsules | Human skin | Increase skin delivery of resveratrol | [162] |
| Niosomes | Cell rabbit skin | Increase skin delivery of resveratrol Increased antioxidant activity | [163] | |
| Hesperetin, hesperidin | Microemulsion | Guinea pigs | Whitening effect | [5,164] |
| Topical matrix film | Albino rabbits | Release of hesperetin in posterior of eye | [5,165] | |
| Microemulsion based ointment | Wistar rats | Skin irritation | [5,166] | |
| Naringenin | Gel | HRS/J mice | Antioxidant and anti-inflammatory agent | [5,162] |
| Nanoparticles | Wistar rats | Photoprotective, antioxidant agent | [5,162] | |
| Apigenin | Phospholipid phytosomes | Albino rats | Antioxidant agent | [5,167] |
| Ethosomes | Konmin mice | Anti-inflammatory agent | [5,168] | |
| Anthocyanin | Niosome gel | Male Wistar rats | Anti-inflammatory agent | [5,169] |
| Luteolin | Luteolin in olive oil | ICR mice | Anti-inflammatory agent | [5,170] |
| Luteolin-loaded niosomes/Niosomal transgel | Albino Wistar rats | Treatment of arthritis | [148] | |
| Nanoemulsion | C57BL/6 mice | Growth promoting effect | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. https://doi.org/10.3390/antiox10091376
Costa R, Costa Lima SA, Gameiro P, Reis S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants. 2021; 10(9):1376. https://doi.org/10.3390/antiox10091376
Chicago/Turabian StyleCosta, Raquel, Sofia A. Costa Lima, Paula Gameiro, and Salette Reis. 2021. "On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations" Antioxidants 10, no. 9: 1376. https://doi.org/10.3390/antiox10091376
APA StyleCosta, R., Costa Lima, S. A., Gameiro, P., & Reis, S. (2021). On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants, 10(9), 1376. https://doi.org/10.3390/antiox10091376









