Abstract
Flavonoids are one of the vital classes of natural polyphenolic compounds abundantly found in plants. Due to their wide range of therapeutic properties, which include antioxidant, anti-inflammatory, photoprotective, and depigmentation effects, flavonoids have been demonstrated to be promising agents in the treatment of several skin disorders. However, their lipophilic nature and poor water solubility invariably lead to limited oral bioavailability. In addition, they are rapidly degraded and metabolized in the human body, hindering their potential contribution to the prevention and treatment of many disorders. Thus, to overcome these challenges, several cutaneous delivery systems have been extensively studied. Topical drug delivery besides offering an alternative administration route also ensures a sustained release of the active compound at the desired site of action. Incorporation into lipid or polymer-based nanoparticles appears to be a highly effective approach for cutaneous delivery of flavonoids with good encapsulation potential and reduced toxicity. This review focuses on currently available formulations used to administer either topically or systemically different classes of flavonoids in the skin, highlighting their potential application as therapeutic and preventive agents.
1. Introduction
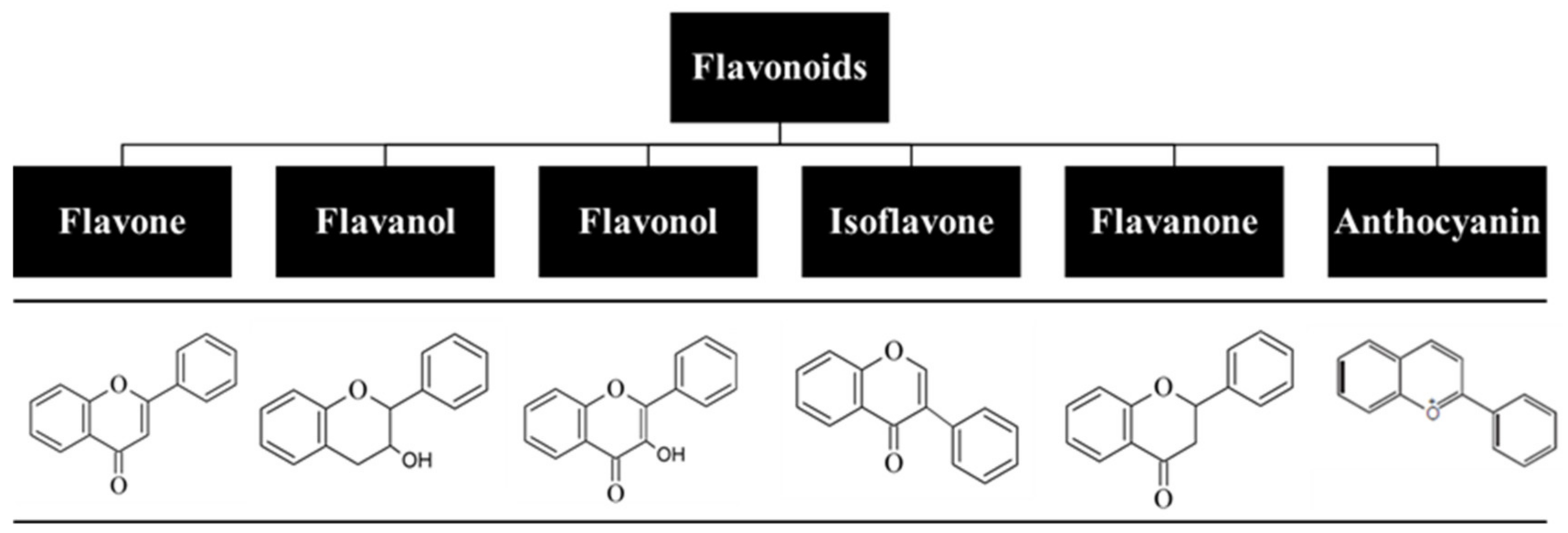
For centuries, flavonoids have been used to treat various human diseases, and despite the fast-growing development of new and innovative synthetic drugs, continuous use of these natural compounds has prevailed to this day [1,2]. Flavonoids are one of the key classes of bioactive compounds abundantly found in plants and have a general structure of a 15-carbon backbone, consisting of two benzene rings connected by a 3-carbon bridge, which forms a heterocycle. They are low-molecular-weight polyphenolic compounds derived from plant metabolites, and the presence of different substitutes creates different subclasses (Figure 1) [3,4,5]. Due to their broad spectrum of biological activity and attractive pharmacological properties, which include antioxidant, anti-inflammatory, antiproliferative, photoprotective, and antiaging effects, flavonoids have been explored as a therapeutic option towards a great number of diseases, including several skin disorders [6,7]. However, their lipophilic nature, which results in a reduced capacity to be orally absorbed, and the fact that they undergo extensive first-pass metabolism and rapid elimination hamper their oral bioavailability [8,9,10]. Thus, alternative research focuses on the development of the cutaneous delivery of flavonoids, with high patient compliance and potential to surpass drawbacks associated with oral and parental routes of administration. Although skin acts as a physical barrier to drug absorption, the development of delivery systems, such as nanoparticles, hydrogels, and microneedles, allows for the delivery of both hydrophilic and lipophilic compounds as well as drugs with shorter half-time and limited therapeutic index. This results in a higher bioavailability at the target site under a controlled release rate and avoids interactions with gastric and intestinal fluids as well as flavonoid degradation [11,12].
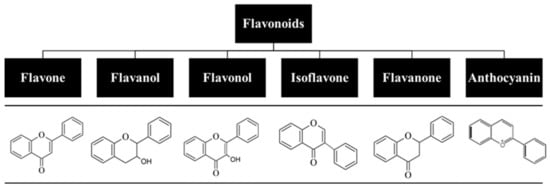
Figure 1.
Classification of flavonoids based on their chemical structure.
This review focuses on the therapeutic potential of flavonoids, including their mechanisms of action and influence of several delivery systems for topical application on the improvement of their bioavailability, safety, and therapeutic capacity. In addition, current in vitro and in vivo studies of different classes of flavonoids under study for its application on the treatment of skin conditions are highlighted.
2. Human Skin: Structure and Function
The skin is the largest organ of the body and acts as a physical barrier that separates the body from the external environment. It constitutes a first line of defense in protecting the body against physical, chemical, and microbial insults and assists in a wide range of functions such as prevention of body’s dehydration, thermoregulation, sensation, and synthesis of vitamin D.
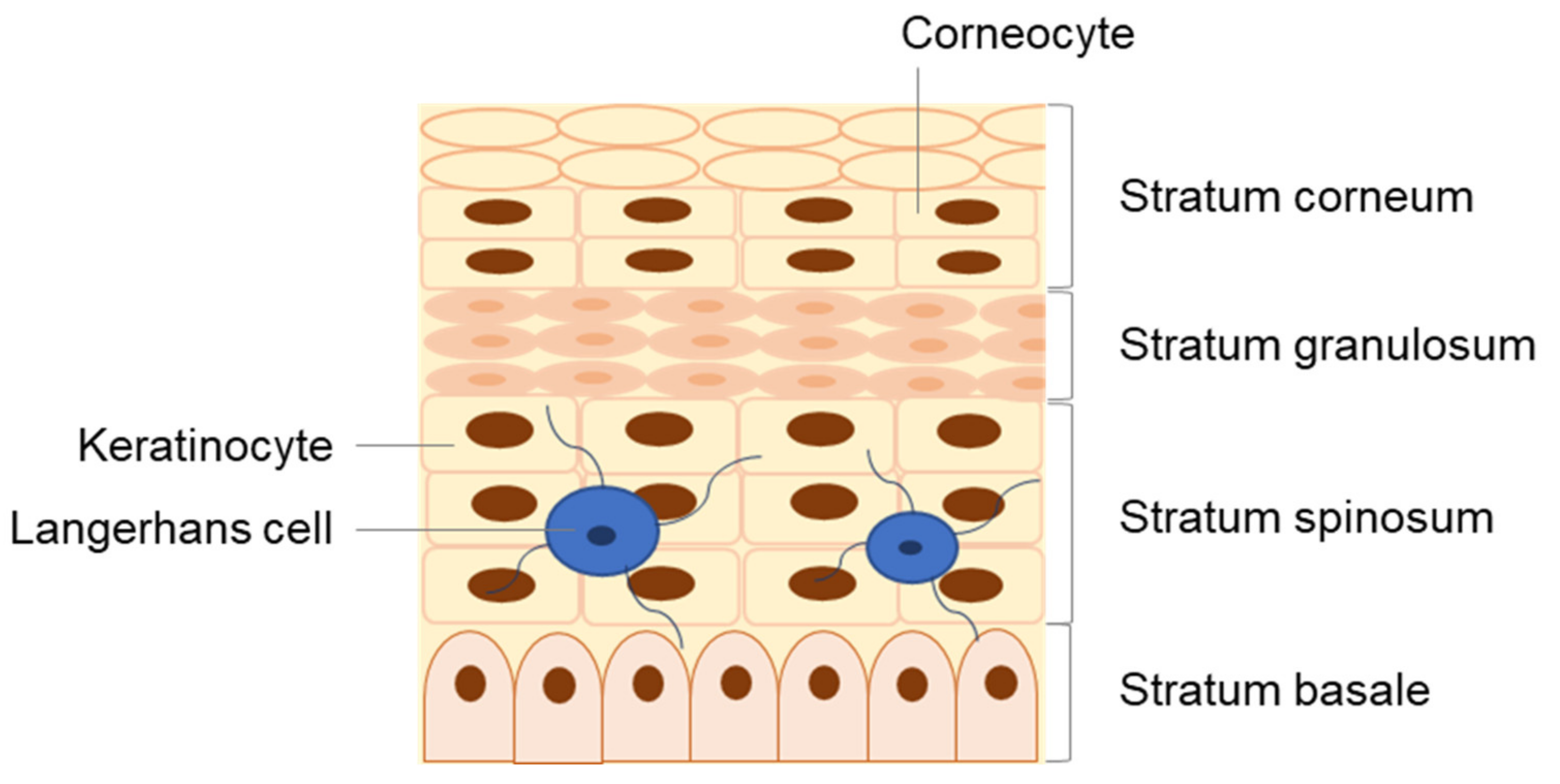
The skin is divided into three major layers, namely the epidermis, dermis, and hypodermis [13,14,15,16]. The epidermis is the outermost viable layer of the skin and constitutes a barrier between the body and the external environment. As represented in Figure 2, the epidermis is composed of four layers: the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum (SC). An additional layer, the stratum lucidum, which is often considered the lower part of the stratum corneum as opposed to an individual epidermal layer, can be found on the palm and sole of the foot, parts of the body with thickened skin. In addition, appendageal features such as hair follicles and sweat ducts are transversal to multiple skin layers [14].
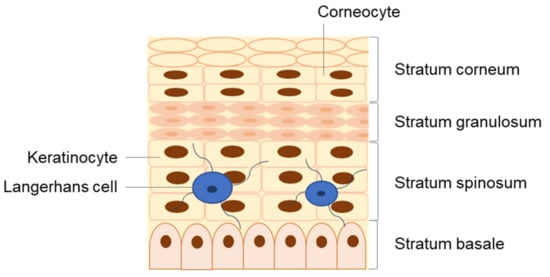
Figure 2.
Detailed structure of the epidermis, composed of four distinct strata: the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum.
The dermis, with a thickness of typically 1–2 mm, comprises the bulk layer of the skin and provides its elasticity, flexibility, and tensile strength. It is composed of collagenous and elastin fibbers, which accommodate epidermally derived appendages such as hair follicles, nails, sebaceous glands, and sweat glands as well as sensory nerve endings, lymphatic vessels and blood capillaries, which extend to the dermal side of the dermo-epidermal junction, thus allowing for metabolic exchanges and waste removal between the epidermis and the blood system [15]. The dermis contains resident cells, primarily fibroblasts that synthesize type I collagen for the extracellular matrix, as well as cells from the immune system, including macrophages and dermal dendritic cells (DCs). Below this layer, the fibrous connective tissue starts to transition to the adipose tissue of the hypodermis, where adipocytes interconnect with the collagen fibers, forming a thermal barrier for energy storage and protection from physical shock [15,17].
The hypodermis is the innermost layer of the skin and may be considered part of the endocrine system. It provides the nerves, and the lymphatic and blood vessels, which permeate into the upper layers, thus playing a critical role in re-epithelization, wound healing, and angiogenesis [14,18].
3. The Skin as an Immune Organ
The skin is undoubtably a complex organ that harbors a highly specialized immune microenvironment essential for maintaining tissue homeostasis, defense, and repair. Through a sophisticated network of resident immune and non-immune cells, biomolecules, and skin structures, the skin is able to protect the host from pathogen invasion as well as chemical and physical stress [13,14,15].
Resident immune cells (e.g., melanocytes and Langerhans cells) ensure tissue function in homeostasis and actively seek environmental antigens. Following an infection or tissue injury, these cells create a defense network in order to fight the insult and to restore the tissue to its original state [19,20]. Both epidermal keratinocytes and Langerhans cells (LCs) as well as dermal DCs, mast cells, and macrophages function as sentinels that not only provide a protective barrier but also trigger an early response to pathogen invasion by releasing stored antimicrobial peptides (AMPs), chemotactic proteins, and cytokines [20].
3.1. Non-Immune Cells as Key Immunological Mediators
Keratinocytes in response to multiple stimuli produce large amounts of interleukins (ILs), tumor-necrosis factor (TNF), and antimicrobial peptides, which trigger local immune responses. Moreover, they produce chemokines and immunoregulatory cytokines that act on resident immune cells such as DCs, mast cells, and macrophages, triggering the upregulation of inducible mediator expression and the recruitment of additional immune cells to the site of inflammation [21].
Similar to keratinocytes, fibroblasts also exert key immunomodulatory features. They express pattern recognition receptors (PRRs), produce AMPs, and synthesize many cytokines.
3.2. Immune Skin Cells
Langerhans cells are the only myeloid cell type in the epidermis. These cells act as key immunological mediators, with both an antigen-presenting role and a possible tolerance induction during an infection. These cells take up and process microbial fragments and lipid antigens and present them to effector T cells [19]. LCs are naturally migratory cells that continuously search the skin for signs of infection and that drain lymph nodes in order to build tolerance in homeostasis or to initiate adaptive immune responses. In addition, they can further exert immunoregulatory and tolerogenic functions [22,23,24].
Mast cells are commonly found in the upper dermal layer of the skin, actively protecting it and responding to infections, venoms, and stress caused by wound healing [20]. Mast cells produce and release significant amounts of histamine, thus being naturally involved in allergic reactions, and are recognized as typical allergy cells. Recent studies show their critical role in wound healing, inflammation, angiogenesis, immune tolerance, and cancer [19].
Dermal DCs, similar to LCs, are prime antigen-presenting cells, the main role of which is to provide immunosurveillance against pathogens. These cells activate and promote the clonal expansion of skin-resident memory CD4+ or CD8+ T cells. T cell-derived pro-inflammatory cytokines and chemokines can in turn stimulate epithelial and mesenchymal cells, therefore intensifying the inflammatory response [25]. Plasmacytoid DCs are a type of DC found in the skin exclusively during an inflammatory stage. These cells produce large quantities of interferon-α (IFN-α), essential for viral defense. In addition, they have also been implicated in autoimmune disease such as psoriasis as well as fibrosis [26].
Table 1 summarizes the functions of the main cell types found in the skin and their role in the skin immunology, which leads the outcome of molecules delivered cutaneously.

Table 1.
Main immunological functions of skin cells.
4. The Skin as a Barrier in Cutaneous Delivery
Cutaneous delivery is one of the most attractive routes of administration for drugs and cosmetics, since it can overcome the many drawbacks of most common routes (e.g., parenteral and oral), including low bioavailability and cytotoxicity, while ensuring a sustained drug release at the desired site of action [32]. However, normal skin presents a serious barrier to drug absorption, mostly due to the unique lipid composition and organization of the SC, which plays a key role in skin permeability and therefore drug permeation through the skin [32,33,34].
Despite recent advances in the identification and elucidation of the mechanisms of drug transport through the skin and the generation of structure–activity relations that allow for an accurate prediction of the permeation profile of a drug, the development of new formulations and drug delivery systems capable of improving drug uptake via the skin barrier are still needed [5]. This is particularly relevant when it comes to routes for flavonoid administration. It is now well-established that, due to its lipophilic nature, the cutaneous route is the best delivery approach for flavonoids. In fact, an array of novel formulations for topical delivery have been developed and optimized in order to increase the solubility and permeability of flavonoids across the skin barrier [5]. Nonetheless, there are still major challenges to overcome in order to successfully deliver these compounds to the skin for therapeutic purposes, including inadequate residence time and sustained release profile as well as the scalability of formulation and manufacturing process [1,3,4,5].
Targeting the optimal skin penetration pathway is an essential step for effective topical drug delivery. On that matter, drugs can be administrated through the skin in an invasive and noninvasive way. In the invasive route of administration, drugs can permeate through the skin via needle injections (subcutaneous, intramuscular, or intravenous routes) or via the implantation of a device [35]. In the subcutaneous route, the needle is inserted directly into the fatty tissue, thus reaching the bloodstream. For instance, insulin, similar to other proteins that are destroyed in the digestive tract, is administrated through this route. For larger volumes of drugs, the intramuscular route is preferred in comparison with the subcutaneous one. On the other hand, in the intravenous route, the drug is delivered directly into the bloodstream, in a well-controlled and rapid manner. The implantation of a device inserted under the skin is another invasive drug administration method and is usually considered when a controlled release of the drug with time is needed.
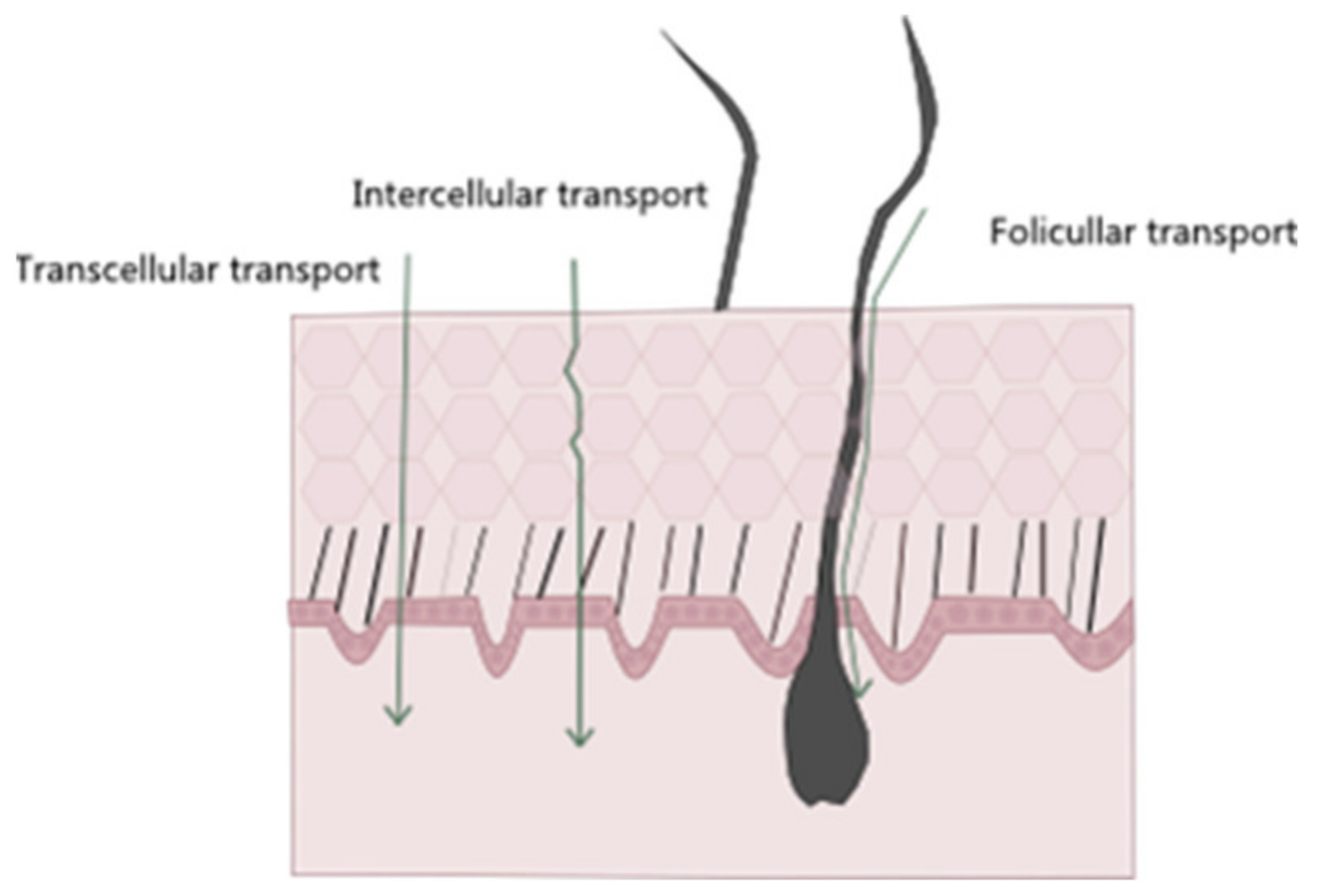
Regarding noninvasive drug administration methods, there are four possible pathways of drug permeation across the skin: the intracellular, intrafollicular, transcellular, and polar pathways (Figure 3) [36]. The intrafollicular route, sometimes classified as the appendageal route, encompasses drug permeation through the skin appendages, such as lipophilic follicular ducts, sebaceous glands, or hydrophilic sweat ducts [14,37]. In the most commonly used pathway, the intercellular one, the drug travels through the lipid matrix that occupies the intercellular spaces between the corneocytes, thus making it the preferred permeation route for lipophilic molecules. On the other hand, in the transcellular way, also known as the intracellular pathway, the drug diffuses through the various skin layers and dead cells, allowing for the transport of hydrophilic or polar molecules. Finally, in the polar pathway, the drugs permeate through the skin via polar pores available at its surface. This observed flux of drugs across the various layers of the skin is called transdermal drug delivery [15,18,38,39].
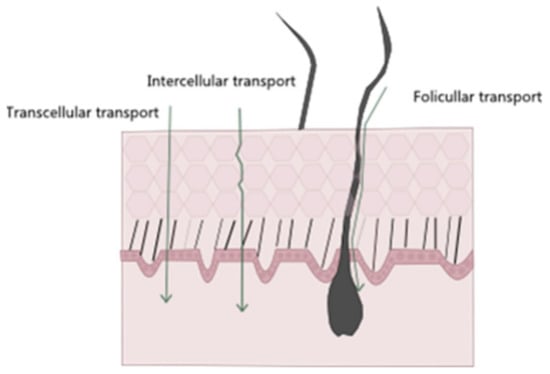
Figure 3.
Schematic representation of different entry pathways for molecules into the skin.
After passing through the SC and diffusing through the viable epidermis and dermis, the drug becomes available for its uptake into the systemic circulation [5]. Systemic absorption depends on the application site, its area, and the nature of the delivery system. Another alternative to the oral administration of drugs is topical delivery, in which the drug is intended to be absorbed at specific areas of the skin rather than being targeted for systemic delivery. Examples of drugs topically delivered to the skin include corticosteroids, antifungals, antivirals, antibiotics, antiseptics, and local anesthetics [40].
5. Flavonoids: Relevant Biochemical and Biological Properties
In addition to their well-reported strong antioxidant activity, flavonoids also exhibit the ability to modulate key cellular signaling pathways and enzymatic reactions involved in a wide range of pathophysiological events such as cell proliferation, inflammation, immune response, platelet aggregation, and cytotoxicity [41,42,43,44,45]. Studies indicate that the biological properties of flavonoids are beneficial in solving or controlling skin disorders. The following subsections briefly describe the antioxidant, anti-inflammatory, anticancer, and antibacterial activities of flavonoids, elucidating the molecular targets and mechanism of actions with an effect on skin disorders (Table 2).

Table 2.
Synopsis of the main molecular targets and mechanisms of action of flavonoids.
5.1. Antioxidant Properties
One of the best-described properties of flavonoids is their capacity to act as powerful antioxidants. In fact, flavonoids have the ability to act as free-radical scavengers and metal ion chelators as well as the capacity to affect enzymatic and non-enzymatic systems that regulate cellular redox balance [41,45,53]. Their mechanisms of antioxidant action can include (1) the suppression of reactive oxygen species formation (ROS) either through inhibition of certain enzymes or by chelating trace elements involved in the generation of free radicals, (2) scavenging ROS, and (3) the upregulation or protection of antioxidant defenses [3].
Depending on their structure, there is considerable evidence that flavonoids are effective scavengers of ROS, such as peroxyl, alkyl peroxyl, hydroxyl, and superoxide radicals as well as reactive nitrogen species (RNS), in particular, nitric oxide (NO) and peroxynitrite (ONOO−). Due to the presence of vicinal hydroxyl groups, several flavonoids can also act as chelators of redox-active metal ions, such as copper and iron, thus preventing free radical formation and lipid peroxidation [54,55,56,57].
Free metal ions enhance the formation of ROS through the reduction of hydrogen peroxide to the highly reactive hydroxyl radical. Flavonoids, due to their lower redox potential are able to reduce highly oxidizing free radicals such as hydroxyl, superoxide, and peroxyl radicals by donating a hydrogen atom. The presence of a 3′,4′-catechol group in the flavonoid structure, for example, is known to enhance their capacity to inhibit lipid peroxidation. This trait makes flavonoids highly effective scavengers of peroxyl, superoxide, and peroxynitrite radicals [58,59,60,61]. Epicatechin and rutin, for instance, were shown to be strong radical scavengers and inhibitors of lipid peroxidation in vitro [3]. Moreover, they are known to inhibit enzymes involved in ROS formation, such as the case of xanthine oxidase, myeloperoxidase, and NADPH oxidase.
On the other hand, flavonoids have the capacity to upregulate both enzymatic and non-enzymatic systems involved in the removal and detoxification of oxidant species, particularly reduced glutathione (GSH), GSH peroxidase, GSH reductase, GSH S-transferase, superoxide dismutase, and catalase, as it has been demonstrated in animal models for rutin; quercetin; daidzein; and to a lesser extent, genistein [62,63]. Certain flavonoids, such as quercetin and the catechins, have been shown to regenerate ascorbate and α-tocopherol via electron transfer reactions, thus displaying an additional antioxidant mechanism [64,65,66].
It is noteworthy that, under certain conditions, flavonoids might also exert a marked pro-oxidant activity, becoming cytotoxic. In fact, they can undergo transition metal or peroxidase-catalyzed reactions, which lead to the formation of highly reactive oxygen species that can damage proteins and DNA [67].
5.2. Anti-Inflammatory Properties
Inflammation is a biological response to tissue injury, microbial infection, and chemical irritation. During an inflammatory process, the migration of immune cells from blood vessels and the release of mediators to the site of damage are followed by the recruitment of inflammatory cells and the release of ROS and pro-inflammatory cytokines that work together to eliminate pathogens and to repair injured tissues. Flavonoids are known to significantly affect the immune system [47,68]. For instance, hesperidin, apigenin, and quercetin are among a broad spectrum of flavonoids known for their anti-inflammatory and analgesic capacity. In fact, studies have shown that, both in vitro and in vivo, flavonoids have the capacity to downregulate the expression of a wide range of pro-inflammatory genes, including the inducible nitric oxide synthase (iNOS), cyclooxygenase (COX), lipoxygenase (LOX), and several key cytokines, mainly through the inhibition of the mitogen-activated protein kinase (MAPK)- and nuclear factor-kappa B (NF-κB)-mediated signaling pathways [68,69,70]. This ability to inhibit the arachidonic acid pathway at the level of phospholipase A2, COX and LOX is of particular importance since it results in a decrease in the production of prostaglandins and leukotrienes, essential mediators of the acute inflammatory response [71,72]. Flavonoids are in fact known to modulate several steps of the inflammatory cascade both in human and animal cell types. Quercetin, kaempferol, genistein, and epigallocatechin-3-gallate (EGCG) are among the flavonoids that have been extensively studied on their ability to affect iNOS activity and NO production. They have been found to inhibit iNOS expression via the downregulation of extracellular signal regulated protein kinase 1/2 (ERK 1/2) and p38 MAPK phosphorylation and by preventing the binding of NF-κB to the iNOS gene promoter [71,73,74,75,76,77]. In addition, several flavonoids have been shown to interfere with the production and function of various pro-inflammatory cytokines, chemokines, and adhesion molecules, such as TNF-α; IL-1β, -6, and -8; monocyte chemotactic protein-1 (MCP-1); macrophage inflammatory protein-2 (MIP-2); vascular cell adhesion molecule (VCAM); and P-selectin by inhibiting the MAPK pathways, by blocking NF-κB nuclear translocation, and via COX-2 synthesis [78,79,80,81,82,83,84].
5.3. Anticancer Properties
Recent studies have demonstrated a direct link between flavonoids and their ability to prevent the development of different types of malignant tumors both in human and animal models. In fact, it is now well-established that flavonoids exert an anticarcinogenic effect by quenching oxidative stress and inflammatory response; by inducing apoptosis; by suppressing MMP secretion; and by inhibiting cell growth, tumor cell invasion, and angiogenesis [85,86,87,88,89,90,91].
Flavonoids might act on the initial steps of cancer development by preventing the DNA damage that can be induced by free radicals and carcinogens. In addition, flavonoids have been demonstrated to inhibit various types of cancer cell proliferation by inducing cell cycle arrest at the G1/S or G2/M phases through the downregulation of cyclins and cyclin-dependent kinases. They were also shown to stimulate apoptosis through the activation of caspases 3, 9, and 8 and proapoptotic proteins p53, p27, and Bax as well as via the inhibition of antiapoptotic proteins (Bcl-2 and Bid) and the release of cytochrome c [92,93,94,95,96,97,98].
Their antiproliferative and proapoptotic activity might implicate the inhibition of growth factors and its receptors such as platelet-derived growth factor (PDGF), PDGF receptor (PDGFR), and epidermal growth factor receptor (EGFR) in addition to the activation of NF-κB and the inhibition of the Akt/PI3K, ERK and activating protein-1 (AP-1) pathways [99,100]. For instance, flavonoids such as EGCG, quercetin, genistein, luteolin, and the anthocyanins were able to reduce angiogenesis, a key event in tumor growth, invasion, and metastasis via the downregulation of VEGF, VEGFR, PDGF, PDGFR, EGFR, and MMP. Other flavonoids were also shown to affect cancer cell adhesion and movement by inducing cytoskeletal modifications, by inhibiting cell adhesion to fibronectin, by reducing integrin expression and disrupting the stress fibers, and by reducing myosin II regulatory light chain phosphorylation [86,87,88,101,102,103].
5.4. Antibacterial Properties
Flavonoids are naturally synthesized by plants in response to microbial infection. Similarly, it has been found that they exert in vitro antimicrobial activity against a wide range of microorganisms. In fact, flavonoids such as apigenin, galangin, flavonol glycosides, isoflavones, and flavanones have all been shown to possess strong antibacterial activity [1]. Given their antibacterial properties, flavonoids are being used as wound healing agents.
6. Bioavailability of Flavonoids
One of the major concerns regarding the use of flavonoids as therapeutic agents is their relatively low bioavailability. Even in the presence of a large daily intake of flavonoids in dietary sources, their plasma and tissue concentrations are often insufficient to exert the desired pharmacological effects [3]. Due to several factors that include chemical structure and molecular weight, relatively low water solubility, absorption and metabolism in the gastrointestinal tract, lack of site specificity in distribution, and rapid elimination, flavonoids have generally low bioavailability, which largely affects their therapeutic potential. Moreover, this class of compounds is highly susceptive to degradation upon oxygen exposure, temperature changes, ultraviolet radiation, or pH change [104,105,106].
After being absorbed by the intestinal epithelium, flavonoids undergo extensive biotransformation into conjugated products, namely glucuronides, sulphates, and methylated derivatives, first in the intestine and then in the liver, where they are secreted into bile [107,108]. Thus, the bioavailability and the subsequent cell and tissue accumulation of the different flavonoids essentially depend on the multidrug-resistance-associated proteins (MRP-1 and MRP-2), ubiquitously expressed as ATP-dependent efflux transporters. The actual flux of a flavonoid from the gut lumen to the blood stream and the various organs depends on the tissue distribution of MRP-1 and MRP-2 as well as on their substrate’s affinity. This metabolic pathway is called phase III metabolism. However, it appears that certain phase II metabolic derivates of flavonoids can act as competitive substrates of the MRP-mediated membrane transporters and the potential use of flavonoids as a mean to overcome transporter-mediated chemotherapy resistance due to the frequent overexpression of MRP in several types of cancer is based on this property. The intestinal absorption of quercetin, for instance, is favored in the aglycone form, and its metabolism in the gut and liver appears to be relatively high, so that less than 2% of ingested quercetin is recovered on the plasma [3]. Additionally, after oral administration of flavonoids, a significant amount can reach the colon and can interact with microbiota. Microbiota can, for instance, metabolize some flavonoids to smaller phenolic compounds with similar biological effects and improved bioavailability; however, on the other hand, it can also extensively metabolize flavonoids via the glucuronidase and sulfatase enzymes, cleaving the heterocycle break and producing inert polar compounds that are rapidly excreted without producing any biological effect [104].
In addition, flavonoids have been reported to significantly inhibit the activity of the cytochrome P450 system, which can result in an increase of the half-life and concentration of many drugs, thus enhancing their toxicity and side effects [109]. Flavonoids such as quercetin, ECG, EGCG, and sylibin have been shown to downregulate the cytochrome CYP3A4, which is the major cytochrome P450 isoenzyme in the intestine and is responsible for the metabolism of approximately 50% of all prescribed drugs, thus increasing the risk of potential toxicity, especially of drugs with a limited therapeutic window [110]. Flavonoids can also interact with ATP-binding cassette (ABC) transporters, inhibiting them, which can increase the bioavailability of poorly available drugs, on the one hand, but it can also potentiate the toxicity of other ABC transporters substrates [111]. Thus, flavonoid encapsulation in effective nano-carrier systems can not only improve their pharmacokinetics and therapeutic potential but also avoid enhancement of the toxicity and side effects of drugs that can concomitantly be administrated with these compounds [104].
The rapid metabolic elimination of flavonoids, together with the evidence that they are able to interact with the metabolism of other drugs, highlights the need to develop novel ways to improve the delivery of flavonoids. Cutaneous administration emerges as an alternative option to common oral and parenteral routes [112,113]. Skin drug delivery is one of the most preferred administration routes with higher patient compliance and satisfaction. The advantages also include the avoidance of liver first pass metabolism effects, metabolic degradation associated with oral administration, and minimal systemic side effects.
7. The Need for Nanocarriers in Cutaneous Flavonoid Delivery
Despite flavonoids’ pharmacological potential, dietary flavonoids present several disadvantages, mentioned in Section 6, hindering their clinical potential. In addition, the fact that flavonoids can suffer an enhanced complexation or precipitation when ingested with other food components as well as degradation by microbiota greatly reduces their bioavailability and stability. On that matter, cutaneous delivery is one of the most advantageous routes in overcoming the challenges associated with flavonoid administration [3,104].
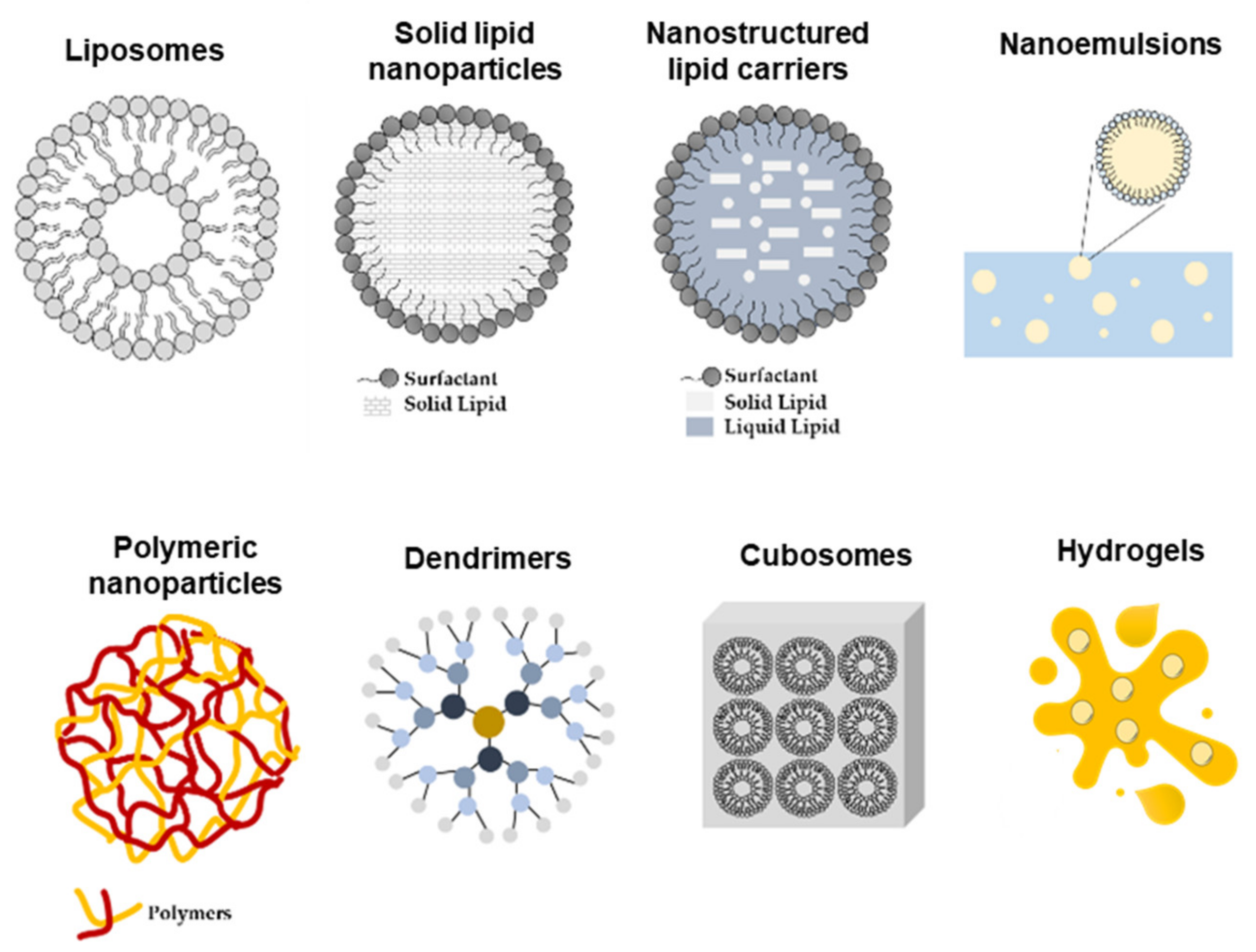
Nonetheless, the impermeable nature of the skin presents a serious challenge to cutaneous delivery, where in most of the cases the therapeutic effect produced by the conventional drug dosage is not sufficiently effective. Thus, the development of nano-engineered delivery systems for flavonoids capable of increasing the solubility and bioavailability and of providing a site-specific delivery with improved pharmacokinetic properties is imperative. Thus far, gels are the most common form of topical drug administration, including hydrogels and olegels. However, other delivery systems such as lipid and polymeric nanoparticles, microparticles, and transferosomes, among others, are currently being developed (Figure 4). These carriers can later be formulated into creams and gels, improving patient compliance [5].
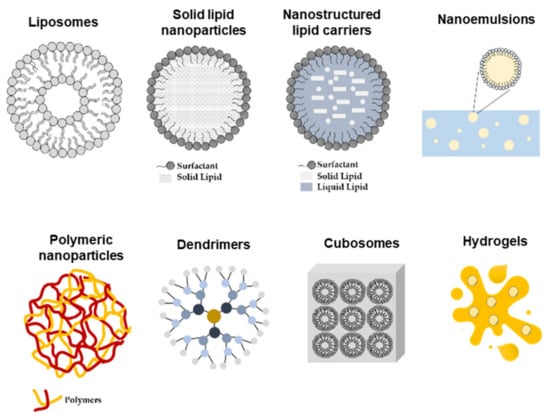
Figure 4.
Schematic representation of nano-delivery systems used for topical skin delivery.
7.1. Nano-Delivery Systems: Advantages and Limitations
The development of novel drug delivery systems, which allow for the cutaneous delivery of otherwise poorly effective compounds with undesirable physicochemical and pharmacokinetics parameters, can improve their efficacy and safety. Nanotechnology tools designed for skin drug delivery include microdevices (1–1000 µm) and nanodevices (1–1000 nm) for drug delivery [112]. Micro-delivery vehicles can act as reservoirs for a drug that is released into the tissue interstitial space. Due to their size, they can cross the skin barrier and directly deliver the drug to the site of action, minimizing toxicity and prolonging release [3,51].
Despite great progress, the development of a successful drug delivery system is still a challenging task that requires meticulous selection of the vehicle according to the active agent. In fact, the safety of the chosen materials, eventual harmful degradation products, and high cost of the final product are major limitations that need to be addressed.
The use of nanocarriers allows for an improvement in crucial drug properties, including solubility, diffusivity, blood circulation half-life, and immunogenicity. However, there are some essential prerequisites for the development of a successful targeted drug delivery vehicle, including the physicochemical and biological properties of the vehicle [114]. For instance, size, charge, and surface hydrophilicity are all properties that can impact the circulating half-life of the particles as well as their biodistribution. Small molecule-, peptide-, or nucleic acids-loaded nanoparticles are not as easily recognized by the immune system; in addition, the presence of targeting ligands can increase the interaction of drug delivery systems with the cells and can enhance cellular uptake by receptor-mediated endocytosis [115]. Nevertheless, there are some limitations on the use of nanocarriers, namely storage, generation of pro-oxidant chemical species, and unexpected pro-inflammatory response, which need to be considered in their design.
In summary, the advantages of nanocarriers application for cutaneous drug delivery include (1) targeted delivery, with maximized efficacy and minimized systemic side effects; (2) controlled drug release; (3) prolonged half-life in the systemic circulation; (4) improved patient compliance; (5) improved drug solubility and permeability; (6) protection against degradation; (7) delivery of multiple drugs with a synergistic effect; and (8) improved biocompatibility [3,115,116,117].
7.2. Nano-Delivery Systems Applied for Flavonoid Cutaneous Administration
Among the numerous nano-based drug delivery systems that have been developed so far, lipid-based nanoparticles, including liposomes and lipid nanoparticles as well as polymeric-based nanoparticles, are most commonly used for flavonoid delivery [3]. Liposomes are concentric vesicles consisting of an aqueous core surrounded by a membranous lipid bilayer that, thanks to their structure, can encapsulate hydrophilic, hydrophobic (in the lipid bilayers), and amphipathic molecules. To avoid the rapid elimination of liposomes from the blood by the cells of the reticuloendothelial system (RES), primarily in the liver and spleen, their structure can be modified by coating their surface with inert and biocompatible polymers such as polyethylene glycol (PEG) [118,119,120,121].
Solid lipid nanoparticles (SLN) are nanocarriers composed by a solid hydrophobic core and stabilized by a surfactant. Among the main advantages of using SLN as drug carriers, their high stability and capacity to protect the incorporated drugs from degradation, the controlled drug release, site-specific targeting, and good biocompatibility stand out. However, they often display low loading capacity as well as a short storage time with frequent drug expulsion. SLN can be administered by the parenteral, oral, transdermal, dermal, and ocular routes. In addition, they have higher stability compared with liposomes and, due to their easy biodegradability, are less toxic than polymeric nanoparticles, making them highly versatile drug delivery vehicles. Their primary applications target skin disorders; for example, curcumin loaded in SLNs featured a controlled drug release over 24 h and effective skin deposition for the reduction in pigmentation and inflammation in Balb/c mouse skin [117,122,123].
Regarding its potential application as a cutaneous drug delivery system, SLN-enhanced SC permeation is attributed to (1) prolonged contact with the skin surface; (2) their occlusive nature, since they form a film on the surface of the skin that combines with the skin lipids promoting a reduction in water loss and hydration of the skin; and (3) the interaction between the lipids in the nanoparticles and SC lipids, which facilitates permeation of lipid-soluble compounds.
The use of cationic lipids on the nanoparticle’s composition allows for an interaction with the negatively charged skin surface. For instance, a highly positively charged (+51 mV) SLN using cationic phospholipids, tween 20 as a surfactant, tricaprin as a solid lipid core, and encapsulating plasma DNA was shown to have enhanced in vitro permeation into mouse skin and the expression of mRNA in vivo after topical application [124].
Liquid lipids (oils) can be added to a solid lipid, creating an irregular lipid matrix, called the nanostructured lipid carriers (NLC). The lipids’ spatial structure allows for an increased drug loading capacity and better stability compared with SLN. Studies have shown that both NLC and SLN display similar mechanisms of skin permeation enhancement, through occlusion and mixing between the formulation and the SC lipids, although the presence of a liquid lipid is known to increase the solubilization and loading capacity, thus resulting in greater skin deposition [3,124].
Polymeric nanoparticles are colloidal structures composed of natural or synthetic polymers. Depending on their shape, they can be classified as nanocapsules, vesicular systems with the drug in a core surrounded by a polymeric membrane, and nanospheres, which are porous matrixes in which the drug is uniformly dispersed [125,126]. The most common synthetic polymers used in the preparation of these nanoparticles are poly(lactic acid) (PLA), poly(lactide-co-glycolide) (PLGA), poly(methyl methacrylate) (PMMA), and poly(alkylcyanoacrylate) (PACA) [127,128,129,130,131,132]. In addition, natural polymers such as alginate, gelatin, chitosan, and albumin are also frequently used since they are less toxic compared with synthetic polymers. Polymeric nanoparticles feature biocompatibility, biodegradability, stability, and surface modification potential, therefore allowing for the controlled release of both hydrophobic and hydrophilic compounds as well as proteins, peptides, or nucleotides to the specific site of action. To avoid rapid removal from blood and to reduce its cytotoxicity, polymeric nanoparticles can be covered with a non-ionic surfactant or coated with hydrophilic substances such as PEG or carbohydrates, thus reducing opsonization [3].
8. Cutaneous Delivery Systems of Flavonoids for Treatment of Skin Pathologies
Cutaneous delivery of flavonoids is a powerful strategy to avoid systemic toxicity while restricting the therapeutic effects to the specific site of action. However, one of the major challenges that a topical delivery system faces is the ability to overcome the SC barrier against foreign substances [5]. In addition, most flavonoids are highly lipophilic compounds and their permeation across the SC into viable skin layers is hindered by their affinity for SC components and the tendency to be retained in this layer. Thus, there has been a growing interest in the use of nanotechnology as a strategy for a more efficient flavonoid delivery to the human body (Figure 5). Nano-delivery systems are in fact excellent tools to overcome the challenges associated not only with the cutaneous absorption of the drug per se but also with flavonoid pharmacology, including low solubility, short half-life, and poor bioavailability [5,124].
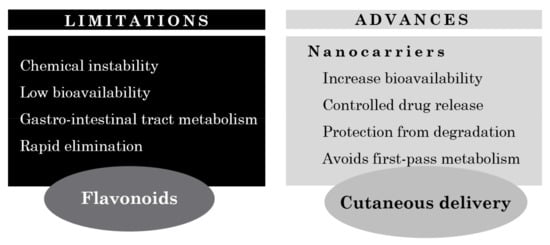
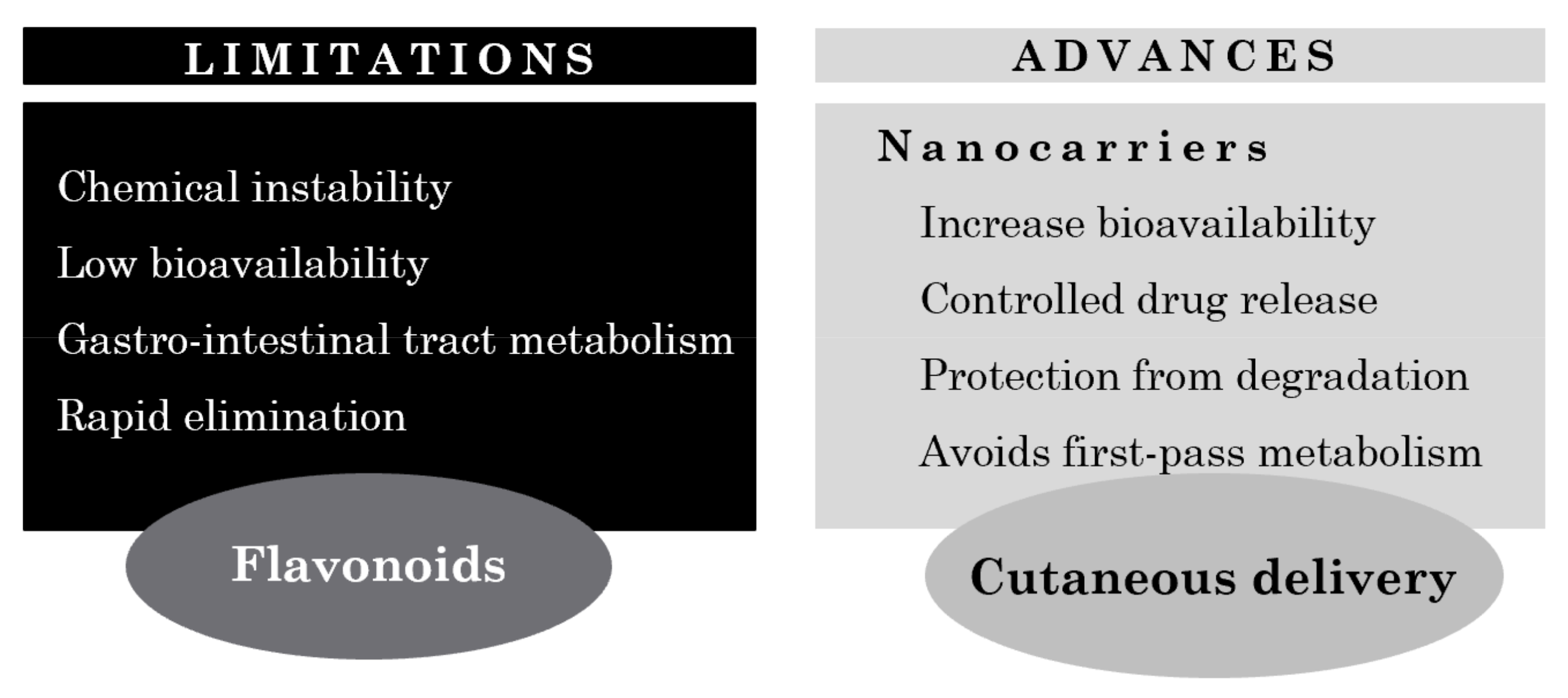
Figure 5.
Limitations and advances on cutaneous flavonoid delivery.
8.1. Examples of Nanocarriers Designed for Flavonol Cutaneous Delivery
Flavonols are O-glycosidic ketonic compounds with a sugar moiety at the 3-position that act as powerful antioxidants, protecting the skin from ROS formation. Compounds belonging to this family of flavonoids are quercetin, kaempferol, and myricetin, among others [133].
Quercetin, one of the best studied and most common flavonoid found in nature, was shown to have poor permeability across excised human skin [4]. For that, this flavonoid has been incorporated into different delivery systems, including nanoemulsions, nanocapsules, lipid nanoparticles, and microemulsions, to increase its solubility and skin permeability [5]. Casagrande and colleagues incorporated quercetin into two different oil-in-water emulsions with a distinct lipid content in order to evaluate their potential application as a topical delivery system. The in vivo results demonstrated that these formulations were an effective vehicle for topical application of quercetin with the goal of controlling ultraviolet B (UVB)-induced skin damage [4,134]. Based on these results, other studies were conducted to design novel delivery systems to increase quercetin effectiveness when topically applied. For example, quercetin was incorporated into a liquid, crystalline formulation and the influence of this vehicle in the antioxidant activity of this flavonoid was evaluated in vitro. The presence of a liquid, crystalline structure allowed for an easier diffusion through the skin and a considerable solubilizing capacity for both oil- and water-soluble compounds. Scalia and colleagues also demonstrated that the incorporation of quercetin in lipid microparticles improved its photostability and chemical stability as well as its biocompatibility [135,136]. In another study, Tan and colleagues investigated the potential of using lecithin-chitosan nanoparticles as a topical delivery system for quercetin. Compared with quercetin in its free form, the quercetin-loaded nanoparticles displayed higher permeation ability and significant accumulation of quercetin in the skin, particularly in the epidermis. In addition, microstructure observations of the skin surface following administration showed that the interaction between constituents of the nanoparticles and the skin surface markedly changed the morphology of the SC and disrupted the corneocyte layers, therefore facilitating permeation and accumulation of quercetin in the skin [137].
Nan and colleagues evaluated the efficacy of topically applying quercetin-loaded chitosan nanoparticles against UVB radiation. The authors demonstrated that quercetin, if entrapped into chitosan nanoparticles, could be efficiently up taken by HaCaT cells (keratinocytes) and could easily permeate through the epidermis layer while displaying better stability and lower cytotoxicity. Moreover, they also found that quercetin-loaded nanoparticles could enhance the effects of this flavonoid when inhibiting the NF-kB/COX-2 signaling pathway as well as when ameliorating the skin edema caused by UVB radiation [138].
Bose and Michniak-Kohn developed a solvent-free NLC formulation of quercetin using probe ultrasonication and evaluated the feasibility for topical delivery. Formulation factors such as the nature of the lipid (solid/combination of solid and liquid) in the SLN and NLC systems and the drug loading capacity were evaluated to produce the optimal formulation with an adequate physical stability. Overall, the NLC system showed the highest improvement in the topical delivery of quercetin, manifested by the amount of quercetin retained in full-thickness human skin compared with a control formulation with a similar composition and particle size in the micrometer range, thus demonstrating the feasibility of NLC systems for improved cutaneous delivery of this compound [139].
Penetration enhancer containing vesicles (PEVs) are also known to be powerful enhancers for dermal delivery due to the presence of both phospholipids and penetration enhancers (PE), which provide a synergistic effect on skin permeation. PE increases the fluidity of the lipids of the SC, facilitating the delivery of drug-loaded vesicles and its subsequent diffusion through the skin [5]. Hence, in 2011, Chessa and colleagues developed quercetin-loaded PEVs, formulated using four different hydrophilic PE, and characterized them by size, surface charge, loading capacity, and morphological and viscoelastic features. In addition, their penetration capability and distribution through pig skin were assessed to obtain the optimal formulation for the delivery of quercetin to the skin [140]. In another study, performed by Caddeo and colleagues, quercetin-loaded phospholipid vesicles, in particular liposomes and PEVs, were developed in order to study their efficacy on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation. In vivo results demonstrated that the vesicles, in particular PEVs, were capable of delivering the drug to the inflammation site, that is the dermis, inhibiting oxidative stress and leukocyte accumulation as well as stimulating the repair of skin damaged induced by TPA. Through this work, the authors highlighted the use of vesicular systems, particularly PEVs, as a delivery vehicle for flavonoids, with a therapeutic potential to treat inflammatory skin disorders [141].
Kaempferol is another well-known flavonol with antioxidant, anti-inflammatory, anticancer, and antiallergic properties [142]. In fact, Wang and colleagues reported that kaempferol inhibited the iNOS mRNA expression and prostaglandin E2 production in a dose-dependent manner, by inhibiting in part the NF-kB signaling pathway [143]. Furthermore, Park et al. reported that kaempferol was also able to inhibit the activation of inflammatory NF-kB transcription factor via nuclear factor-inducing kinase (NIK)/IkB kinase (IKK) and MAPKs in aged rat kidney [120]. Nonetheless, studies have shown that this flavonoid undergoes excessive first pass metabolism and, as a consequence, displays a bioavailability rate of only 2%, thus making it a good candidate for cutaneous application [142]. Keeping that in mind, Yun Chao and colleagues developed submicron emulsions to be employed as a delivery system for the topical application of kaempferol. These submicron emulsion systems are oil-in-water dispersions with small droplet sizes in the range of 100–600 nm. In comparison with traditional drug delivery vehicles, they are easy to manufacture, are more thermodynamically stable, and exhibit enhanced drug solubilization as well as increased drug permeation rates. Additionally, submicron emulsions were demonstrated to be a potential vehicle for the transdermal and topical delivery of lipophilic and hydrophilic drugs. In this study, kaempferol-loaded submicron emulsions with different water/oil/surfactant/cosurfactant ratios were prepared, and different physiochemical properties (e.g., viscosity, droplet size, permeation rate, lag time, and deposition amount in skin) were determined in order to evaluate the effectiveness of this delivery system for the cutaneous application of kaempferol. Overall, the authors demonstrated that, based on the permeation parameters, including the increase in the cumulative amount of drug over 12 h and deposition in the skin, in addition to a shorter lag time, submicron emulsions may be a promising vehicle for cutaneous application of kaempferol [142].
8.2. Examples of Nanocarriers Designed for Other Flavonoid Classes’ Topical Delivery
Isoflavones, are naturally occurring isoflavonoids mainly found in soybeans, soy foods, and legumes. They are non-steroidal compounds that act as phytoestrogens as they exert pseudohormonol activity by binding to estrogen receptors in mammals. The most common isoflavones are genistein and daidzein [5]. Huang and colleagues assessed the potential topical delivery and dermal use of soy isoflavones genistein and daidzein, using α-terpineol and oleic acid as PE, both in vitro and in vivo. As demonstrated in vivo, there was an increase in the uptake of genistein an daidzein, with no toxic effects, and a decrease in the erythema. In vitro studies showed an inhibition of UVB-induced intracellular H2O2 production and the consequent protection of keratinocytes against UVB radiation, suggesting that a reduction in photodamage to the skin via the topical application of antioxidants could be an efficient way to enrich the endogenous cutaneous protection system [143,144].
Apigenin is a hydrophobic, polyphenolic flavonoid known to possess antioxidant, antimicrobial, anti-inflammatory, antiviral, antidiabetic, and tumor inhibitory activities. In particular, this flavonoid was demonstrated to act as a chemo-preventive by inhibiting the enzyme CYP2C and by preventing the metabolism of many drugs and xenobiotics. Similar to the already mentioned flavonoids, the clinical potential of apigenin is suppressed by its poor aqueous solubility, low oral bioavailability, and rapid metabolism. Thus, the development of novel formulations is a necessary step to overcome these limitations and to improve apigenin delivery [5]. On that matter, several formulations have been developed so far, including liposomes, nanocrystal gel formulations, and self-micro-emulsifying drug delivery systems. Munyendo and colleagues reported that the formulation of D-α-tocopherol acid and polyethylene glycol 1000 succinate (TPGS) stabilized the mixed micelles of apigenin and phospholipids, creating an effective drug delivery vehicle capable of enhancing the bioavailability of this flavonoid [145]. Karthivashan and colleagues prepared “flavonosomes”, which are phytosomes loaded with multiple flavonoids, using phosphatidylcholine as a carrier and evaluated their in vitro pharmacokinetics and toxicity [146]. Shen and co-workers evaluated a novel topical delivery system for apigenin by using soy lecithin-based ethosomes, demonstrating a higher skin targeting capacity and a significant reduction in COX-2 levels in mouse skin inflammation induced by UVB light [147].
Luteolin is another promising flavonoid with potential antiarthritic activity. In addition, due to its lipophilicity, it can be used in topical formulations to treat psoriasis [148]. Niosomes are non-ionic surfactant-based colloidal systems that have the ability to encapsulate both hydrophobic and hydrophilic drugs. Abidin and co-workers prepared luteolin-loaded niosomes using different non-ionic surfactants and characterized them for their in vitro and in vivo antiarthritic activity. The optimized formulation was later converted into gel using Carbopol as a gelling agent for enhanced transdermal luteolin delivery. The in vivo bioactive studies revealed that the niotransgel formulation of luteolin was able to provide good antiarthritic activity, with the results being comparable with standard diclofenac gel formulation [149]. In another study, Shin and colleagues, established a nanoemulsion-based follicular delivery system, in which luteolin was incorporated into oil-in-water nanoemulsions. In vivo studies proved that these luteolin-loaded nanoemulsions possessed hair-growth promotion ability. In fact, when nanoemulsions are formed by the assembly of amphiphilic polymers at the oil/water (O/W) interface, they provide an efficient system for the encapsulation of poorly water-soluble substances, resulting in better bioavailability, accurate dosing, and minimal side effects [150].
Catechins are a group of flavonoids that belong to the flavanol family and are present in high concentrations in a variety of plant-based fruits, vegetables, and beverages. Belonging to this family are catechin, epicatechin (EG), and EGCG. EGCG, in particular, has captured a lot of attention due to its broad spectrum of biological properties, including antioxidant, photoprotective, antiviral, and antibacterial as well as anticancer and neuroprotective effects. Nevertheless, its clinical use has been limited due to its poor systemic absorption and low bioavailability [5]. With the goal to overcome this problem and to increase EGCG clinical applicability, Avadhani and co-workers developed nano-transfersomal formulations of EGCG for an efficient permeation into the SC and delivery into the skin [151]. In addition, hyaluronic acid (HA) was also encapsulated in the transfersomes not only because it is widely distributed in connective tissues and is a main component of the extracellular matrix but also because it is a non-irritating biopolymer and antiaging agent with high biocompatibility, specific viscoelasticity, and hydration and lubrification properties. The optimized transfersomal formulation containing EGCG and HA displayed a high free radical scavenging effect while showing no cell toxicity. In addition, the formulation was able to suppress the MDA and ROS levels to a significant extent in human keratinocytes as well as the expression levels of MMP-2 and MMP-9. The encapsulation of EGCG in the transfersomes resulted in higher skin permeation and deposition of this flavonoid in the skin, compared with plain EGCG. Interestingly, the co-entrapment of HA in the formulation increased both the skin permeation and deposition of EGCG, thus demonstrating that this system constitutes a useful and effective EGCG cutaneous delivery vehicle, with synergistic antiaging and antioxidant benefits [151].
Fang and colleagues assessed the possibility of using multilamellar phosphatidylcholine (PC) liposomes studied for topical and intratumor delivery administration of catechin, EC, and EGCG in nude mice [152,153]. The authors showed that the inclusion of anionic species such as deoxycholic acid and dicetyl phosphate increased the encapsulation of the catechins and the permeability of the lipid bilayers. EGCG performed differently due to its higher lipophilicity. In addition, the authors reported an even higher EGCG encapsulation for deoxycholic acid-liposomes prepared in the presence of 15% ethanol as well as an increased catechin in vitro and in vivo skin permeation and deposition in basal cell carcinomas compared with both the free form and ethanol-free liposomes. This might be attributed to the fact that ethanol-enriched liposomes penetrate easily in the skin due to the increased elasticity conferred by the insertion of alcohol into the PC membranes. The results showed that optimization of the physicochemical features and composition of liposomes could control and improve the delivery of catechins. Moreover, the results suggested that the intratumor administration of liposomes might be an effective approach for the local treatment of solid tumors [152,153].
Overall, there are several strategies that can be adopted to increase the solubility and subsequent bioavailability of flavonoids with therapeutic potential. Although much progress has been recently made, novel drug delivery systems suitable for an optimized topical application should continue to be explored [112,154,155,156,157]. A summary of the therapeutic application of flavonoids and the different nanocarriers used to enhance their delivery to the skin is described in Table 3.

Table 3.
In vitro and in vivo studies using different nanocarriers for enhanced topical delivery of flavonoids to the skin.
9. Concluding Remarks
In the last few years, flavonoids have been extensively studied for their remarkable antioxidant, anti-inflammatory, anticancer, and antibacterial properties. However, their lipophilic nature and poor aqueous solubility invariably lead to limited oral bioavailability. In addition, flavonoids are rapidly degraded and metabolized in the human body, which greatly hinders their clinical application. Thus, oral delivery faces many challenges, and recently, there has been a shift towards the development of new formulations and alternative delivery routes, namely cutaneous administration. Flavonoid encapsulation is also an effective way not only to improve their pharmacokinetics but also to avoid degradation and improve safety. Various novel formulations aiming at cutaneous delivery have been developed with the goal to increase the solubility and permeability of flavonoids across the skin barrier, with minimal adverse effects. However, there is still the need to overcome limitations, such as a sustained release profile and skin retention time, to achieve an effective therapeutic dosage. Within the literature, different experimental protocols have been applied, hampering comparisons and progress to clinical evaluation. There is some indication of a relation between flavonoid’s chemical structure and the most suitable delivery system, but given the diversity of the skin models used, it is not possible to establish such a relation. Other technical issues can limit the translation to industrial process, as laboratorial methods are a challenge to scale up.
Currently, several cutaneous formulations for flavonoids have been described in the literature and some have been patented, which indicates the relevance of these natural compounds, and the difficulty to certify for safety and efficacy towards translation to the market. Stakeholders need to come forward and to support long clinical trials that allow for the evaluation of adverse effects and for the identification of a dosage scheme. Clinical trials must be based on solid preclinical results obtained in appropriate models. The use of animal models (e.g., mice and rabbits) in preclinical studies present limitations related to a lack of similarity to human skin. The research community’s awareness to the search for alternative models finds solutions on mimetic skin models (e.g., reconstituted human epidermis and phospholipid-based permeation assays) as data show a good correlation to human skin absorption and permeability features.
Flavonoids will continue to be explored both as a therapeutic and preventive tool for several disease conditions, alone or in combination (several synergistic effects have been described). A continuous growth in the search for novel strategies to empower flavonoid use is expected given their demonstrated potential as active agent. In the future, limitations on the cutaneous application of flavonoids will be overcome and translational advances towards commercialization will bring novel skin products to the market and to society.
Author Contributions
Writing-original draft preparation; R.C.; writing-review and editing; S.A.C.L.; funding acquisition; P.G. and S.R.; revising the manuscript; S.A.C.L., P.G., S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds
(FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da
Ciência, Tecnologia e Ensino Superior) through the project
UIDB/50006/2020 | UIDP/50006/2020.
Acknowledgments
S.C.L. acknowledges funding from FCT
(CEECIND/01620/2017). R.C. acknowledges funding from project
NORTE-08-5369-FSE-000050.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 4, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and Development of Nanovehicle-Based Delivery Systems for Preventive or Therapeutic Supplementation with Flavonoids. Curr. Med. Chem. 2009, 17, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.V.; Casagrande, R. Studies in Natural Products Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36. [Google Scholar]
- Nagula, R.L.; Wairkar, S.J. Recent advances in topical delivery of flavonoids: A review. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Yang Wang, T.; Li, Q.; Shun Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2017, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, K.R.R.; Khan, H.S.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2018, 194, 107–131. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Shelke, S.J.; Shinkar, D.M.; Saudagar, R.B. Topical gel: A novel approach for development of topical drug delivery system. Int. J. Pharm. Technol. 2013, 5, 2739–2763. [Google Scholar]
- Menaa, F.; Menaa, A.; Menaa, B. Polyphenols Nano-Formulations for Topical Delivery and Skin Tissue Engineering. In Polyphenols in Human Health and Disease; Elsevier Inc: Amsterdam, The Netherlands, 2014; pp. 839–848. [Google Scholar]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An Update of the Defensive Barrier Function of Skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Costa Lima, S.A.; Reis, S. Nanoparticles in Life Sciences and Biomedicine; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery. Dermatol 2012, 3, 2065–2073. [Google Scholar]
- Forster, M.; Bolzinger, M.A.; Fessi, H.; Briancon, S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur. J. Dermatol. 2009, 19, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matejuk, A. Skin Immunity. Arch. Immunol. et Ther. Exp. 2018, 66, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Flacher, V.; Tripp, C.H.; Mairhofer, D.G.; Steinman, R.M.; Stoitzner, P.; Idoyaga, J.; Romani, N. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol. Med. 2014, 6, 1191–1204. [Google Scholar] [CrossRef]
- Shklovskaya, E.; O’Sullivan, B.J.; Ng, L.G.; Roediger, B.; Thomas, R.; Weninger, W.; de St Groth, B.F. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 2011, 108, 18049–18054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashem, S.W.; Hani, M.; Kaplan, D.H. Skin Immunity to Candida albicans. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kitashima, D.Y.; Kobayashi, T.; Woodring, T.; Idouchi, K.; Doebel, T.; Voisin, B.; Adachi, T.; Ouchi, T.; Takahashi, H.; Nishifuji, K. Langerhans Cells Prevent Autoimmunity via Expansion of Keratinocyte Antigen-Specific Regulatory T Cells. eBioMedicine 2018, 27, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.K.; Philips, R.L.; Eriksson, A.U.; Kim, P.J.; Halder, R.C.; Lee, D.J.; Singh, R.R. Langerhans cells maintain local tissue tolerance in a model of systemic autoimmune disease. J. Immunol. 2015, 195, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, E.X.; Fang, C.; Bane, K.L.; Long, A.T.; Naudin, C.; Kucukal, E.; Gandhi, A.; Brett-Morris, A.; Mumaw, M.M.; Izadmehr, S.; et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Invest. 2018, 128, 944–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar] [CrossRef]
- Long, H.; Zhang, G.; Wang, L.; Lu, Q. Eosinophilic skin diseases: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 189–213. [Google Scholar] [CrossRef]
- Souyoul, S.A.; Saussy, K.P.; Lupo, M.P. Nutraceuticals: A review. Dermatol. Ther. 2018, 8, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Marks, J.G.; Miller, J.J. Lookingbill Marks’ Principles of Dermatology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019; pp. 2–10. [Google Scholar]
- Zaki, N.M. Progress and Problems in Nutraceuticals Delivery. J. Bioequiv. Bioavailab. 2014, 6, 75–77. [Google Scholar]
- Routes for Drug Administration through the Skin. Available online: https://www.msdmanuals.com/home/drugs/administration-and-kinetics-of-drugs/drug-administration (accessed on 17 May 2019).
- Pouillot, A.; Dayan, N.; Polla, A.S.; Polla, L.L.; Polla, B.S. The stratum corneum: A double paradox. J. Cosmet. Dermatol. 2008, 7, 143–148. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.N.; Attama, A.A. Application of Nanotechnology in Drug Delivery; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Van Gele, M.; Geusens, B.; Brochez, L.; Speeckaert, R.; Lambert, J. Three-dimensional skin models as tools for transdermal drug delivery: Challenges and limitations. Expert Opin Drug Deliv. 2011, 8, 705–720. [Google Scholar] [CrossRef]
- Kulkarni, V.S. Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–36. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sang, S.; Hou, Z.; Lambert, J.D.; Yang, C.S. Redox properties of tea polyphenols and related biological activities. Antioxid. Redox Signal. 2005, 7, 1704–1714. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Robards, K. Bioactivity and structure of biophenols as mediators of chronic diseases. Crit. Rev. Food Sci. Nutr. 2008, 48, 929–966. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.H.; Khera, R.; Ohno-Machado, L.; Sandborn, W.J.; Singh, S. Annual burden and costs of hospitalization for high-need, high-cost patients with chronic gastrointestinal and liver diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zheng, Y.; Chen, X. Drugs for autoimmune inflammatory diseases: From small molecule compounds to anti-TNF biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Baker, D.; Marta, M.; Pryce, G.; Giovannoni, G.; Schmierer, K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017, 16, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mak, P.; Leung, Y.K.; Tang, W.Y.; Harwood, C.; Ho, S.M. Apigenin Suppresses Cancer Cell Growth through ERβ1. Neoplasia 2006, 8, 896–904. [Google Scholar]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Yen, G.C.; Lai, H.H. Inhibition of reactive nitrogen species effects in vitro and in vivo by isoflavones and soy-based food extracts. J. Agric. Food Chem. 2003, 51, 7892–7900. [Google Scholar] [CrossRef]
- D’Ischia, M.; Panzella, L.; Manini, P.; Napoletano, A. The chemical basis of the antinitrosating action of polyphenolic cancer chemopreventive agents. Curr. Med. Chem. 2006, 13, 3133–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, T.; Prakosay, I.; Date, R.A.; Sies, H.; Schewe, T. Oxidative modification of low-density lipoprotein: Lipid peroxidation by myeloperoxidase in the presence of nitrite. Biol. Chem. 2004, 385, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; Tromp, M.N.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A.A. Flavonoids as scavengers of nitric oxide radical. Biophys. Res. Commun. 1995, 214, 755–759. [Google Scholar] [CrossRef]
- Trujillo, M.; Ferre-Sueta, G.; Radi, R. Peroxynitrite detoxification and its biologic implications. Antioxid. Redox Signal. 2008, 10, 1607–1619. [Google Scholar] [CrossRef]
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with alpha-linolenic acid. Free Radic. Biol. Med. 1999, 27, 1313–1323. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Structure–Activity Relationships for Antioxidant Activities of a Series of Flavonoids in a Liposomal Syste. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic Compounds Rutin and o-Coumaric Acid Ameliorate Obesity Induced by High-Fat Diet in Rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef]
- Amália, P.M.; Possa, M.N.; Augusto, M.C.; Francisca, L.S. Quercetin prevents oxidative stress in cirrhotic rats. Dig. Dis. Sci. 2007, 52, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, L.M.; Yang, L.; Liu, Z.L. Evidence for α-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef]
- Frank, J.; Budek, A.; Lundh, T.; Parker, R.S.; Swanson, J.E.; Lourenço, C.F.; Gago, B.; Laranjinha, J.; Vessby, B.; Kamal-Eldin, A. Dietary flavonoids with a catechol structure increase α-tocopherol in rats and protect the vitamin from oxidation in vitro. J. Lipid Res. 2006, 47, 2718–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, S.; Ishihara, M.; Atsumi, T.; Kadoma, Y. A quantitative approach to the free radical interaction between alpha-tocopherol or ascorbate and flavonoids. In Vivo 2006, 20, 445–452. [Google Scholar] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Ristagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filasi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanità. 2007, 43, 394–405. [Google Scholar]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care. 2007, 10, 724–728. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang. S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquay, J.B.; Haenen, G.R.; Stender, G.; Wiseman, S.A.; Tijburg, L.B.; Bast, A.J. Protection against nitric oxide toxicity by tea. Agric. Food Chem. 2000, 48, 5768–5772. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Rahman, R.M.; Appleton, I.J. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef]
- Cíz, M.; Pavelková, M.; Gallová, L.; Králová, J.; Kubala, L.; Lojek, A. The influence of wine polyphenols on reactive oxygen and nitrogen species production by murine macrophages RAW 264.7. Physiol. Res. 2008, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, T.Y.; Kim, Y.K.; Baik, J.H.; So, S.; Hahm, K.B.; Surh, Y.J. Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: Stress-responsive transcription factors and MAP kinases as potential targets. Mutat. Res. 2005, 579, 214–224. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, J.K. (−)-Epigallocatechin-3-gallate Blocks the Induction of Nitric Oxide Synthase by Down-Regulating Lipopolysaccharide-Induced Activity of Transcription Factor Nuclear Factor-κB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1β mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jeong, H.J.; Lee, K.M.; Myung, N.Y.; An, N.H.; Yang, W.M.; Park, S.K.; Lee, H.J.; Hong, S.H.; Kim, H.M.; et al. Epigallocatechin-3-gallate suppresses NF-κB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J. Nutr. Biochem. 2007, 18, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Epigallocatechin Gallate Inhibits Phorbol Ester-Induced Activation of NF-κB and CREB in Mouse Skin: Role of p38 MAPK. Ann. N. Y. Acad. Sci. 2007, 1095, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Clichici, S.; Daicoviciu, D.; Adriana, M.; Postescu, I.D. Photochemoprevention of cutaneous neoplasia through natural products. Exp. Oncol. 2009, 31, 9–15. [Google Scholar] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 2006, 27, 19–35. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Chen, D.; Milacic, V.; Chen, M.S.; Wan, S.B.; Lam, W.H.; Huo, C.; Landis-Piwowar, K.R.; Cui, Q.C.; Wali, A.; Chan, T.H.; et al. Tea polyphenols, their biological effects and potential molecular targets. Histol. Histopathol. 2008, 23, 487–496. [Google Scholar]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green Tea Component, Catechin, Induces Apoptosis of Human Malignant B Cells via Production of Reactive Oxygen Species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S.J. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). Nutrients 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C.K. Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: Mediated by p53 pathway and inhibition in U1B, U4-U6 UsnRNAs expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. Hepatology 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Sah, J.F.; Balasubramanian, S.; Eckert, R.L.; Rorke, E.A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway: Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2004, 279, 12755–12762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.A.; Adhami, V.M.; Afaq, F.; Ahmad, N.; Mukhtar, H.J. Modulation of phosphatidylinositol-3-kinase/protein kinase B-and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. Cell. Biochem. 2004, 91, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell. Biochem. 2006, 287, 109–116. [Google Scholar] [CrossRef]
- Zhen, M.C.; Huang, X.H.; Wang, Q.; Sun, K.; Liu, Y.J.; Li, W.; Zhang, L.J.; Cao, L.Q.; Chen, X.L. Green tea polyphenol epigallocatechin-3-gallate suppresses rat hepatic stellate cell invasion by inhibition of MMP-2 expression and its activation. Acta Pharmacol. Sin. 2006, 27, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of Various Temperatures and pH Values on the Extraction Yield of Phenolics from Litchi Fruit Pericarp Tissue and the Antioxidant Activity of the Extracted Anthocyanins. Int. J. Mol. 2008, 9, 1333–1341. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lee, M.J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.T.; et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.; Eriksson, U.; Edgar, B.; Cullberg, G.; Hedner, T. Flavonoids in grapefruit juice inhibit the in vitro hepatic metabolism of 17β-estradiol. Eur. J. Drug Metab. Pharmacok. 1995, 20, 219–224. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 11, 1–34. [Google Scholar]
- Cermak, R.; Wolffram, S. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2008, 4, 17–35. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.K. Nanomedicine: Application of nanobiotechnology in medical practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano. 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Simões, S.; Moreira, J.N.; Fonseca, C.; Düzgüneş, N.; de Lima, M.C. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef]
- Gasco, M.R. Lipid nanoparticles: Perspectives and challenges. Adv. Drug Deliv. Rev. 2007, 59, 377–378. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and transdermal drug delivery: From simple potions to smart technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles. In Encyclopaedia of Pharmaceutical Technology; Marcel Dekker Inc: New York, NY, USA, 1994. [Google Scholar]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Panyam, J.; Sahoo, S.K.; Prabha, S.; Bargar, T.; Labhasetwar, V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly (D, L-lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2003, 262, 1–11. [Google Scholar] [CrossRef]
- Bala, I.; Hariharan, S.; Kumar, M.N. Critical Reviews™ in Therapeutic Drug Carrier Systems. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Serizawa, T.J. Preparation and Characterization of Surfactant-Free Nanoparticles Composed of Stereoregular Poly(methyl methacrylate)s. Nanosci. Nanotechnol. 2009, 9, 591–597. [Google Scholar] [CrossRef]
- Chana, J.; Forbes, B.; Jones, S.A. The synthesis of high molecular weight partially hydrolysed poly (vinyl alcohol) grades suitable for nanoparticle fabrication. J. Nanosci. Nanotechnol. 2008, 8, 5739–5747. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly (alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res. Int. 2001, 34, 223–227. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Borin, M.F.; Lopez, R.F.; Fonseca, M.J. In vitro evaluation of quercetin cutaneous absorption from topical formulations and its functional stability by antioxidant activity. Int. J. Pharm. 2007, 328, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; Casagrande, R.; Verri, W.A., Jr.; Georgetti, S.R.; Bentley, M.V.; Fonseca, M.J. Quercetin in lyotropic liquid crystalline formulations: Physical, chemical and functional stability. AAPS Pharm. Sci. Technol. 2008, 9, 591–596. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M.J. Incorporation of quercetin in lipid microparticles: Effect on photo-and chemical-stability. Pharm. Biomed. Anal. 2009, 49, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Design of self-assembling peptides and their biomedical applications. Int. J. Nanomed. 2011, 6, 1621. [Google Scholar]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.U.; Yu, L.; Sui, X.; Shi, X. Discovery of the consistently well-performed analysis chain for SWATH-MS based pharmacoproteomic quantification. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar]
- Bose, S.; Michniak-Kohn, B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur. J. Pharm. Sci. 2013, 48, 442–452. [Google Scholar] [CrossRef]
- Chessa, M.; Caddeo, C.; Valenti, D.; Manconi, M.; Sinico, C.; Fadda, A.M. Effect of penetration enhancer containing vesicles on the percutaneous delivery of quercetin through new born pig skin. Pharmaceutics 2011, 3, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical Anti-Inflammatory Potential of Quercetin in Lipid-Based Nanosystems: In Vivo and In Vitro Evaluation. Pharmaceut. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.; Huang, C.-T.; Fu, L.-T.; Huang, Y.-B.; Tsai, Y.-H.; Wu, P.-C. The Effect of Submicron Emulsion Systems on Transdermal Delivery of Kaempferol. Chem. Pharmaceut. Bull. 2012, 60, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive Antioxidant and Antiinflammatory Effects of Flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-R.; Hung, C.-F.; Lin, Y.-K.; Fang, J.-Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Munyendo, W.L.L.; Zhang, Z.; Abbad, S.; Waddad, A.Y.; Lv, H.; Baraza, L.D.; Zhou, J. Micelles of TPGS modified apigenin phospholipid complex for oral administration: Preparation, in vitro and in vivo evaluation. J. Biomed. Nanotechnol. 2013, 9, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.N.; Zhang, Y.T.; Wang, Q.; Xu, L.; Feng, N.P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Karthivashan, G.; Masarudin, M.J.; Umar Kura, A.; Abas, F.; Fakurazi, S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int. J. Nanomed. 2016, 11, 3417–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1069–1074. [Google Scholar] [CrossRef]
- Shin, K.; Choi, H.; Song, S.K.; Yu, J.W.; Lee, J.Y.; Choi, E.J.; Lee, D.H.; Do, S.H.; Kim, J.W. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. ACS Biomat. Sci. Eng. 2018, 4, 1723–1729. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 4, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target 2005, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hwang, T.L.; Huang, Y.L.; Fang, C.L. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int. J. Pharm. 2006, 310, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Al-Karaki, R. Nanotechnological Innovations Enhancing the Topical Therapeutic Efficacy of Quercetin: A Succinct Review. Curr. Drug Deliv. 2020, 17, 270–278. [Google Scholar] [CrossRef]
- Guan, F.; Wang, Q.; Bao, Y.; Chao, Y. Anti-rheumatic effect of quercetin and recent developments in nano formulation. RSC Adv. 2021, 11, 7280–7293. [Google Scholar] [CrossRef]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative Evaluation of the Effect of Permeation Enhancers, Lipid Nanoparticles and Colloidal Silica on in vivo Human Skin Penetration of Quercetin. Skin Pharmacol. Physiol. 2013, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mandal, S.; Sinha, J.; Mukhopadhyay, S.; Das, N.; Basu, M.K. Quercetin: Critical evaluation as an antileishmanial agent in vivo in hamsters using different vesicular delivery modes. J. Drug Target 2002, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Shamseddin, A.; Aubert-Pouëssel, A.; Devoisselle, J.M.; Begu, S. Dermal quercetin lipid nanocapsules: Influence of the formulation on antioxidant activity and cellular protection against hydrogen peroxide. Int. J. Pharm. 2017, 518, 167–176. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, W.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Silva, A.P.; Nunes, B.R.; De Oliveira, M.C.; Koester, L.S.; Mayorga, P.; Bassani, V.L.; Teixeira, H.F. Development of topical nanoemulsions containing the isoflavone genistein. Pharmazie 2009, 64, 32–35. [Google Scholar] [PubMed]
- Carlotti, M.; Sapino, S.; Ugazio, E.; Gallarate, M.; Morel, S.J. Resveratrol in Solid Lipid Nanoparticles. Dispers. Sci. Technol. 2012, 33, 465–471. [Google Scholar] [CrossRef]
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H.L.; Beck, R.C.; Windbergs, M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 2015, 78, 204–213. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B. 2014, 114, 144–149. [Google Scholar] [CrossRef]
- Tsai, Y.; Lee, K.; Huang, Y.; Huang, C. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int. J. Pharm. 2010, 388, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Adelli, G.R.; Hingorani, T.; Punyamurthula, N.; Prachetan, S.; Majumdar, S. Evaluation of topical hesperetin matrix film for back-of-the-eye delivery. Eur. J. Pharm. Biopharm. 2015, 92, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Kilor, V.; Sapkal, N.; Vaidya, G. Design and development of novel microemulsion based topical formulation of Hesperidin. Int. J. Pharm. Pharm. Sci. 2015, 7, 142–148. [Google Scholar]
- Haritima, J. Papr reduction using scs-slm technique in stfbc mimo-ofdm. ARPN J. Eng. Appl. Sci. 2017, 12, 3218–3221. [Google Scholar]
- Telange, D.R.; Patin, A.T. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2016, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Priprem, A.; Limsitthichaikoon, S.; Thappasarapong, S.; Complex, A.A. Preparation, Anti-inflammatory Activity of Topical Anthocyanins by Complexation and Niosomal Encapsulation. Int. J. Chem. Mol. Eng. 2015, 9, 133–137. [Google Scholar]
- Baolin, L.; Weiwei, W.; Ning, T. Topical Application of Luteolin Inhibits Scratching Behavior Associated with Allergic Cutaneous Reaction in Mice. Planta Med. 2005, 71, 424–428. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).