The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease
Abstract
:1. Introduction
2. Inflammation and Oxidative Stress
3. GM, Oxidative Stress and Inflammation
4. Alzheimer’s Disease
4.1. Microbiota-Gut-Brain Axis and AD
4.2. Oxidative Stress, Inflammation and AD: The Role of GM
5. Polyphenols
5.1. Anti-Oxidative Properties of Polyphenols
5.2. Anti-Inflammatory Properties of Polyphenols
| Polyphenols | Study | Findings | Reference |
|---|---|---|---|
| Anthocyanins | Mouse microglial cells | ↓IL-1β, TNF-α, and NO release, NF-κB nuclear translocation, COX-2 and iNOS expressions. | [84] |
| Human | ↓IL-6, IL-18, and TNF-α | [85] | |
| Quercetin | Mouse BV2 microglial cells and mice | ↓Oxygen glucose deprivation induced expression of inflammatory factors and TLR4/MyD88/NF-κB signalling. Ameliorated cognitive, cerebral infarct volume and motor function in mice. | [86] |
| Wistar rats | ↑Activity of enzymatic antioxidants and sirtuin 1, ↓NF-κB and IL-1β levels, increased IL-10 and modulated AMPK/SIRT1/NF-κB signaling pathway. | [87] | |
| Resveratrol | SH-SY5Y neuronal cells | ↓TNF-α, IL-1β, mitochondrial, and cytosolic ROS, improved the intracellular Ca2+ responses and mitochondrial function. | [88] |
| Curcumin | Sprague-Dawley rats | ↓iNOS, COX-2 expression and inflammatory factor | [89] |
| Epigallocatechin Gallate | SPF Wistar rats | ↓Acetyl-CoA carboxylase, NF-κB, and free fatty acid synthase and ↑fatty acid binding protein-1, carnitine palmitoyltransferase II and sirtuin 1. | [90] |
| WI-38 cells | ↑Antioxidant enzymes, superoxide dismutase 1 and 2 and ↓IL-32 and TNF-α expression. | [91] | |
| Luteolin | Wistar rats | ↓ Oxidative stress parameters, levels of NF-κB, malondialdehyde, and hydrogen peroxide and ↑glutathione S-transferase. | [92] |
| Kaempferol | C57 BL/6J mice | ↓TNF-α and IL-6, and the activation of NF-κB and ↑NRF2/HO-1 signaling pathway and level antioxidants | [93] |
| Myricetin | Wistar rats | ↓Markers of inflammation such as NF-κB, IL-6, TNF-α, and NRF2, ↑xanthine oxidase activity and phase-II detoxifying enzyme activity and ameliorated lipid peroxidation | [94] |
| Green Tea polyphenols | C57BL/6 mice | ↓NLRP3 inflammasome expression, NRF2 pathways, hepatic inflammatory damage and immunological reaction | [95] |
| Grape Seed Extract | Human colorectal adenocarcinoma cell line Caco-2 | ↓Pro-inflammatory cytokine gene expression, intracellular ROS and mitochondrial superoxide production, ↑anti-inflammatory cytokines, and mitochondrial membrane potential. | [96] |
5.3. GM and Polyphenols
5.4. Polyphenols and AD
6. Research Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GM | Gut microbiota |
| AD | Alzheimer’s disease |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SCFA | Short-chain fatty acids |
| MGBX | Microbiota-gut-brain axis |
| NOXs | NADPH oxidases |
| NO | Nitric oxide |
| TLR4 | Toll-like receptor-4 |
| IL-6 | Interleukin |
| GIT | Gastrointestinal tract |
| APP | Amyloid precursor protein |
| TNF-α | Tumor necrosis factor-α |
| HMGB1 | High mobility group box-1 |
| DAMP | Damage associated molecular pattern |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide binding oligomerization domain leucine rich repeat containing protein 3 |
| TMAO | Trimethylamine N-oxide |
| LPS | Lipopolysaccharides |
| Aβ | Amyloid-beta |
| MCI | Mild cognitive impaired |
| MDD | Major depressive disorder |
| PD | Parkinson’s disease |
| BBB | Blood-brain barrier |
| NRF2 | Nuclear factor-erythroid factor 2-related factor 2 |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| CREB | Cyclic AMP response element binding protein. |
References
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, J.-F.; Lu, Z.Y.; Massart, C.; Levon, K. Dynamic immune/inflammation precision medicine: The good and the bad inflammation in infection and cancer. Front. Immunol. 2021, 12, 97. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Raucci, A.; Di Maggio, S.; Scavello, F.; D’Ambrosio, A.; Bianchi, M.E.; Capogrossi, M.C. The Janus face of HMGB1 in heart disease: A necessary update. Cell. Mol. Life Sci. 2019, 76, 211–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2021, 86, 153170. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.-H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Łuc, M.; Misiak, B.; Pawłowski, M.; Stańczykiewicz, B.; Zabłocka, A.; Szcześniak, D.; Pałęga, A.; Rymaszewska, J. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110039. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport: Oxidative Phosphorylation, Mitochondrial Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ortiz, E.; Browning, K.L.; Damgaard, L.S.; Nordström, R.; Micciulla, S.; Bucciarelli, S.; Malmsten, M. Effects of oxidation on the physicochemical properties of polyunsaturated lipid membranes. J. Colloid Interface Sci. 2019, 538, 404–419. [Google Scholar] [CrossRef]
- Chen, T.; Luo, W.; Wu, G.; Wu, L.; Huang, S.; Li, J.; Wang, J.; Hu, X.; Huang, W.; Liang, G. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol. Appl. Pharmacol. 2019, 370, 44–55. [Google Scholar] [CrossRef]
- Serbulea, V.; Upchurch, C.M.; Ahern, K.W.; Bories, G.; Voigt, P.; DeWeese, D.E.; Meher, A.K.; Harris, T.E.; Leitinger, N. Macrophages sensing oxidized DAMPs reprogram their metabolism to support redox homeostasis and inflammation through a TLR2-Syk-ceramide dependent mechanism. Mol. Metab. 2018, 7, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef]
- Cezar, T.L.; Martinez, R.M.; da Rocha, C.; Melo, C.P.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Burt, K.G.; Jacobsen, T.; Fernandes, T.D.; Alipui, D.O.; Weber, K.T.; Levine, M.; Chavan, S.S.; Yang, H.; Tracey, K.J. High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway. J. Orthop. Res. 2019, 37, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Hatayama, K.; Stonestreet, B.S. High mobility group box-1 protein as a therapeutic target in perinatal hypoxic-ischemic brain injury. Neural Regen. Res. 2021, 16, 2006–2007. [Google Scholar]
- Nishibori, M.; Mori, S.; Takahashi, H.K. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 2019, 140, 94–101. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Q.; Hu, X.; Lu, D.; Yu, W.; Bai, H. N4-acetylcytidine is required for sustained NLRP3 inflammasome activation via HMGB1 pathway in microglia. Cell. Signal. 2019, 58, 44–52. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Perry, G. Neuropsychiatric Disturbances and Diabetes Mellitus: The Role of Oxidative Stress; Hindawi: London, UK, 2019. [Google Scholar]
- Beaumont, M.; Andriamihaja, M.; Lan, A.; Khodorova, N.; Audebert, M.; Blouin, J.-M.; Grauso, M.; Lancha, L.; Benetti, P.-H.; Benamouzig, R. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic. Biol. Med. 2016, 93, 155–164. [Google Scholar] [CrossRef]
- He, Z.; Kwek, E.; Hao, W.; Zhu, H.; Liu, J.; Ma, K.Y.; Chen, Z.-Y. Hawthorn fruit extract reduced trimethylamine-N-oxide (TMAO)-exacerbated atherogenesis in mice via anti-inflammation and anti-oxidation. Nutr. Metab. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Loffredo, L.; Ettorre, E.; Zicari, A.M.; Inghilleri, M.; Nocella, C.; Perri, L.; Spalice, A.; Fossati, C.; De Lucia, M.C.; Pigozzi, F. Oxidative stress and gut-derived lipopolysaccharides in neurodegenerative disease: Role of NOX2. Oxidative Med. Cell. Longev. 2020, 2020, 8630275. [Google Scholar] [CrossRef] [Green Version]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2020, 264, 118627. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110076. [Google Scholar] [CrossRef]
- Jiang, H.-y.; Zhang, X.; Yu, Z.-h.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Chen, Y.-h.; Bai, J.; Wu, D.; Yu, S.-F.; Qiang, X.-L.; Bai, H.; Wang, H.-N.; Peng, Z.-W. Association between fecal microbiota and generalized anxiety disorder: Severity and early treatment response. J. Affect. Disord. 2019, 259, 56–66. [Google Scholar] [CrossRef]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age-and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut microbiota altered in mild cognitive impairment compared with normal cognition in sporadic Parkinson’s disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.; Houser, M.C.; Pereira, P.A.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, X.; Pang, L.; Miao, Y.; Wang, S.; Zhang, X.; Hu, S.; Wang, Y.; Andreassen, O.A.; Song, X. Gut Microbiota Markers for Antipsychotics Induced Metabolic Disturbance in Drug Naïve Patients with First Episode Schizophrenia—A 24 Weeks Follow-up Study. medRxiv 2021, 2020-12. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Subramaniapillai, M.; Shekotikhina, M.; Carmona, N.E.; Lee, Y.; Mansur, R.B.; Brietzke, E.; Fus, D.; Coles, A.S.; Iacobucci, M. Characterizing the gut microbiota in adults with bipolar disorder: A pilot study. Nutr. Neurosci. 2021, 24, 173–180. [Google Scholar] [CrossRef]
- Lai, W.-T.; Zhao, J.; Xu, S.-X.; Deng, W.-F.; Xu, D.; Wang, M.-B.; He, F.-S.; Liu, Y.-H.; Guo, Y.-Y.; Ye, S.-W. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in bipolar disorder with current major depressive episode patients. J. Affect. Disord. 2021, 278, 311–319. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L. Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer’s disease: Signals in host–microbe interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef]
- Şafak, B.; Altunan, B.; Topçu, B.; Topkaya, A.E. The gut microbiome in epilepsy. Microb. Pathog. 2020, 139, 103853. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, D.-H.; Kim, D.W. A comparison of the gut microbiota among adult patients with drug-responsive and drug-resistant epilepsy: An exploratory study. Epilepsy Res. 2021, 172, 106601. [Google Scholar] [CrossRef]
- Stan, T.L.; Soylu-Kucharz, R.; Burleigh, S.; Prykhodko, O.; Cao, L.; Franke, N.; Sjögren, M.; Haikal, C.; Hållenius, F.; Björkqvist, M. Increased intestinal permeability and gut dysbiosis in the R6/2 mouse model of Huntington’s disease. Sci. Rep. 2020, 10, 18270. [Google Scholar] [CrossRef]
- WHO. W.H.O. Dementia Fact Sheets. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 10 July 2021).
- Alzheimer’s Disease International. Dementia Statistics. 2020. Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/ (accessed on 10 July 2021).
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and its derivatives as theranostic agents in Alzheimer’s disease: The implication of nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Stages of Alzheimer’s. 2021. Available online: https://www.alz.org/alzheimers-dementia/stages (accessed on 7 July 2021).
- He, Y.; Li, B.; Sun, D.; Chen, S. Gut microbiota: Implications in Alzheimer’s disease. J. Clin. Med. 2020, 9, 2042. [Google Scholar] [CrossRef]
- Zetterberg, H.; Bendlin, B.B. Biomarkers for Alzheimer’s disease—Preparing for a new era of disease-modifying therapies. Mol. Psychiatry 2021, 26, 296–308. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 1–22. [Google Scholar] [CrossRef]
- Sun, M.; Ma, K.; Wen, J.; Wang, G.; Zhang, C.; Li, Q.; Bao, X.; Wang, H. A review of the brain-gut-microbiome axis and the potential role of microbiota in Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 73, 849–865. [Google Scholar] [CrossRef]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.; Hulme, J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsom, E.M.; Lee, K.; Cope, E.K. Do the bugs in your gut eat your memories? Relationship between gut microbiota and Alzheimer’s disease. Brain Sci. 2020, 10, 814. [Google Scholar] [CrossRef]
- Botchway, B.O.; Okoye, F.C.; Chen, Y.; Arthur, W.E.; Fang, M. Alzheimer Disease: Recent Updates on Apolipoprotein E and Gut Microbiome Mediation of Oxidative Stress, and Prospective Interventional Agents. Aging Dis. 2021, 13, 55–85. [Google Scholar]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Zhang, A.H.; Ma, Z.m.; Kong, L.; Gao, H.L.; Sun, H.; Wang, X.Q.; Yu, J.B.; Han, Y.; Yan, G.L.; Wang, X.J. High-throughput lipidomics analysis to discover lipid biomarkers and profiles as potential targets for evaluating efficacy of Kai-Xin-San against APP/PS1 transgenic mice based on UPLC–Q/TOF–MS. Biomed. Chromatogr. 2020, 34, e4724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y. Gut microbiome alterations precede cerebral amyloidosis and microglial pathology in a mouse model of Alzheimer’s disease. Biomed. Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Murotani, K.; Hisada, T.; Kunihiro, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between dementia and gut microbiome-associated metabolites: A cross-sectional study in Japan. Sci. Rep. 2020, 10, 8088. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Neth, B.J.; Wang, S.; Mishra, S.P.; Craft, S.; Yadav, H. Gut mycobiome and its interaction with diet, gut bacteria and Alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 2020, 59, 102950. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Shabbir, U.; Khalid, S.; Abbas, M.; Suleria, H.A.R. Natural carotenoids: Weapon against life-style-related disorders. In Phytochemicals from Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 159–178. [Google Scholar]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- Tyagi, A.; Shabbir, U.; Chelliah, R.; Daliri, E.B.-M.; Chen, X.; Oh, D.-H. Limosilactobacillus reuteri Fermented Brown Rice: A Product with Enhanced Bioactive Compounds and Antioxidant Potential. Antioxidants 2021, 10, 1077. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in neuroprotection by phytochemicals: Bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Spagnuolo, C.; Russo, M.; Tedesco, I.; Moccia, S.; Cervellera, C. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 2020, 173, 113719. [Google Scholar] [CrossRef]
- Gay, N.H.; Suwanjang, W.; Ruankham, W.; Songtawee, N.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Butein, isoliquiritigenin, and scopoletin attenuate neurodegeneration via antioxidant enzymes and SIRT1/ADAM10 signaling pathway. RSC Adv. 2020, 10, 16593–16606. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Huang, Q.; Li, S.; Zhou, Y.; Li, Z. Cardioprotection of CAPE-oNO2 against myocardial ischemia/reperfusion induced ROS generation via regulating the SIRT1/eNOS/NF-κB pathway in vivo and in vitro. Redox Biol. 2018, 15, 62–73. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Modulation of Akt-p38-MAPK/Nrf2/SIRT1 and NF-κB pathways by wine pomace product in hyperglycemic endothelial cell line. J. Funct. Foods 2019, 58, 255–265. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wu, Q.-H.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Gal-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, 2000746. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Lee, K.T.; Choi, Y.H.; Jayasingha, J.A.C.C.; Kim, G.-Y. Anthocyanins from Hibiscus syriacus L. Inhibit NLRP3 Inflammasome in BV2 Microglia Cells by Alleviating NF-κB-and ER Stress-Induced Ca2+ Accumulation and Mitochondrial ROS Production. Oxidative Med. Cell. Longev. 2021, 2021, 1246491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Liu, T.; Wang, X.; Huang, H.; Liu, W. Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis. Mol. Med. Rep. 2019, 20, 802–812. [Google Scholar] [CrossRef]
- Nikbakht, E.; Singh, I.; Vider, J.; Williams, L.T.; Vugic, L.; Gaiz, A.; Kundur, A.R.; Colson, N. Potential of anthocyanin as an anti-inflammatory agent: A human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflamm. Res. 2021, 70, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Song, Z.; Deng, J.; Peng, X.; Zhang, J.; Wang, L.; Zhou, L.; Bi, H.; Liao, Z.; Feng, Z. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation. Inflamm. Res. 2020, 69, 1201–1213. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef]
- Akyuva, Y.; Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020, 10, 6449. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.H.; Shen, C.F.; Wang, G.J.; Kang, Y.; Song, Y.H.; Liu, J.W. Curcumin alleviates acute kidney injury in a dry-heat environment by reducing oxidative stress and inflammation in a rat model. J. Biochem. Mol. Toxicol. 2021, 35, e22630. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Guan, Y.; Ling, F.; Li, Y.; Niu, Y. Epigallocatechin gallate prevents senescence by alleviating oxidative stress and inflammation in WI-38 human embryonic fibroblasts. RSC Adv. 2019, 9, 26787–26798. [Google Scholar] [CrossRef] [Green Version]
- Oyagbemi, A.A.; Akinrinde, A.S.; Adebiyi, O.E.; Jarikre, T.A.; Omobowale, T.O.; Ola-Davies, O.E.; Saba, A.B.; Emikpe, B.O.; Adedapo, A.A. Luteolin supplementation ameliorates cobalt-induced oxidative stress and inflammation by suppressing NF-kB/Kim-1 signaling in the heart and kidney of rats. Environ. Toxicol. Pharmacol. 2020, 80, 103488. [Google Scholar] [CrossRef]
- Yao, H.; Sun, J.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y. Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rather, I.A. Myricetin abrogates cisplatin-induced oxidative stress, inflammatory response, and goblet cell disintegration in colon of wistar rats. Plants 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, M.; Wang, T.; Liu, T.; Guo, Y.; Granato, D. Green tea polyphenols mitigate the plant lectins-induced liver inflammation and immunological reaction in C57BL/6 mice via NLRP3 and Nrf2 signaling pathways. Food Chem. Toxicol. 2020, 144, 111576. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-rich grape seed extract reduces inflammation and oxidative stress and restores tight junction barrier function in Caco-2 colon cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Sreng, N.; Champion, S.; Martin, J.-C.; Khelaifia, S.; Christensen, J.E.; Padmanabhan, R.; Azalbert, V.; Blasco-Baque, V.; Loubieres, P.; Pechere, L. Resveratrol-mediated glycemic regulation is blunted by curcumin and is associated to modulation of gut microbiota. J. Nutr. Biochem. 2019, 72, 108218. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, R.; Gao, X.; Li, J.; Jiang, L.; Zheng, J.; Cui, S.; Ying, M.; Yang, B.; Cao, J. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol. Res. 2019, 148, 104460. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.; Bruins, M.E.; Vincken, J.-P. Reciprocal interactions between epigallocatechin-3-gallate (EGCG) and human gut microbiota in vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s Disease and in the Gut–Brain Axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, B.N.; Parylak, S.L.; Gage, F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Asp. Med. 2018, 61, 50–62. [Google Scholar] [CrossRef]
- Pinheiro, R.; Granja, A.; Loureiro, J.; Pereira, M.; Pinheiro, M.; Neves, A.; Reis, S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer’s disease. Pharm. Res. 2020, 37, 1–12. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, A.; Ahmad, S.; Ahmad, R.; Rehman, I.U.; Ikram, M.; Kim, M.O. Inhibition of JNK alleviates chronic hypoperfusion-related ischemia induces oxidative stress and brain degeneration via Nrf2/HO-1 and NF-κB signaling. Oxidative Med. Cell. Longev. 2020, 2020, 5291852. [Google Scholar] [CrossRef]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid β1–42 peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M. Flavonoid-Derived Human Phenyl-γ-Valerolactone Metabolites Selectively Detoxify Amyloid-β Oligomers and Prevent Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2020, 64, 1900890. [Google Scholar] [CrossRef]
- Carregosa, D.; Carecho, R.; Figueira, I.S.; Santos, C.U. Low-molecular weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2019, 68, 1790–1807. [Google Scholar] [CrossRef] [Green Version]
- Corral-Jara, K.F.; Nuthikattu, S.; Rutledge, J.; Villablanca, A.; Morand, C.; Schroeter, H.; Milenkovic, D. Integrated Multi-Omic Analyses of the Genomic Modifications by Gut Microbiome-Derived Metabolites of Epicatechin, 5-(4′-Hydroxyphenyl)-γ-Valerolactone, in TNFα-Stimulated Primary Human Brain Microvascular Endothelial Cells. Front. Neurosci. 2021, 15, 622640. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ1-42-Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, 2000660. [Google Scholar] [CrossRef]
- Selvakumar, K.; Bavithra, S.; Krishnamoorthy, G.; Arunakaran, J. Impact of quercetin on tight junctional proteins and BDNF signaling molecules in hippocampus of PCBs-exposed rats. Interdiscip. Toxicol. 2018, 11, 294. [Google Scholar] [CrossRef] [Green Version]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L. Prevention by dietary polyphenols (resveratrol, quercetin, apigenin) against 7-ketocholesterol-induced oxiapoptophagy in neuronal N2a cells: Potential interest for the treatment of neurodegenerative and age-related diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, J.; Guo, L.; Xu, Y.; Sun, L.; Wang, S.; Feng, Y.; Gou, L.; Zhang, L.; Liu, Y. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharmacol. Biochem. Behav. 2014, 126, 122–130. [Google Scholar] [CrossRef]
- Facchinetti, R.; Valenza, M.; Bronzuoli, M.R.; Menegoni, G.; Ratano, P.; Steardo, L.; Campolongo, P.; Scuderi, C. Looking for a Treatment for the Early Stage of Alzheimer’s Disease: Preclinical Evidence with Co-Ultramicronized Palmitoylethanolamide and Luteolin. Int. J. Mol. Sci. 2020, 21, 3802. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Jiang, Y.; Xu, Y.; Zhao, H.; Lyu, X.; Wu, T. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. 2020, 11, 1572–1584. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, Y.; Cheng, L.; Shi, M.; Li, X.; Zhang, L.; Zhao, H. Tea polyphenols ameliorates memory decline in aging model rats by inhibiting brain TLR4/NF-κB inflammatory signaling pathway caused by intestinal flora dysbiosis. Exp. Gerontol. 2021, 111476. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Dev. Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Sarroca, S.; Gatius, A.; Rodríguez-Farré, E.; Vilchez, D.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.; Abd El-moneam, N. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of polyphenols as antioxidant and anti-inflammatory compounds in brain–liver–gut axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]

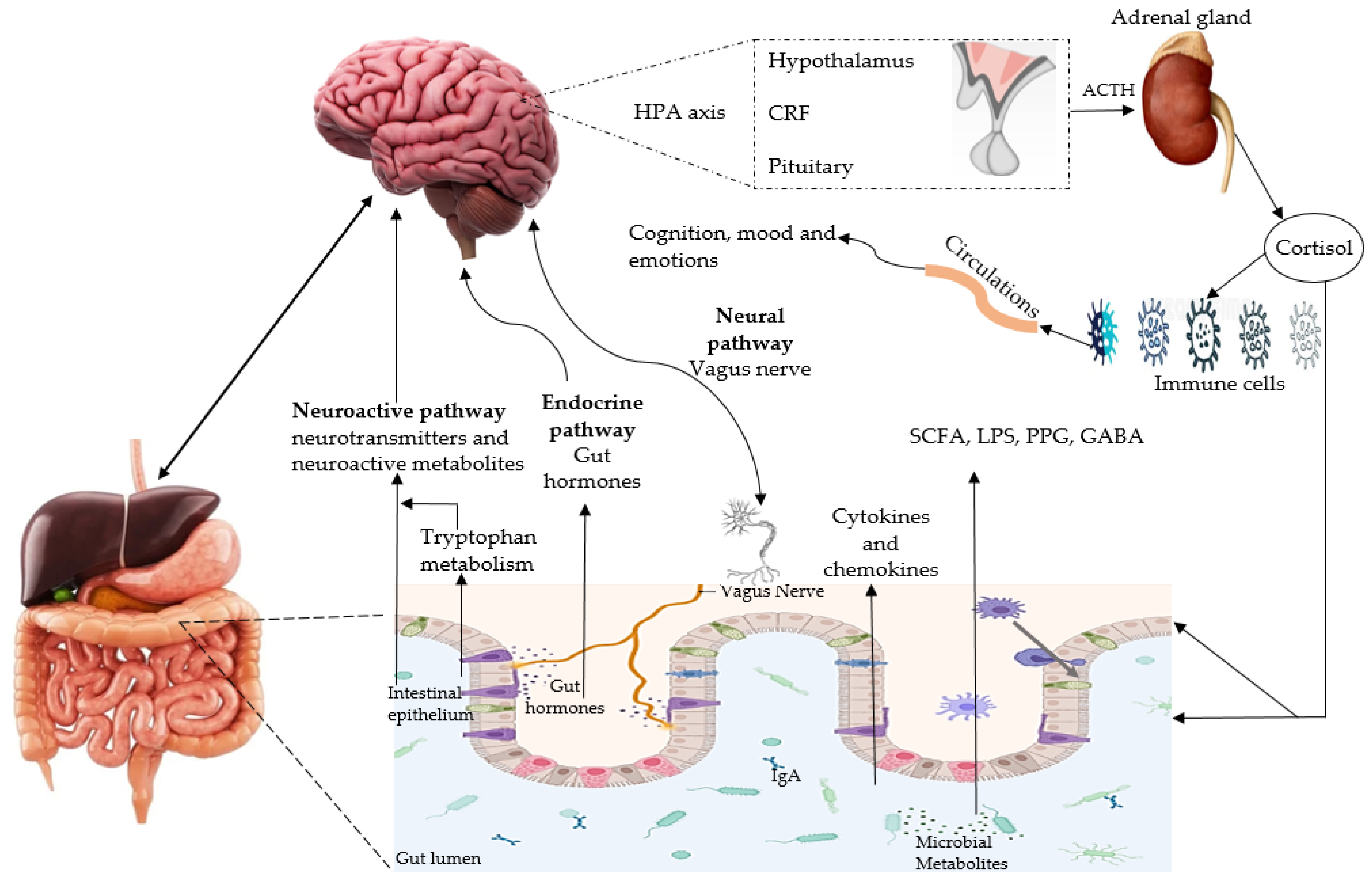
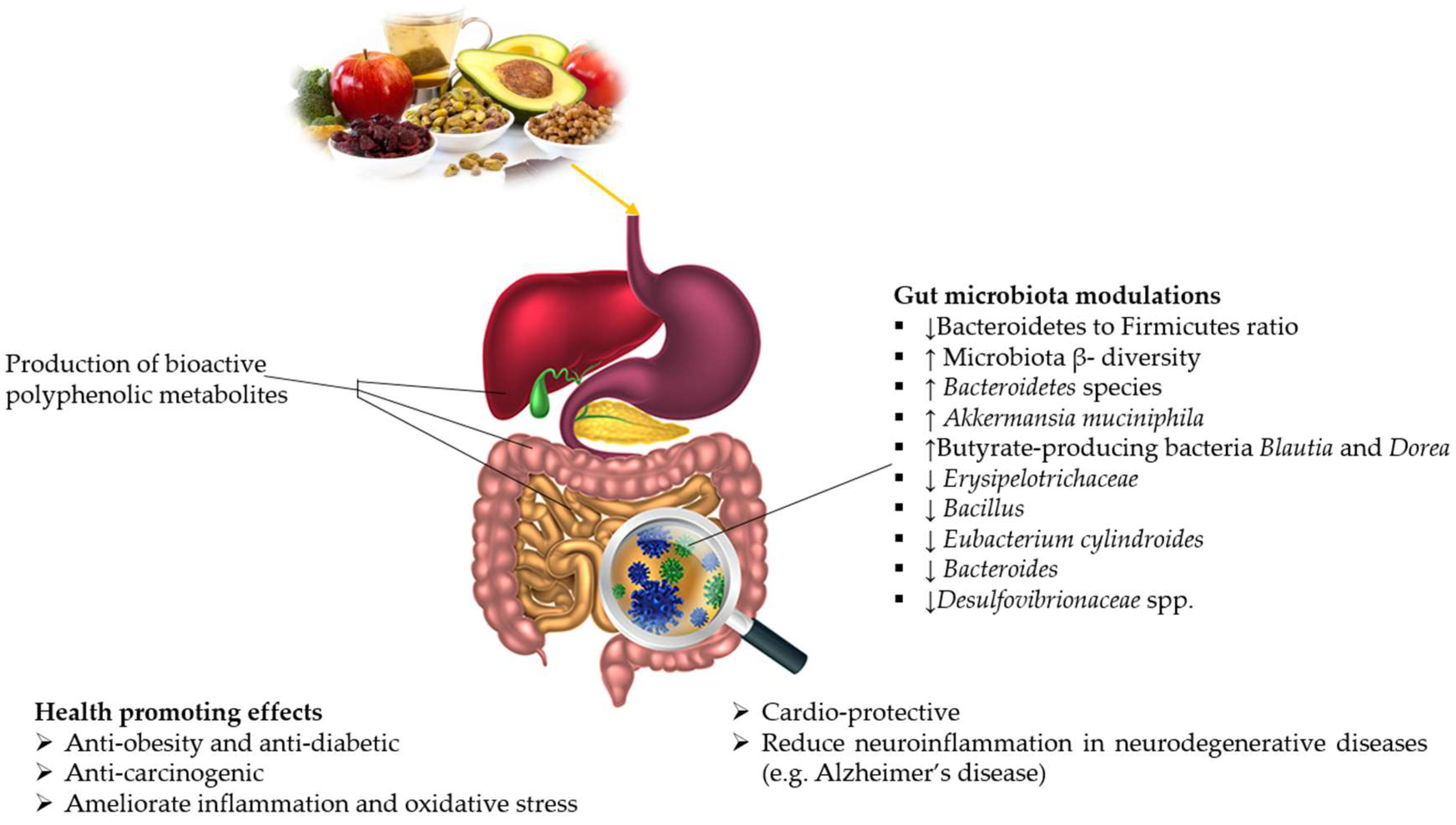
| Disease | Study | Change in GM | Findings | Reference |
|---|---|---|---|---|
| Major Depressive Disorder | Human (n = 36) | Phylum Firmicutes and Actinobacteria were overrepresented, ↑Bifidobacterium and Blautia at the genus level. | Sucrose, starch and pentose phosphate metabolism were important pathways for depression via GM functions. | [30] |
| Human (n = 90) | Paraprevotella showed positive correlation while Clostridia, Clostridiales, Firmicutes, and the RF32 order negatively correlated with depression. | Integrity intestinal and inflammation markers were linked with the response to treat the MDD. | [31] | |
| Anxiety | Human (n = 9) | ↑Fusobacterium, Ruminococcus gnavus, and Escherichia/Shigella↓Microbial richness and diversity. | Enhanced gut permeability and the abundance of pro-inflammatory bacteria linked with neuroinflammation. | [32] |
| Human (n = 36) | ↑Bacteroidaceae, Bacteroides, Betaproteobacteriales, Burkholderiaceae, Tyzzerella 3, Escherichia/Shigella, Hungatella, Enterobacteriales, and Enterobacteriaceae. | The abundance of Ruminococcaceae_UCG-014, Eubacterium_coprostanoligenes group, and Prevotella_9 was negatively associated with anxiety severity and positively with anxiety reduction, whereas Escherichia/Shigella and Bacteroides was positively correlated with anxiety severity. | [33] | |
| Obsessive-Compulsive Disorder | Human (n = 43) | ↓species richness, evenness, and abundance of Anaerostipes, Odoribacter, and Oscillospira. | C-reactive protein was increased that demonstrated mild to strong linkage with psychiatric symptomatology. | [34] |
| Parkinson’s Disease | Human (n = 40) | ↑relative abundance of Ruminococcaceae and Rikenellaceae family and Barnesiella, Alistipes, Odoribacter, and Butyricimonas genera. | Significant enhancement in genera from the Porphyromonadaceae family and decrease in the abundance of genera Blautia and Ruminococcus was observed in PD patients with compromised cognitive ability. | [35] |
| Human (n = 111) | ↑Firmicutes enterotype ↓Prevotella enterotype | Increased intestinal inflammatory responses, reduced SCFA level, and shifts in microbiota-host interactions between earlier PD onset. | [36] | |
| Schizophrenia | Human (n = 194) | ↑Bacteroidetes, ↓Firmicutes and Actinobacteria | Metabolic disturbance (levels of glucose, low-density lipid-cholesterol, high-density lipid-cholesterol, triglyceride, and homeostasis model assessment of insulin resistance) was observed in the patients. | [37] |
| Bipolar Disorder | Human (n = 46) | ↓microbiota diversity, ↑Clostridiaceae and Collinsella | Differences in GM colonization may modulate metabolic and metabolomic alterations and other biological processes such as inflammation. | [38] |
| Human (n = 53) | ↓Bacteroidetes, ↑Actinobacteria and Firmicutes | Change in GM can be a potential biomarker. | [39] | |
| Dementia | Human (n = 77) | ↓Clostridia, Clostridiales Ruminococcaceae, Firmicutes, and Ruminococcus | Decrease in indole-3-pyruvic acid and SCFA producing bacteria as a signature for discrimination and prediction of dementia. | [40] |
| Epilepsy | Human (n = 40) | ↑Delftia, Campylobacter, Lautropia, Haemophilus, and Neisseria genera among Proteobacteria phylum and Leptotrichia and Fusobacterium genera among Fusobacteria phylum | Inflammation and autoimmune mechanisms due to the taxonomic drift and differences in the intestinal microbiota have a role in the etiology of epilepsy. | [41] |
| Human (n = 44) | ↑Ruminococcus_g2 and Bacteroides finegoldii in drug-resistant group, Negativicutes from Firmicutes in drug-resistant group and Bifidobacterium in all patients. | Alteration in GM can be a biomarker to evaluate and diagnose the treatment response in patients. | [42] | |
| Huntington’s Disease | R6/2 HD mice | ↑abundance of Bacteroidetes and ↓Firmicutes | Different compositions of Bacteroides, Coprobacillus, Enterobacteriaceae, Lactobacillus, and Parabacteroides were found in diseased animals. | [43] |
| Polyphenols | Study | Findings | Reference |
|---|---|---|---|
| Curcumin | APP/PS1 double transgenic mice | Change in Lactobacillaceae, Rikenellaceae, Prevotellaceae, and Bacteroidaceae at family level, and Bacteroides, Prevotella, and Parabacteroides at genus level. Curcumin reduced the Aβ plaques burden and improved the cognitive abilities. | [114] |
| Quercetin-3-O-Glucuronide | Mice and SH-SY5Y Cells | Ameliorated tau phosphorylation, and Aβ plaques. Restored CREB and brain-derived neurotrophic factor levels in the hippocampus, and gut dysbiosis. | [115] |
| Quercetin | Adult male albino rats | Protected and prevented neuronal damage in the hippocampus. | [116] |
| RSV, QCT and API | Human SK-N-BE and SH-SY5Y cells | Reduced mitochondrial and peroxisomal dysfunction, 7KC-induced toxicity and cell death. | [117] |
| Luteolin | Sprague-Dawley rats | Down-regulated the expression of BASE1 and NF-κB and reduced Aβ levels in the hippocampus and cortex. Moreover, increased antioxidant potential, and suppressed inflammation and lipid peroxide production. | [118] |
| Palmitoylethanolamide and Luteolin | Sprague-Dawley rats | Up-regulated the gene expression of enzymes, pro-inflammatory cytokines, and reduction of mRNA levels.Moreover, inhibited the Aβ-induced astrogliosis and microgliosis. | [119] |
| Bilberry Anthocyanins | Sprague-Dawley rats | Enhanced the growth of Aspergillus oryzae, Bacteroidales-S24-7-group, Bacteroides, Clostridiaceae-1, Lactobacillus, and Lachnospiraceae_NK4A136_group and inhibited the growth of Verrucomicrobia and Euryarchaeota in aging rats. | [120] |
| APP/PSEN1 transgenic AD mice | Down-regulated the expression of inflammatory factors, chemokine receptor CX3CR1, serum and brain LPS. Reversed the brain, kidney, and liver injury caused by AD. | [121] | |
| Tea Polyphenols | Aging model rats | Prevented memory decline and TLR4/NF-κB inflammatory signal pathway. Besides, significantly improved the composition and diversity of intestinal microflora, shape and function of epithelium, and brain inflammation. | [122] |
| Epigallocatechin-3-Gallate | Sprague-Dawley rats | Decreased the tau hyperphosphorylation in hippocampus and expression of BACE1 and Aβ1-42 by improving the antioxidant system, learning and memory function. | [123] |
| Resveratrol | AD transgenic 5XFAD | Prevented memory loss and reduced the amyloid burden and tau pathology. | [124] |
| Berberine | Sprague-Dawley rats | Production of COX-2, TNF-α, IL-12, IL-6 and IL-1β was normalized, inhibited the production of Aβ42 and evoked the formation of antioxidant Aβ40. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, U.; Tyagi, A.; Elahi, F.; Aloo, S.O.; Oh, D.-H. The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants 2021, 10, 1370. https://doi.org/10.3390/antiox10091370
Shabbir U, Tyagi A, Elahi F, Aloo SO, Oh D-H. The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants. 2021; 10(9):1370. https://doi.org/10.3390/antiox10091370
Chicago/Turabian StyleShabbir, Umair, Akanksha Tyagi, Fazle Elahi, Simon Okomo Aloo, and Deog-Hwan Oh. 2021. "The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease" Antioxidants 10, no. 9: 1370. https://doi.org/10.3390/antiox10091370
APA StyleShabbir, U., Tyagi, A., Elahi, F., Aloo, S. O., & Oh, D.-H. (2021). The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants, 10(9), 1370. https://doi.org/10.3390/antiox10091370









