Involvement of microRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review
Abstract
:1. Introduction
- (1)
- Organize the current knowledge involving light-based therapy and miRNAs;
- (2)
- Pinpoint the most recurrent and interesting skin-related miRNAs;
- (3)
- Explore possible differences in results for the same disease regarding one light source rather than various light sources.
- (4)
- Propose new applications of the current knowledge for future light-based therapies exploiting miRNAs as targets.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs–microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Minciullo, P.L.; Calapai, G.; Navarra, M.; Gangemi, S. Involvement of microRNAs in skin disorders: A literature review. Allergy Asthma Proc. 2017, 38, 9–15. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free. Radic Res. 2019, 53, 829–840. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bagnato, G.; Cristani, M.; Borgia, F.; Spatari, G.; Tigano, V.; Saja, A.; Guarneri, F.; Cannavò, S.P.; Gangemi, S. Oxidation products are increased in patients affected by non-segmental generalized vitiligo. Arch. Dermatol. Res. 2017, 309, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Z.M.; Xue, H.Y.; Nie, F.F. Effects of photoelectric therapy on proliferation and apoptosis of scar cells by regulating the expression of microRNA-206 and its related mechanisms. Int. Wound J. 2020, 17, 317–325. [Google Scholar] [CrossRef]
- Gu, X.; Nylander, E.; Coates, P.J.; Nylander, K. Effect of narrow-band ultraviolet B phototherapy on p63 and microRNA (miR-21 and miR-125b) expression in psoriatic epidermis. Acta Dermatol. Venereol. 2011, 91, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Soonthornchai, W.; Tangtanatakul, P.; Meephansan, J.; Ruchusatsawat, K.; Reantragoon, R.; Hirankarn, N.; Wongpiyabovorn, J. Down-regulation of miR-155 after treatment with narrow-band UVB and methotrexate associates with apoptosis of keratinocytes in psoriasis. Asian Pac. J. Allergy Immunol. 2019. [Google Scholar] [CrossRef]
- Chowdhari, S.; Saini, N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J. Cell Physiol. 2014, 229, 1630–1638. [Google Scholar] [CrossRef]
- Chowdhari, S.; Saini, N. Gene expression profiling reveals the role of RIG1 like receptor signaling in p53 dependent apoptosis induced by PUVA in keratinocytes. Cell Signal. 2016, 28, 25–33. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Wu, Y.; Hou, J.; Nie, Y.; Gu, H.; Li, J.; Hu, S.; Zhang, H. MicroRNA-193 pro-proliferation effects for bone mesenchymal stem cells after low-level laser irradiation treatment through inhibitor of growth family, member 5. Stem Cells Dev. 2021, 21, 2508–2519, Erratum in 2021, 30, 163. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Y.; Zhang, Y.; Han, C.; Gao, D.; Jin, W.; Liang, J.; Xia, X. The comparison of skin rejuvenation effects of vitamin A, fractional laser, and their combination on rat. J. Cosmet. Laser Ther. 2019, 21, 19–27. [Google Scholar] [CrossRef]
- Khori, V.; Alizadeh, A.M.; Gheisary, Z.; Farsinejad, S.; Najafi, F.; Khalighfard, S.; Ghafari, F.; Hadji, M.; Khodayari, H. The effects of low-level laser irradiation on breast tumor in mice and the expression of Let-7a, miR-155, miR-21, miR125, and miR376b. Lasers Med. Sci. 2016, 31, 1775–1782. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, H.; Lv, G.; Zhou, Q.; Yang, B.; Zheng, J.; Cao, W. 5-Aminolevulinic acid-mediated sonodynamic therapy induces anti-tumor effects in malignant melanoma via p53-miR-34a-Sirt1 axis. J. Dermatol. Sci. 2015, 79, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, B.; Nan, F.; Guan, D.; Zhang, Y. 5-Aminolevulinic acid photodynamic therapy in human cervical cancer via the activation of microRNA-143 and suppression of the Bcl-2/Bax signaling pathway. Mol. Med. Rep. 2016, 14, 544–550. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.K.; Kim, J. Apoptosis-related microRNA-145-5p enhances the effects of pheophorbide a-based photodynamic therapy in oral cancer. Oncotarget 2017, 8, 35184–35192. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.Y.; Chen, P.Y.; Ho, D.C.; Tsai, L.L.; Hsieh, P.L.; Lu, M.Y.; Yu, C.C.; Yu, C.H. miR-145 mediates the anti-cancer stemness effect of photodynamic therapy with 5-aminolevulinic acid (ALA) in oral cancer cells. J. Formos. Med. Assoc. 2018, 117, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, W.; Liu, Y.; Sun, Y.; Jiang, Y.; Zhang, S. Electrochemiluminescence-Microscopy for microRNA Imaging in Single Cancer Cell Combined with Chemotherapy-Photothermal Therapy. Anal. Chem. 2019, 91, 12581–12586. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Guan, Z.; Wang, X.; Wang, Z.; Zeng, R.; Xu, L.; Cao, P. ALA-PDT promotes HPV-positive cervical cancer cells apoptosis and DCs maturation via miR-34a regulated HMGB1 exosomes secretion. Photodiagn. Photodyn. Ther. 2018, 24, 27–35. [Google Scholar] [CrossRef]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X.; et al. Photoacoustic-imaging-guided therapy of functionalized melanin nanoparticles: Combination of photothermal ablation and gene therapy against laryngeal squamous cell carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef]
- Wu, C.; Tian, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Guan, X.; Li, T.; Yang, H.; Li, S.; Qin, X.; et al. Acid-Triggered Charge-Convertible Graphene-Based All-in-One Nanocomplex for Enhanced Genetic Phototherapy of Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2020, 9, e1901187. [Google Scholar] [CrossRef] [PubMed]
- Assali, A.; Akhavan, O.; Adeli, M.; Razzazan, S.; Dinarvand, R.; Zanganeh, S.; Soleimani, M.; Dinarvand, M.; Atyabi, F. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomedicine 2018, 14, 1891–1903. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Chen, C.; Wang, D.; Wu, T.; Dong, H.; Zhang, X. Rattle-type Au@Cu2-xS hollow mesoporous nanocrystals with enhanced photothermal efficiency for intracellular oncogenic microRNA detection and chemo-photothermal therapy. Biomaterials 2018, 158, 23–33. [Google Scholar] [CrossRef]
- Qian, R.C.; Cao, Y.; Long, Y.T. Binary System for MicroRNA-Targeted Imaging in Single Cells and Photothermal Cancer Therapy. Anal. Chem. 2016, 88, 8640–8647. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Tepper, C.; Jagdeo, J. MicroRNA expression analysis of human skin fibroblasts treated with high-fluence light-emitting diode-red light. J. Biophotonics 2019, 12, e201800207. [Google Scholar] [CrossRef]
- McGirt, L.Y.; Baerenwald, D.A.; Vonderheid, E.C.; Eischen, C.M. Early changes in miRNA expression are predictive of response to extracorporeal photopheresis in cutaneous T-cell lymphoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2269–2271. [Google Scholar] [CrossRef] [Green Version]
- Ele-Refaei, A.M.; El-Esawy, F.M. Effect of Narrow-Band Ultraviolet B Phototherapy and Methotrexate on MicroRNA (146a) Levels in Blood of Psoriatic Patients. Dermatol. Res. Pract. 2015, 2015, 145769. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.; Fuereder, J.; Karbiener, M.; Scheideler, M.; Ress, A.L.; Neureiter, D.; Kemmerling, R.; Dietze, O.; Wiederstein, M.; Berr, F.; et al. Comprehensive analysis of alterations in the miRNome in response to photodynamic treatment. J. Photochem. Photobiol. B 2013, 120, 74–81. [Google Scholar] [CrossRef]
- Li, P.T.; Tsai, Y.J.; Lee, M.J.; Chen, C.T. Increased Histone Deacetylase Activity Involved in the Suppressed Invasion of Cancer Cells Survived from ALA-Mediated Photodynamic Treatment. Int. J. Mol. Sci. 2015, 16, 23994–24010. [Google Scholar] [CrossRef] [Green Version]
- Kushibiki, T. Photodynamic therapy induces microRNA-210 and -296 expression in HeLa cells. J Biophotonics 2010, 3, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lv, X.; Li, J.; Li, J.; Li, X.; Li, W.; Li, Y. The status of microRNA-21 expression and its clinical significance in human cutaneous malignant melanoma. Acta Histochem. 2012, 114, 582–588. [Google Scholar] [CrossRef]
- Pace, E.; Di Vincenzo, S.; Di Salvo, E.; Genovese, S.; Dino, P.; Sangiorgi, C.; Ferraro, M.; Gangemi, S. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J. Cell Physiol. 2019, 234, 22183–22194. [Google Scholar] [CrossRef]
- Aguennouz, M.; Guarneri, F.; Oteri, R.; Polito, F.; Giuffrida, R.; Cannavò, S.P. Serum levels of miRNA-21-5p in vitiligo patients and effects of miRNA-21-5p on SOX5, beta-catenin, CDK2 and MITF protein expression in normal human melanocytes. J. Dermatol. Sci. 2021, 101, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Cretoiu, S.M.; Zurac, S. miRNAs in the Diagnosis and Prognosis of Skin Cancer. Front. Cell Dev. Biol. 2020, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Eissa, M.G.; Artlett, C.M. The MicroRNA miR-155 Is Essential in Fibrosis. Noncoding RNA 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, K.; Liu, W.; Zhang, J.; Fan, X.; Liu, J.; Zhao, N.; Yao, C.; Miao, G. MicroRNA-125b exerts antitumor functions in cutaneous squamous cell carcinoma by targeting the STAT3 pathway. Cell. Mol. Biol. Lett. 2020, 25, 12. [Google Scholar] [CrossRef] [Green Version]
- Ghaderi, R.; Haghighi, F. Immunohistochemistry assessment of p53 protein in Basal cell carcinoma. Iran. J. Allergy Asthma Immunol. 2005, 4, 167–171. [Google Scholar]
- Amin, K.N.; Umapathy, D.; Anandharaj, A.; Ravichandran, J.; Sasikumar, C.S.; Chandra, S.K.R.; Kesavan, R.; Kunka Mohanram, R. miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc. Res. 2020, 127, 103924. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Tonacci, A.; Bertino, L.; Casciaro, M.; Borgia, F.; Gangemi, S. The role of oxidative stress in the biology of melanoma: A systematic review. Pathol. Res. Pract. 2019, 215, 21–28. [Google Scholar] [CrossRef] [PubMed]
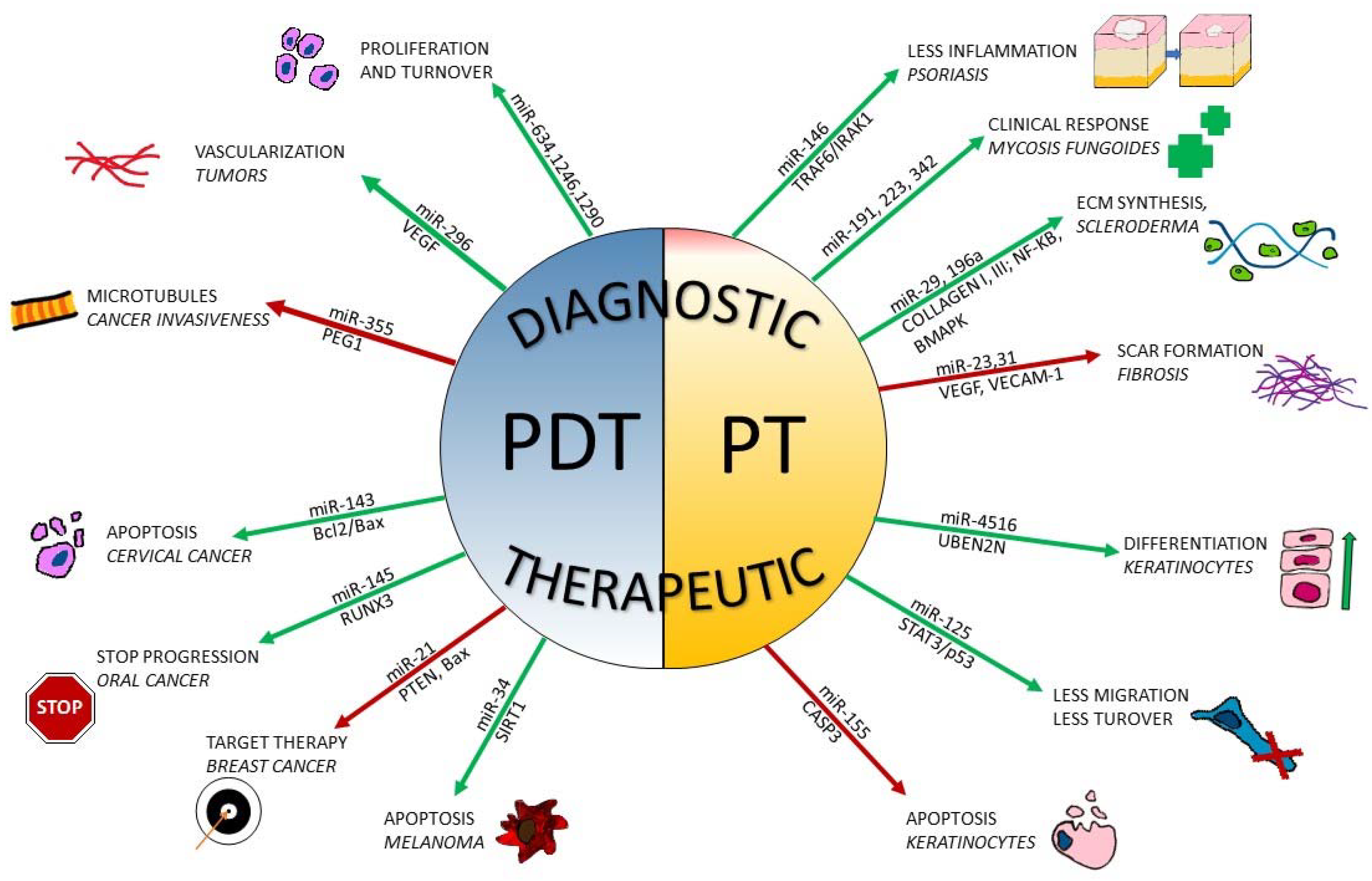
| Authors | Increased miRNA | Decreased miRNA | Target |
|---|---|---|---|
| Phototherapy, laser therapy, PUVA, photoacoustic therapy | |||
| Zhang et al. | 206 | fibroblasts | |
| Zhang et al. | 21 | HeLa cells | |
| Fan et al. | 145-5p | laringeal cancer | |
| Mamalis et al. * | 29, 196a | 21, 23b, 31 | fibrosis |
| Qu et al. | 29a | skin rejuvination | |
| Chowdhari et al. | 4516 | psoriasis | |
| Chowdhari et al. | 4516 | psoriasis | |
| Mcgirt et al. * | 191, 223, 342 | T cell lymphoma | |
| Gu et al. | 125b | 21 | psoriasis |
| Wang et al. | 193 | mesenchymal cells | |
| Ele-refaei et al. * | 146a | psoriasis | |
| Soonthornchai et al. | 155 | psoriasis | |
| Khori et al. | 125a | 155, 21, 376b | breast cancer |
| PDT | |||
| Kushibiki et al. * | 210, 296 | HeLa cells | |
| Bach et al. * | 487b, 634, 1246, 1290 | tumoral cells | |
| Hu et al. | 34a | melanoma | |
| Li et al. * | 355 | tumor cells | |
| Guo et al. | 143 | cervical cancer | |
| Moon et al. | 9-5p, 192-5p, 193a-5p | 32-5p, 143-5p, 145-5p | oral cancer |
| Jin et al. | 34a | cervical cancer | |
| Fang et al. | 145 | oral cancer | |
| Wu et al. | 21 | breast cancer | |
| Assali et al. | 101 | breast cancer | |
| Cao et al. | 155 | target therapy | |
| Qian et al. | 21 | target therapy | |
| miR | Pathways Involved | Effects If Decreased | Effects If Increased | Conditions |
|---|---|---|---|---|
| 21 | Akt, PTEN, Bcl2/Bax, RAF/MEK/ERK p53 | Cellular differentiation | Carcinogenesis, immune cell activation | Lung cancer, skin aging, melanoma, basal cell carcinoma |
| 29 | TGF-beta, Akt, COL1A1, COL1A2, COL3A1 and FBN1 | Upregulation of collagen and fibrillin deposition; tumor-suppressing properties | Negative regulator of collagen expression | Fibrosis-related processes, skin aging, sclerodermia, melanoma, liver, colon, cervical and lung cancer |
| 125 | STAT3; p53 | Cell migration, cell turnover | Apoptosis, cell killing | Breast cancer Basal cell carcinoma |
| 145 | RUNX3 | Cell migration and proliferation | Increase in apoptotic processes, inhibition of tumor progression | Oral cancer, diabetes |
| plese155 | CASP3, NF-kB, SOCS1 | Inflammation inhibition, less tumoral growth, activation of Th2 pathway | Survival factor, cell polarization | Melanoma Lymphomas Skin fibrosis Breast cancer |
| 23 31 | VEGF; HOX; VECAM-1; Ephrin | Lesser collagen deposition | Fibrosis, angiogenesis | Inflammatory bowel diseases, tumors, skin wound healing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgia, F.; Custurone, P.; Peterle, L.; Pioggia, G.; Guarneri, F.; Gangemi, S. Involvement of microRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review. Antioxidants 2021, 10, 1310. https://doi.org/10.3390/antiox10081310
Borgia F, Custurone P, Peterle L, Pioggia G, Guarneri F, Gangemi S. Involvement of microRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review. Antioxidants. 2021; 10(8):1310. https://doi.org/10.3390/antiox10081310
Chicago/Turabian StyleBorgia, Francesco, Paolo Custurone, Lucia Peterle, Giovanni Pioggia, Fabrizio Guarneri, and Sebastiano Gangemi. 2021. "Involvement of microRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review" Antioxidants 10, no. 8: 1310. https://doi.org/10.3390/antiox10081310
APA StyleBorgia, F., Custurone, P., Peterle, L., Pioggia, G., Guarneri, F., & Gangemi, S. (2021). Involvement of microRNAs as a Response to Phototherapy and Photodynamic Therapy: A Literature Review. Antioxidants, 10(8), 1310. https://doi.org/10.3390/antiox10081310









