Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits
Abstract
:1. Introduction
2. Bioavailability and Pharmacokinetics
3. Inflammation
4. Metabolic Diseases
4.1. Liver Disease
4.2. Obesity and Diabetes
4.3. Cardiovascular Disease
4.4. Kidney Disease
5. Cancer
6. Neurological Disorders and Other Diseases of the Brain
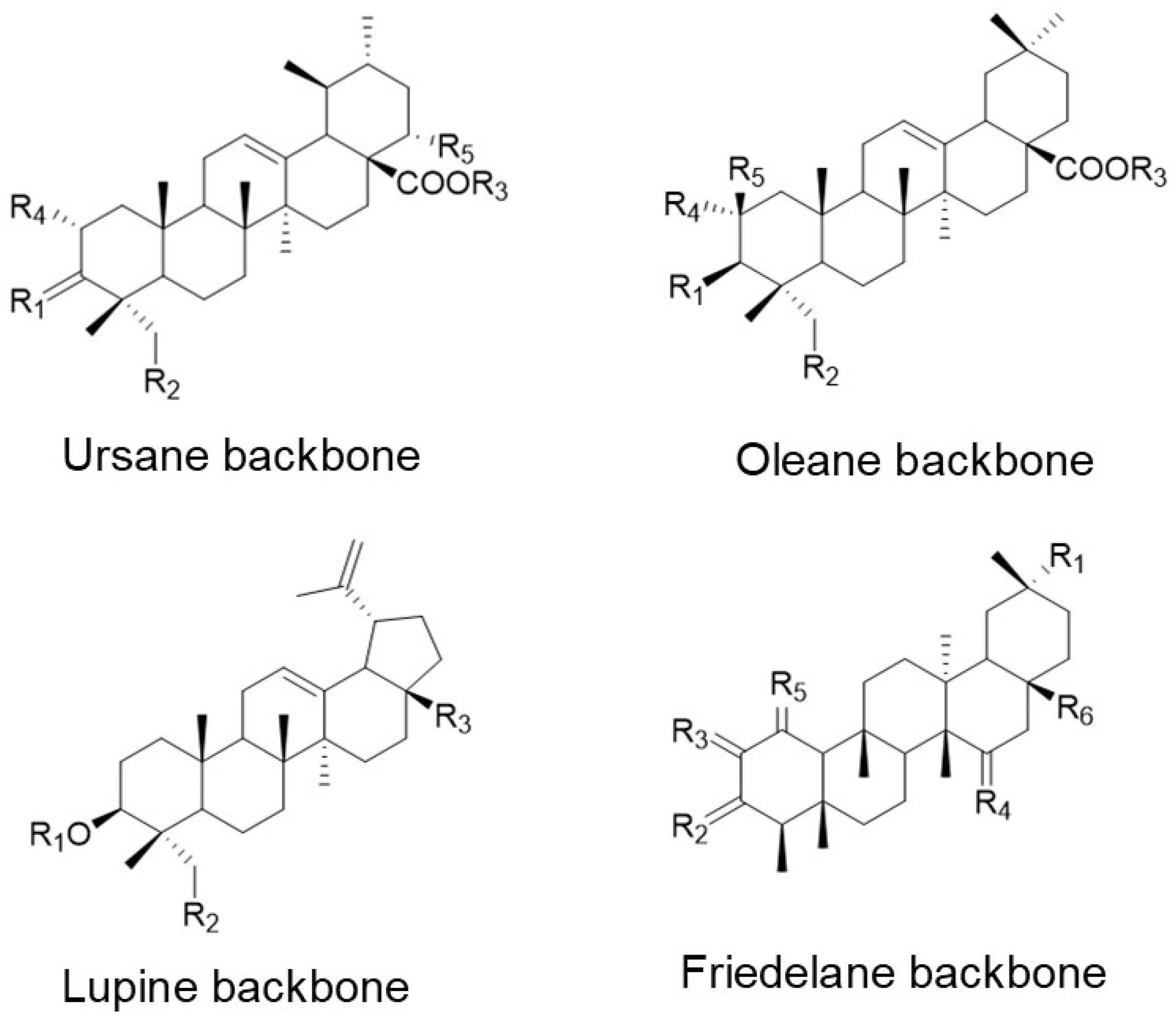
7. Biological Effects of Naturally Occurring Analogues of UA
7.1. Asiatic Acid
7.2. Corosolic Acid
7.3. 23-Hydroxy Ursolic Acid
7.4. Other Ursolic Acid Analogues and Related Pentacyclic Triterpenoids
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heber, D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J. Postgrad. Med. 2004, 50, 145–149. [Google Scholar] [PubMed]
- Taiz, L.; Zeiger, E. Chapter 13: Secondary Metabolites and Plant Defense. In Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Murakami, A.; Ohnishi, K. Target molecules of food phytochemicals: Food science bound for the next dimension. Food Funct. 2012, 3, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharkey, T.D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 2014, 31, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordoba, E.; Salmi, M.; León, P. Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J. Exp. Bot. 2009, 60, 2933–2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the Methylerythritol Phosphate Pathway for Isoprenoid Biosynthesis in Bacteria and Plastids. A Metabolic Milestone Achieved through Genomics. Plant. Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant. Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Robinson, T. The Organic Constituents of Higher Plants, 5th ed.; Cordus Press: North Amherst, MA, USA, 1983. [Google Scholar]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Bringe, K.; Schumacher, C.F.A.; Schmitz-Eiberger, M.; Steiner, U.; Oerke, E.-C. Ontogenetic variation in chemical and physical characteristics of adaxial apple leaf surfaces. Phytochemistry 2006, 67, 161–170. [Google Scholar] [CrossRef]
- Yin, M.-C.; Lin, M.-C.; Mong, M.-C.; Lin, C.-Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a liquid chromatography–mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011, 399, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yang, W.; Jia, Y.; Chen, X.; Gao, Q.; Bi, K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi 2005, 125, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Hirsh, S.; Huber, L.; Zhang, P.; Stein, R.; Joyal, S. A single ascending dose, initial clinical pharmacokinetic and safety study of ursolic acid in healthy adult volunteers (1044.6). FASEB J. 2014, 28, 1044–1046. [Google Scholar] [CrossRef]
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H.; Ying, G. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2013, 8, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.N.; Ahn, Y.J.; Medina, E.A.; Asmis, R. Dietary 23-hydroxy ursolic acid protects against atherosclerosis and obesity by preventing dyslipidemia-induced monocyte priming and dysfunction. Atherosclerosis 2018, 275, 333–341. [Google Scholar] [CrossRef]
- Somova, L.; Nadar, A.; Rammanan, P.; Shode, F. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Geerlofs, L.; He, Z.; Xiao, S.; Xiao, Z.C. Repeated dose (90 days) oral toxicity study of ursolic acid in Han-Wistar rats. Toxicol. Rep. 2020, 7, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Najid, A.; Simon, A.; Cook, J.; Chable-Rabinovitch, H.; Delage, C.; Chulia, A.; Rigaud, M. Characterization of ursolic acid as a lipoxygenase and cyclooxygenase inhibitor using macrophages, platelets and differentiated HL60 leukemic cells. FEBS Lett. 1992, 299, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Finlay, H.J.; Gribble, G.W.; Suh, N.; Spor, M.B. New Enone Derivatives of Oleanolic Acid and Ursolic Acid as Inhibitors of Nitric Oxide Production in Mouse Macrophages. Bioorganic Med. Chem. Lett. 1997, 7, 1623–1628. [Google Scholar] [CrossRef]
- Suh, N.; Honda, T.; Finlay, H.J.; Barchowsky, A.; Williams, C.; Benoit, N.E.; Xie, Q.W.; Nathan, C.; Gribble, G.W.; Sporn, M.B. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998, 58, 717–723. [Google Scholar] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-κB activation induced by carcinogenic agents through suppression of IκBα kinase and p65 phosphorylation correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar]
- Tsai, S.J.; Yin, M.C. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, C.; Hwang, S.W.; Im, J.P.; Kim, J.S. Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci. 2014, 110, 23–34. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Liu, X.; Zhu, W.; Guo, F.; Wang, R.; Chen, K.; Huang, C.; Li, Y. Inhibition of human neutrophil elastase by pentacyclic triterpenes. PLoS ONE 2013, 8, e82794. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Murakami, A.; Fujimura, Y.; Tachibana, H.; Yamada, K.; Masuda, D.; Hirano, K.; Yamashita, S.; Ohigashi, H. Aggregated ursolic acid, a natural triterpenoid, induces IL-1beta release from murine peritoneal macrophages: Role of CD36. J. Immunol. 2007, 178, 4854–4864. [Google Scholar] [CrossRef]
- Ullevig, S.; Kim, H.S.; Asmis, R. S-glutathionylation in monocyte and macrophage (dys)function. Int. J. Mol. Sci 2013, 14, 15212–15232. [Google Scholar] [CrossRef] [Green Version]
- Asmis, R.; Begley, J.G.; Jelk, J.; Everson, W.V. Lipoprotein aggregation protects human monocyte-derived macrophages from OxLDL-induced cytotoxicity. J. Lipid Res. 2005, 46, 1124–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Mao, Q.; Klaassen, C.D. The Effects of 10 Triterpenoid Compounds on Experimental Liver Injury in Mice. Fundam. Appl. Toxicol. 1994, 22, 34–40. [Google Scholar] [CrossRef]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, B.; Visen, P.K.S.; Agarwal, D.P. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother. Res. 2000, 14, 163–166. [Google Scholar] [CrossRef]
- Saravanan, R.; Viswanathan, P.; Pugalendi, K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006, 78, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic Acid Increases Skeletal Muscle and Brown Fat and Decreases Diet-Induced Obesity, Glucose Intolerance and Fatty Liver Disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-M.; Kim, M.-J.; Choi, M.-S.; Kwon, E.-Y.; Lee, M.-K. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metab. Clin. Exp. 2010, 59, 512–519. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Li, S.; Meng, F.; Liao, X.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic Role of Ursolic Acid on Ameliorating Hepatic Steatosis and Improving Metabolic Disorders in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Rats. PLoS ONE 2014, 9, e86724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-A.; Lee, J.-S.; Park, H.-J.; Kim, J.-W.; Kim, C.-J.; Shim, I.-S.; Kim, N.-J.; Han, S.-M.; Lim, S. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ikejima, K.; Kon, K.; Arai, K.; Aoyama, T.; Okumura, K.; Abe, W.; Sato, N.; Watanabe, S. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J. Hepatol. 2011, 55, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Z.; Liu, Y.; Kang, X.; Zhang, H.; Meng, M. Ursolic acid via LKB1-AMPK signaling suppresses oxidative stress and improves liver functions in mice. J. Gastroenterol. Hepatol. 2014. [Google Scholar] [CrossRef]
- Jia, Y.; Bhuiyan, M.J.H.; Jun, H.-j.; Lee, J.H.; Hoang, M.H.; Lee, H.-J.; Kim, N.; Lee, D.; Hwang, K.Y.; Hwang, B.Y.; et al. Ursolic acid is a PPAR-α agonist that regulates hepatic lipid metabolism. Bioorganic Med. Chem. Lett. 2011, 21, 5876–5880. [Google Scholar] [CrossRef]
- Lv, Y.-Y.; Jin, Y.; Han, G.-Z.; Liu, Y.-X.; Wu, T.; Liu, P.; Zhou, Q.; Liu, K.-X.; Sun, H.-J. Ursolic acid suppresses IL-6 induced C-reactive protein expression in HepG2 and protects HUVECs from injury induced by CRP. Eur. J. Pharm. Sci. 2012, 45, 190–194. [Google Scholar] [CrossRef]
- Rao, V.S.; Melo, C.L.d.; Queiroz, M.G.R.; Lemos, T.L.G.; Menezes, D.B.; Melo, T.S.; Santos, F.A. Ursolic Acid, a Pentacyclic Triterpene from Sambucus australis, Prevents Abdominal Adiposity in Mice Fed a High-Fat Diet. J. Med. Food 2011, 14, 1375–1382. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a Natural Compound that Increases Muscle Mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wong, H.S.; Leung, H.Y.; Leong, P.K.; Chan, W.M.; Ko, K.M. An ursolic acid-enriched Cynomorium songarium extract attenuates high fat diet-induced obesity in mice possibly through mitochondrial uncoupling. J. Funct. Foods 2014, 9, 211–224. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.-I.; Seo, K.-I.; Cho, H.W.; Kim, M.-J.; Park, E.-M.; Lee, M.-K. Effects of ursolic acid on glucose metabolism, the polyol pathway and dyslipidemia in non-obese type 2 diabetic mice. Indian J. Exp. Biol. 2014, 52, 683–691. [Google Scholar]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic Acid Inhibits Adipogenesis in 3T3-L1 Adipocytes through LKB1/AMPK Pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kang, Z.; Li, S.; Kong, T.; Liu, X.; Sun, C. Ursolic acid stimulates lipolysis in primary-cultured rat adipocytes. Mol. Nutr. Food Res. 2010, 54, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, M.; Luo, H.; Dong, L.Q.; Liu, F. Ursolic Acid Inhibits Leucine-Stimulated mTORC1 Signaling by Suppressing mTOR Localization to Lysosome. PLoS ONE 2014, 9, e95393. [Google Scholar] [CrossRef]
- Ogasawara, R.; Sato, K.; Higashida, K.; Nakazato, K.; Fujita, S. Ursolic Acid Stimulates mTORC1 Signaling after Resistance Exercise in Rat Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E760–E765. [Google Scholar] [CrossRef] [Green Version]
- Radhiga, T.; Rajamanickam, C.; Senthil, S.; Pugalendi, K.V. Effect of ursolic acid on cardiac marker enzymes, lipid profile and macroscopic enzyme mapping assay in isoproterenol-induced myocardial ischemic rats. Food Chem. Toxicol. 2012, 50, 3971–3977. [Google Scholar] [CrossRef]
- Radhiga, T.; Rajamanickam, C.; Sundaresan, A.; Ezhumalai, M.; Pugalendi, K.V. Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie 2012, 94, 1135–1142. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, J.; Zhang, Y.; Ling, J.; Xu, X. Attenuation of aortic injury by ursolic acid through RAGE-Nox-NFκB pathway in streptozocin-induced diabetic rats. Arch. Pharmacal Res. 2012, 35, 877–886. [Google Scholar] [CrossRef]
- Ullevig, S.L.; Zhao, Q.; Zamora, D.; Asmis, R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 2011, 219, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.M.; Yee, S.T.; Choi, J.; Choi, M.S.; Do, G.M.; Jeon, S.M.; Yeo, J.; Kim, M.J.; Seo, K.I.; Lee, M.K. Ursolic acid enhances the cellular immune system and pancreatic beta-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int. Immunopharmacol. 2009, 9, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, A.; Ranganathan, H.; Pugalendi, K.V. Ursolic Acid and rosiglitazone combination alleviates metabolic syndrome in high fat fed C57BL/6J mice. Gen. Physiol. Biophys. 2012, 31, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, A.; Radhiga, T.; Pugalendi, K.V. Effect of ursolic acid and Rosiglitazone combination on hepatic lipid accumulation in high fat diet-fed C57BL/6J mice. Eur. J. Pharmacol. 2014, 741, 297–303. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.S.; Zhang, X.; Wu, Y.J.; Huang, K.; Zheng, L. Ursolic acid inhibits early lesions of diabetic nephropathy. Int. J. Mol. Med. 2010, 26, 565–570. [Google Scholar]
- Wang, J.; Jiang, Y.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, Y.; Yu, J. The different responses of glutathione-dependent detoxification pathway to fungicide chlorothalonil and carbendazim in tomato leaves. Chemosphere 2010, 79, 958–965. [Google Scholar] [CrossRef]
- Ling, C.; Jinping, L.; Xia, L.; Renyong, Y. Ursolic Acid Provides Kidney Protection in Diabetic Rats. Curr. Res. Clin. Exp. 2013, 75, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, P.G.; Chamari Nawarathna, S.; Kulkarni, A.; Habeeba, U.; Reddy C, S.; Teerthanath, S.; Shenoy, J.P. Nephroprotective effect of ursolic acid in a murine model of gentamicin-induced renal damage. ISRN Pharm. 2012, 2012, 410902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chein, Y.-C.; Wang, J.-Y.; Fu, Y.-S. Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci. Lett. 2004, 362, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, Y.L.; Wu, D.M.; Luo, L.; Sun, D.X.; Shan, Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem. Pharm. 2007, 74, 1078–1090. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Hu, B.; Zhang, Z.-F.; Ye, Q.; Liu, C.-M.; Shan, Q.; Wang, Y.-J. Ursolic Acid Attenuates D-Galactose-Induced Inflammatory Response in Mouse Prefrontal Cortex through Inhibiting AGEs/RAGE/NF-κB Pathway Activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-J.; Lu, J.; Wu, D.-M.; Zheng, Z.-H.; Zheng, Y.-L.; Wang, X.-H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.-F. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Cheng, W.; Zhang, Z.F.; Shan, Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav. Immun. 2011, 25, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Boyd, J.D.; Glicksman, M.; Moore, K.J.; El Khoury, J. A High Content Drug Screen Identifies Ursolic Acid as an Inhibitor of Amyloid β Protein Interactions with Its Receptor CD36. J. Biol. Chem. 2011, 286, 34914–34922. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Su, J.; Wang, K.; Zhu, T.; Li, X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 2014, 579, 12–17. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef]
- Mortiboys, H.; Aasly, J.; Bandmann, O. Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain 2013, 136, 3038–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullevig, S.L.; Kim, H.S.; Short, J.D.; Tavakoli, S.; Weintraub, S.T.; Downs, K.; Asmis, R. Protein S-Glutathionylation Mediates Macrophage Responses to Metabolic Cues from the Extracellular Environment. Antioxid. Redox Signal. 2016, 25, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Ullevig, S.L.; Kim, H.S.; Nguyen, H.N.; Hambright, W.S.; Robles, A.J.; Tavakoli, S.; Asmis, R. Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of Nox4. Redox Biol. 2014, 2, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Messner, B.; Zeller, I.; Ploner, C.; Frotschnig, S.; Ringer, T.; Steinacher-Nigisch, A.; Ritsch, A.; Laufer, G.; Huck, C.; Bernhard, D. Ursolic acid causes DNA-damage, P53-mediated, mitochondria- and caspase-dependent human endothelial cell apoptosis, and accelerates atherosclerotic plaque formation in vivo. Atherosclerosis 2011, 219, 402–408. [Google Scholar] [CrossRef]
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef]
- Flores-Flores, A.; Hernandez-Abreu, O.; Rios, M.Y.; Leon-Rivera, I.; Aguilar-Guadarrama, B.; Castillo-Espana, P.; Perea-Arango, I.; Estrada-Soto, S. Vasorelaxant mode of action of dichloromethane-soluble extract from Agastache mexicana and its main bioactive compounds. Pharm. Biol. 2016, 54, 2807–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hsu, C.C.; Huang, C.N.; Yin, M.C. Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur. J. Pharm. 2010, 628, 255–260. [Google Scholar] [CrossRef]
- Ma, J.-Q.; Ding, J.; Xiao, Z.-H.; Liu, C.-M. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB activities. Int. Immunopharmacol. 2014, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, A.M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Acuna Ortega, N. Effect of Ursolic Acid on Metabolic Syndrome, Insulin Sensitivity, and Inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stree, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schetter, A.J.; Harris, N.H.H.; Harris, C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis 2010, 31, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting Inflammatory Pathways by Triterpenoids for Prevention and Treatment of Cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [Green Version]
- Manu, K.A.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-κB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Li, Y.; Xing, D.; Chen, Q.; Chen, W.R. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-κB using ursolic acid. Int. J. Cancer 2010, 127, 462–473. [Google Scholar] [CrossRef]
- Yan, S.-L.; Huang, C.-Y.; Wu, S.-T.; Yin, M.-C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Huang, H.-C.; Huang, C.-Y.; Lin-Shiau, S.-Y.; Lin, J.-K. Ursolic acid inhibits IL-1β or TNF-α-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-ζ and downregulating the MMP-9 expression. Mol. Carcinog. 2009, 48, 517–531. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic Acid Inhibits STAT3 Activation Pathway Leading to Suppression of Proliferation and Chemosensitization of Human Multiple Myeloma Cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-X.; Kong, C.-Z.; Wang, L.-H.; Li, J.-Y.; Liu, X.-K.; Xu, B.; Xu, C.-L.; Sun, Y.-H. Ursolic acid overcomes Bcl-2-mediated resistance to apoptosis in prostate cancer cells involving activation of JNK-induced Bcl-2 phosphorylation and degradation. J. Cell. Biochem. 2010, 109, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Ren, T.-N.; Xi, T. Ursolic acid induces apoptosis by suppressing the expression of FoxM1 in MCF-7 human breast cancer cells. Med. Oncol. 2012, 29, 10–15. [Google Scholar] [CrossRef]
- Harmand, P.-O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Achiwa, Y.; Hasegawa, K.; Komiya, T.; Udagawa, Y. Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells. Oncol. Rep. 2005, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kwon, H.-Y.; Sohn, E.J.; Kim, K.A.; Kim, B.; Jeong, S.-J.; Song, J.H.; Koo, J.S.; Kim, S.-H. Inhibition of Wnt/Beta catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharm. Rep. 2013, 65, 1366–1374. [Google Scholar] [CrossRef]
- Way, T.-D.; Tsai, S.-J.; Wang, C.-M.; Ho, C.-T.; Chou, C.-H. Chemical Constituents of Rhododendron formosanum Show Pronounced Growth Inhibitory Effect on Non-Small-Cell Lung Carcinoma Cells. J. Agric. Food Chem. 2014, 62, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ji, Q.; Tang, Y.; Chen, T.; Pan, G.; Hu, S.; Bao, Y.; Peng, W.; Yin, P. Mitochondrial translocation of cofilin-1 promotes apoptosis of gastric cancer BGC-823 cells induced by ursolic acid. Tumor Biol. 2014, 35, 2451–2459. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Shi, D.; Cheng, J.; et al. Ursolic Acid Simultaneously Targets Multiple Signaling Pathways to Suppress Proliferation and Induce Apoptosis in Colon Cancer Cells. PLoS ONE 2013, 8, e63872. [Google Scholar] [CrossRef] [Green Version]
- Gai, L.; Cai, N.; Wang, L.; Xu, X.; Kong, X. Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression in T24 human bladder cancer cells. Mol. Med. Rep. 2013, 7, 1673–1677. [Google Scholar] [CrossRef]
- Shin, S.W.; Park, J.-W. Ursolic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Limami, Y.; Pinon, A.; Leger, D.Y.; Mousseau, Y.; Cook-Moreau, J.; Beneytout, J.-L.; Delage, C.; Liagre, B.; Simon, A. HT-29 colorectal cancer cells undergoing apoptosis overexpress COX-2 to delay ursolic acid-induced cell death. Biochimie 2011, 93, 749–757. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Deng, T.; Hu, Z.F.; Zhang, Q.P.; Zhang, J.; Jiang, H. Mechanisms of inhibiting proliferation and inducing apoptosis of human gastric cancer cell line SGC7901 by ursolic acid. Ai Zheng 2006, 25, 432–437. [Google Scholar]
- Li, J.; Liang, X.; Yang, X. Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF-kappaB pathways. Oncol. Rep. 2012, 28, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.; Ho, P.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Pedro, D.F.N.; Wilson, J.M.; Kristiansen, K.; Pereira-Wilson, C. Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells. J. Nutr. Biochem. 2013, 24, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kong, C.; Zeng, Y.; Wang, L.; Li, Z.; Wang, H.; Xu, C.; Sun, Y. Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol. Carcinog. 2010, 49, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.-Z.; Xuan, Y.-Y.; Zheng, S.; Dong, Q.; Zhang, S.-Z. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J. Zhejiang Univ. Sci. B 2009, 10, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Yadav, V.R.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic Acid Inhibits Growth and Metastasis of Human Colorectal Cancer in an Orthotopic Nude Mouse Model by Targeting Multiple Cell Signaling Pathways: Chemosensitization with Capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhao, C.; Jou, D.; Lü, J.; Zhang, C.; Lin, L.; Lin, J. Ursolic Acid Inhibits the Growth of Colon Cancer-initiating Cells by Targeting STAT3. Anticancer Res. 2013, 33, 4279–4284. [Google Scholar]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Yeger, H.; Tsuchida, R.; Torkin, R.; Gee, M.F.W.; Thorner, P.S.; Shibuya, M.; Malkin, D.; Baruchel, S. A Hypoxia-Driven Vascular Endothelial Growth Factor/Flt1 Autocrine Loop Interacts with Hypoxia-Inducible Factor-1α through Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase 1/2 Pathway in Neuroblastoma. Cancer Res. 2005, 65, 7267–7275. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Lin, C.-Y.; Tsai, C.-W.; Yin, M.-C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. In Vitro 2011, 25, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Wei, L.; Hong, Z.; Sferra, T.J.; Peng, J. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int. J. Oncol. 2013, 43, 1666–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. Ursolic Acid, a Pentacyclin Triterpene, Potentiates TRAIL-induced Apoptosis through p53-independent Up-regulation of Death Receptors: Evidence for the Role of Reactive Oxygen Species and JNK. J. Biol. Chem. 2011, 286, 5546–5557. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Huang, C.-Y.; Mong, M.-C.; Chan, C.-Y.; Yin, M.-C. Antiangiogenic Potential of Three Triterpenic Acids in Human Liver Cancer Cells. J. Agric. Food Chem. 2010, 59, 755–762. [Google Scholar] [CrossRef]
- Cha, H.-J.; Bae, S.-K.; Lee, H.-Y.; Lee, O.-H.; Sato, H.; Seiki, M.; Park, B.C.; Kim, K.-W. Anti-Invasive Activity of Ursolic Acid Correlates with the Reduced Expression of Matrix Metalloproteinase-9 (MMP-9) in HT1080 Human Fibrosarcoma Cells. Cancer Res. 1996, 56, 2281–2284. [Google Scholar] [PubMed]
- Kowalczyk, M.C.; Junco, J.J.; Kowalczyk, P.; Tolstykh, O.; Hanausek, M.; Slaga, T.J.; Walaszek, Z. Effects of combined phytochemicals on skin tumorigenesis in SENCAR mice. Int. J. Oncol. 2013, 43, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angel, R.E.; Smith, S.M.; Glickman, R.D.; Perkins, S.N.; Hursting, S.D. Antitumor effects of ursolic acid in a mouse model of postmenopausal breast cancer. Nutr. Cancer 2010, 62, 1074–1086. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, H.; Zhu, L.; Wei, W. Ursolic Acid Ameliorates Early Brain Injury After Experimental Traumatic Brain Injury in Mice by Activating the Nrf2 Pathway. Neurochem. Res. 2017, 42, 337–346. [Google Scholar] [CrossRef]
- Sahu, S.; Li, R.; Kadeyala, P.K.; Liu, S.; Schachner, M. The human natural killer-1 (HNK-1) glycan mimetic ursolic acid promotes functional recovery after spinal cord injury in mouse. J. Nutr. Biochem. 2018, 55, 219–228. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, L.-T.; Lin, C.-C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef]
- Park, B.C.; Bosire, K.O.; Lee, E.-S.; Lee, Y.S.; Kim, J.-A. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 2005, 218, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jin, D.-Q.; Kwon, E.J.; Park, S.H.; Lee, E.-S.; Jeong, T.C.; Nam, D.H.; Huh, K.; Kim, J.-A. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca 2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002, 186, 83–91. [Google Scholar] [CrossRef]
- Yan, S.-L.; Yang, H.-T.; Lee, Y.-J.; Lin, C.-C.; Chang, M.-H.; Yin, M.-C. Asiatic acid ameliorates hepatic lipid accumulation and insulin resistance in mice consuming a high-fat diet. J. Agric. Food Chem. 2014, 62, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Saravanan, R. Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2013, 20, 230–236. [Google Scholar] [CrossRef]
- Pakdeechote, P.; Bunbupha, S.; Kukongviriyapan, U.; Prachaney, P.; Khrisanapant, W.; Kukongviriyapan, V. Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Nutrients 2014, 6, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Yamada, K.; Yoshikawa, N.; Nakamura, K.; Haginaka, J.; Kunitomo, M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006, 79, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hosokawa, M.; Fujimoto, S.; Fujiwara, H.; Fujita, Y.; Harada, N.; Yamada, C.; Fukushima, M.; Ueda, N.; Kaneko, T. Effect of corosolic acid on gluconeogenesis in rat liver. Diabetes Res. Clin. Pract. 2008, 80, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Itoh, Y.; Kaneko, T.; Ueda, N.; Ishida, T.; Fukushima, M.; Matsuyama, F.; Seino, Y. Corosolic acid induces GLUT4 translocation in genetically type 2 diabetic mice. Biol. Pharm. Bull. 2004, 27, 1103–1105. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhang, W.; Zhou, Y.-Y.; Zhang, Y.-N.; Li, J.-Y.; Hu, L.-H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008, 584, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Wang, L.; Foster, S.; Asmis, R. Dietary 23-hydroxy ursolic acid protects against diet-induced weight gain and hyperglycemia by protecting monocytes and macrophages against nutrient stress-triggered reprogramming and dysfunction and preventing adipose tissue inflammation. J. Nutr. Biochem 2020, 86, 108483. [Google Scholar] [CrossRef]
- Bore, L.; Honda, T.; Gribble, G.W. Partial synthesis of 23-hydroxyursolic acid isolated from medicinal plants of the Rubiaceae family. Nat. Prod. Lett. 2002, 16, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells. Mol. Nutr. Food Res. 2008, 52, 595–599. [Google Scholar] [CrossRef]
- Abuobeid, R.; Herrera-Marcos, L.; Navarro, M.A.; Arnal, C.; Martinez-Beamonte, R.; Surra, J.; Osada, J. Dietary Erythrodiol Modifies Hepatic Transcriptome in Mice in a Sex and Dose-Dependent Way. Int. J. Mol. Sci. 2020, 21, 7331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wesemann, S.; Krenn, L.; Ladurner, A.; Heiss, E.H.; Dirsch, V.M.; Atanasov, A.G. Erythrodiol, an Olive Oil Constituent, Increases the Half-Life of ABCA1 and Enhances Cholesterol Efflux from THP-1-Derived Macrophages. Front. Pharm. 2017, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, L.; Cao, X.; Li, W.; Zhao, Y.; Chen, J.; Li, J.; Chen, Y.; Yu, W.; Xu, Y. Hederagenin Attenuates Cerebral Ischaemia/Reperfusion Injury by Regulating MLK3 Signalling. Front. Pharm. 2020, 11, 1173. [Google Scholar] [CrossRef]
- Ma, W.; Huang, Q.; Xiong, G.; Deng, L.; He, Y. The protective effect of Hederagenin on pulmonary fibrosis by regulating the Ras/JNK/NFAT4 axis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Radich, J.; Gonzalez-Blazquez, R.; Alcala, M.; Martin-Ramos, M.; Viana, M.; Arribas, S.; Delporte, C.; Fernandez-Alfonso, M.S.; Somoza, B.; Gil-Ortega, M. Beneficial effects of murtilla extract and madecassic acid on insulin sensitivity and endothelial function in a model of diet-induced obesity. Sci. Rep. 2019, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Marquez-Martin, A.; De La Puerta, R.; Fernandez-Arche, A.; Ruiz-Gutierrez, V.; Yaqoob, P. Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells. Cytokine 2006, 36, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hang, T.-J.; Wang, Y.; Jiang, L.; Wu, X.-L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Bani, S.; Gupta, B.; Banerjee, S. Anti-inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar] [CrossRef]
- Mengoni, F.; Lichtner, M.; Battinelli, L.; Marzi, M.; Mastroianni, C.M. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Pharmacol. Res. 2002, 3, 8. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids 1. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef]
- Lúcio, K.A.; da Graça Rocha, G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, H.; Chen, D.; Ni, J.; Kang, Y.; Wang, S. Oleanolic acid induces apoptosis in human leukemia cells through caspase activation and poly (ADP-ribose) polymerase cleavage. Acta Biochim. Biophys. Sin. 2007, 39, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.F.; Barbosa-Filho, J.M.; Maia, G.L.; Guimaraes, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.; Soares, M.B. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Kamaraj, Y.; Dhayalan, S.; Chinnaiyan, U.; Kumaresan, V.; Subramaniyan, S.; Kumar, D.; Muniyandi, K.; Punamalai, G. Triterpenoid compound betulin attenuates allergic airway inflammation by modulating antioxidants, inflammatory cytokines and tissue transglutaminase in ovalbumin-induced asthma mice model. J. Pharm Pharm. 2021, 73, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, B.; Sun, J.; Chen, H.; Yang, Z. Betulin ameliorates 7,12-dimethylbenz(a)anthracene-induced rat mammary cancer by modulating MAPK and AhR/Nrf-2 signaling pathway. J. Biochem Mol. Toxicol 2021, e22779. [Google Scholar] [CrossRef]
- Liby, K.T.; Sporn, M.B. Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharm. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, J.Y.; Lin, J.J.; Wahler, J.; Liby, K.T.; Sporn, M.B.; Suh, N. A Synthetic Triterpenoid CDDO-Im Inhibits Tumorsphere Formation by Regulating Stem Cell Signaling Pathways in Triple-Negative Breast Cancer. PLoS ONE 2014, 9, e107616. [Google Scholar]
- Lapillonne, H.; Konopleva, M.; Tsao, T.; Gold, D.; McQueen, T.; Sutherland, R.L.; Madden, T.; Andreeff, M. Activation of peroxisome proliferator-activated receptor γ by a novel synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003, 63, 5926–5939. [Google Scholar] [PubMed]
- Wang, Y.; Porter, W.W.; Suh, N.; Honda, T.; Gribble, G.W.; Leesnitzer, L.M.; Plunket, K.D.; Mangelsdorf, D.J.; Blanchard, S.G.; Willson, T.M. A synthetic triterpenoid, 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor γ. Mol. Endocrinol. 2000, 14, 1550–1556. [Google Scholar] [PubMed] [Green Version]

| Disease | UA | Delivery | Model | Outcome | References |
|---|---|---|---|---|---|
| CVD | 60 mg/kg body weight | IP | Dahl salt-sensitive rat model | ↓ HTN, BG, and TC; ↑ GPx and SOD | [20] |
| CVD | 85 mg/kg body weight | subq IP | Windsor rats | against ISO-induced MI, ↓ CK-MB, LDH, LDL, TG, and FFA | [57] |
| CVD | 85 mg/kg body weight | subq IP | Windsor rats | ↑ Bcl-2, Bcl-xl and ↓ of Bax, caspase-3, -8, and -9, cytochrome c, TNF-alpha, and FAS. ↓ lipid peroxidation markers and ↑ antioxidant enzymes and non-antioxidant enzymes in the plasma and heart tissue of ISO-induced MI | [58] |
| CVD | 50 mg/kg | oral gavage | STZ-Diabetic mice | ↓ aortic damage, RAGE, P22, and NFĸB | [59] |
| CVD | 0.05% | HFD | LDLR-KO mice | ↓atherosclerotic plaque size and weight gain | [19] |
| CVD | 0.20% | HFD | STZ-treated LDLR-KO, mice | ↓ atherosclerosis lesion formation, fewer infiltrating macrophages, ↓ BG, Alb/Crt ratio, inflammatory blood monocytes, ↑ low inflammatory blood monocytes | [60] |
| Diabetes | 0.05% | HFD | STZ-Diabetic mice | Protects pancreatic islet cells and ↑ insulin secretion | [61] |
| Diabetes | 5 mg/kg | HFD | C57BL/6J mice | UA combined with rosiglitazone ↓ whole BW gain, and can have profound responses to rosiglitazone or metformin. | [62] |
| Diabetes | 0.01% and 0.05% | AIN-76 semisynthetic diet | STZ/NA-Diabetic mice | Significant improved diabetic outcomes and stimulated T-lymphocytes in the thymus | [52] |
| Diabetes | 5 mg/kg | HFD | C57BL/6J mice | UA combined with rosiglitazone ↓ hepatic marker enzyme activities and ↓ lipid accumulation in liver | [63] |
| Kidney Disease | 0.01% | Standard rat chow | STZ-Diabetic mice | ↓ glomerular hypertrophy, collagen accumulation, and suppressed activation of STAT-3, ERK1/2, JNK and iNOS overexpression | [64] |
| Kidney Disease | 0.05%, 0.1% and 0.2% | 64 g starch, 23 g protein, 3.5 g fat, 5 g fiber, 1 vitamin mixture and 3 salt mixtures | STZ-Diabetic mice | ↑ kidney function ↓ flux through the renal polyol pathway, and ↓ AGEs formation in urine | [65] |
| Kidney Disease | 0.2% | Standard rat chow | STZ-Diabetic mice | ↓ UAE, renal oxidative stress, NF-KB activity, and P-selection expression | [66] |
| Kidney Disease | 25 and 50 mg/kg | oral gavage | ICR mice | UA prevents CCl4-induced nephrotoxicity, ROS, DNA damage, and proinflammatory markers | [37] |
| Kidney Disease | 2, 5, and 10 mg/kg | orally | Wistar albino rats | UA protected kidneys from gentamicin-induced damage | [67] |
| Kidney Disease | 0.2% in diet | Standard rat chow | Wistar rats | ↓ UAE, renal oxidative stress level, NF-κB activity, and P-selectin expression. | [42] |
| Liver Disease | 1–100 µM | Incubation medium | Human liver microsomes | UA regulation of cytochrome P450 shows hepatoprotective properties | [44] |
| Liver Disease | 50 mg/kg | IP | Wistar rats | Induced apoptosis in liver-damaging hepatic stellate cells while maintaining normal hepatocyte function | [45] |
| Liver Disease | 50 mg/kg | oral gavage | C57/BL6 WT mice | ↓ oxidative stress through activation of LKB1-AMPK signaling | [46] |
| Liver Disease | 25 and 50 mg/kg | intragastrically | ICR mice | ↓ CCl(4)-induced lipid peroxidation levels and depleted TAC levels in liver. ↓ CYP2E1, TNF-α, IL-1β and COX-2, JNK, p38 MAPK, ERK, and inactivation of NF-κB | [37] |
| Liver Disease | 1, 10, and 100 µg/mL | cell culture UA treatment | Albino Druckery rats | UA isolated from Eucalyptus tereticornis improved liver function measured by AST, ALT, and ↑ glutathione, α-tocopherol, and ascorbic acid | [38] |
| Liver Disease | 10, 20, and 40 mg/kg/day | intragastrically | Wistar albino rats | Pure UA improved liver function measured by AST, ALT, and ↑ glutathione, α-tocopherol, and ascorbic acid | [39] |
| Liver Disease | 0.125%, 0.25%, and 0.50% | HFD | Sprague-Dawley rats | Significantly reversed HFD-induced hepatic steatosis and liver injury | [43] |
| Liver Disease | 5, 20, and 80 µM | cell culture UA treatment | Cultured HepG2 cells | ↑ PPARα binding to its response element but did not directly bind PPARα in the liver hepatocyte cell line, HepG2 cells | [47] |
| Liver Disease | HepG2 (6.25, 12.5, and 25 µM) and HUVECs (5, 10, 20 µM) | cell culture UA treatment | HUVECs and HepG2 cells | ↓ inflammatory cytokine production induced by IL-6 in HepG2 cells | [48] |
| Liver Disease | 0.1 and 0.05% | AIN-76 semisynthetic diet | STZ/NA-Diabetic mice | ↓ FBG, TG, FFA, TC and VLDL, LDL. ↓ hepatic G6-P activity and ↑ glucokinase activity, the glucokinase/G6-P ratio, GLUT2 mRNA levels and glycogen content. ↑ aldose reductase activity, ↓ SDH | [52] |
| Metabolic Disease | 50 µM | cell culture UA treatment | C2C12 cells | Inhibited mTORC activation by leucine through suppression of mTOR lysosomal localization | [55] |
| Metabolic Disease | 250 mg/kg | IP | Sprague-Dawley rats | UA sustained exercise-induced mTORC1 activity | [56] |
| Metabolic Disease | 40 mg/kg body weight | IP | C57Bl/6 mice | ↑ muscle mass by inhibiting skeletal muscle atrophy and improved metabolic outcomes | [50] |
| Metabolic Disease | 0.5 g/kg | HFD | STZ-Diabetic mice | ↓ blood glucose, TC, FFA, TG, and improved liver function | [41] |
| Metabolic Disease | 125 nM, 250 nM, 500 nM and 1 µM | cell culture UA treatment | CHO/hIR cells | Inhibition of PTP1B ↓ blood glucose. PTP1B is a phosphatase inhibitor of insulin-mediated signaling. | [68] |
| Neuro. Disease | 5, 10, and 15 µM | cell culture UA treatment | Sprague-Dawley rats | ↓ free radical generation in primary rat hippocampus neurons in response to kainite | [69] |
| Neuro. Disease | 10 mg/kg/day | oral gavage | Kunming strain mice | ↑ activity of antioxidant enzymes, SOD, CAT, GPx, and GR and ↓ general lipid peroxidation in the brain | [70] |
| Neuro. Disease | 10 mg/kg/day | oral gavage | Kunming strain mice | ↓ AGEs, ROS, PCO levels, and down-regulated iNOS, COX-2, and various inflammatory cytokines mediated through NFκB, all found in the prefrontal cortex of the brain | [71] |
| Neuro. Disease | 10 or 20 mg/kg/day | oral gavage | C57BL/6J | improved cognitive deficits attributed to ↓ COX2, iNOS, TNFα and various inflammatory interleukins mediated through p38/NFκB signaling pathways | [72] |
| Neuro. Disease | 10 mg/kg/day | oral gavage | C57BL/6J | improves cognitive impairments by inhibiting ER stress and NFκB signaling pathway, restoring insulin signaling and the mTOR pathway | [73] |
| Neuro. Disease | 50 or 100 µM | cell culture UA treatment | CHO-CD36 and primary microglia cells | Potential treatment for Alzheimer’s Disease due to ↓ amyloid β binding to CD36 | [74] |
| Neuro. Disease | 25 and 50 mg/kg | IP | SD rats | ↓ oxidative stress attenuating EBI after SAH | [75] |
| Neuro. Disease | 130 mg/kg | IP | Nrf2−/− and WT rats | Protects brain from ischemic injury through activation of NRF2 pathway | [76] |
| Neuro. Disease | 100 nM | cell culture UA treatment | Patients with parkin or LRRK2 mutations | ↑ activity of the mitochondrial respiratory chain and displayed drug-like dose-response curves for Parkinson’s Disease | [77] |
| Obesity, Diabetes | 0.05% | HFD | C57Bl/6 mice | improved glucose tolerance and wt maintenance while ↓ lipid accumulation in liver | [42] |
| Obesity | 0.05% | Drinking Water | C57Bl/6 mice | ↓ visceral adiposity, total BW, BG, and lipid | [49] |
| Obesity | 0.14% and 0.27% | HFD | C57Bl/6 mice | ↑ muscle mass, skeletal muscle glucose uptake, and BAT resulting in ↓ obesity, hepatic steatosis, and improved glucose tolerance | [40] |
| Obesity | 2.5 to 10 µM | cell culture UA treatment | 3T3-L1 mouse embryo fibroblasts | Attenuated adipogenesis through the LKB1/AMPK pathway | [53] |
| Obesity | 25, 50, and 100 µM | cell culture UA treatment | Sprague-Dawley rats | Anti-obesity mechanism by stimulating lipolysis by upregulation of ATGL in primary rat adipocytes | [54] |
| Obesity | Cynomorri extract, 100–360 mg/kg body weight | HFD | C57Bl/6 mice | ↓ wgt gain likely to ↑ energy expenditure based on observed mitochondrial uncoupling in skeletal muscle | [51] |
| Pathway | Cancer Type | References | |
|---|---|---|---|
| Induction of apoptosis | FoxM1 ↓ | breast cancer cells | [97] |
| Caspase ↑ | melanoma cell | [98] | |
| endometrial cancer cell | [99] | ||
| prostate cancer cells | [100] | ||
| non-small cell lung cancer | [101] | ||
| gastric cancer cell | [102]. | ||
| colon cancer cells | [103] | ||
| bladder cancer cells | [104] | ||
| Trail-mediated | prostate cancer cells | [105] | |
| COX-2 ↓ | colon cancer cells | [103,106] | |
| gastric cancer cell | [107] | ||
| NF-κB ↓ | bladder cancer cells | [104] | |
| pancreatic cancer cells | [108]. | ||
| prostate cancer cells | [109] | ||
| hepatocellular carcinoma cells | [110] | ||
| JNK ↑ | colon cancer cells | [111] | |
| pancreatic cancer cells | [108] | ||
| prostate cancer cells | [112] | ||
| Inhibition of cell proliferation | MAPK ↓ | endometrial cancer | [113] |
| colon cancer cells | [103,114] | ||
| STAT3 ↓ | prostate cancer cells | [109] | |
| multiple myeloma cells | [95] | ||
| colorectal cancer cells | [115,116] | ||
| p53 and p21WAF1 ↑ | non-small cell lung cancer | [117] | |
| Inhibition of metastasis | HIF-1α ↓ | neuroblastoma cells | [118] |
| VEGF ↓ | lung cancer cells | [119] | |
| colorectal cancer cells | [120] | ||
| liver cancer cells | [93] | ||
| neuroblastoma cells | [118] | ||
| MMP-9 ↓ | glioma cells | [94] | |
| liver cancer cells | [93] | ||
| lung cancer cells | [119] | ||
| ICAM-1 ↓ | liver cancer cells | [93] | |
| lung cancer cells | [119] |
| Analog | Dose | Delivery | Model | Outcome | References |
|---|---|---|---|---|---|
| Asiatic acid | 2.5, 5, 10 and 20 µM | cell culture AA treatment | Breast cancer cell lines MCF-7 and MDA-MB-231 | Cell growth inhibition by inducing cancer cells to undergo S-G2/M phase arrest and apoptosis | [130] |
| Asiatic acid | 10, 20, 30, 40 and 50 µM | cell culture AA treatment | SK-MEL-2 human melanoma cell line | ↓ cell viability, induced apoptosis in SK-MEL-2 cells, ↑ ROS, enhanced Bax expression, and induced caspase-3 activity | [131] |
| Asiatic acid | 10, 20, 30, 40, 70 and 100 µM | cell culture AA treatment | HepG2 human hepatoblastoma cell line | ↓ cell viability, induced apoptosis in HepG2 human hepatoma cells, ↑ intracellular Ca2+ level and p53 expression | [132] |
| Asiatic acid | 10 or 20 mg/kg/day | oral gavage | C57BL/6 mice | ↑ insulin sensitivity, protected mice from hepatosteatosis, ↓ ROS production, hepatic lipid accumulation, and IL-13B secretion with high AA dose | [133] |
| Asiatic acid | 5, 10 and 20 mg/kg BW | oral | STZ-diabetic mice | Reversed STZ-induced diabetes, potentially regulates CHO metabolism by modulating diabetic-regulatory enzymes | [134] |
| Asiatic acid | 10 or 20 mg/kg/day | intragastrically | Sprague-Dawley rats | Improved HCHF diet-induced insulin sensitivity, lipid profiles, hemodynamic parameters, oxidative stress markers, plasma TNF-α, NOx, and recovered abnormality of eNOS/iNOS expressions | [135] |
| Corosolic acid | 0.072% | HFD | SHR-cp rats | ↓ blood pressure, serum FFAs, oxidative stress markers, myeloperoxidase markers, and high sensitivity C-reactive protein | [136] |
| Corosolic acid | 20–100 µM | Syringe pump infused | Wistar rats | Inhibited gluconeogenesis in liver by ↑ Fru-2,6-BP, ↓ cAMP levels, inhibiting PKA activity and ↑ glycolysis | [137] |
| Corosolic acid | 10 mg/kg BW | oral | KK-Ay mice | Hypoglycemic effect derived from ↑ GLUT4 translocation in muscle | [138] |
| Corosolic acid | 250 and 500 nM | cell culture CA treatment | CHO/hIR and L6 myoblast cells | Enhanced glucose uptake by ↑ GLUT4 translocation mediated by insulin pathway activation, inhibited PTP1B, T-cell-PTP, src phosphatase 1 and 2 activity | [139] |
| 23-Hydroxy Ursolic Acid | 0.05% | HFD | LDLR-KO mice | ↓ atherosclerotic plaque size and weight gain, more potent than ursolic acid | [19] |
| 23-Hydroxy Ursolic Acid | 0.2% | HFD | C57BL/6 mice | ↑ glucose tolerance, ↓ weight gain, hyperleptinemia, macrophage recruitment, and adipose tissue inflammation | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. https://doi.org/10.3390/antiox10081161
Nguyen HN, Ullevig SL, Short JD, Wang L, Ahn YJ, Asmis R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants. 2021; 10(8):1161. https://doi.org/10.3390/antiox10081161
Chicago/Turabian StyleNguyen, Huynh Nga, Sarah L. Ullevig, John D. Short, Luxi Wang, Yong Joo Ahn, and Reto Asmis. 2021. "Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits" Antioxidants 10, no. 8: 1161. https://doi.org/10.3390/antiox10081161
APA StyleNguyen, H. N., Ullevig, S. L., Short, J. D., Wang, L., Ahn, Y. J., & Asmis, R. (2021). Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants, 10(8), 1161. https://doi.org/10.3390/antiox10081161






