Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen
Abstract
1. Introduction
- (a)
- to determine, for the first time, general (total extractable and alkaline hydrolyzable phenolics, total extractable and alkaline hydrolyzable flavonoids, total extractable and alkaline hydrolyzable dihydroxycinnamic derivatives, total carotenoids) as well as a detailed phytochemical profile (extractable and alkaline hydrolyzable phenolics and fatty acids) of bee-collected artichoke pollen.
- (b)
- to determine its antioxidant properties based on five different assays- total antioxidant capacity (TAC), ferric reducing power (FRP), ABTS radical cation and DPPH radical scavenging activities as well as Fe2+ chelating capacity (FCC).
- (c)
- to determine two nutritional parameters (UFA/SFA ratio, ω-6/ω-3 ratio) based on the presence of saturated/unsaturated fatty acids.
2. Materials and Methods
2.1. Collection of Bee-Collected Artichoke Pollen
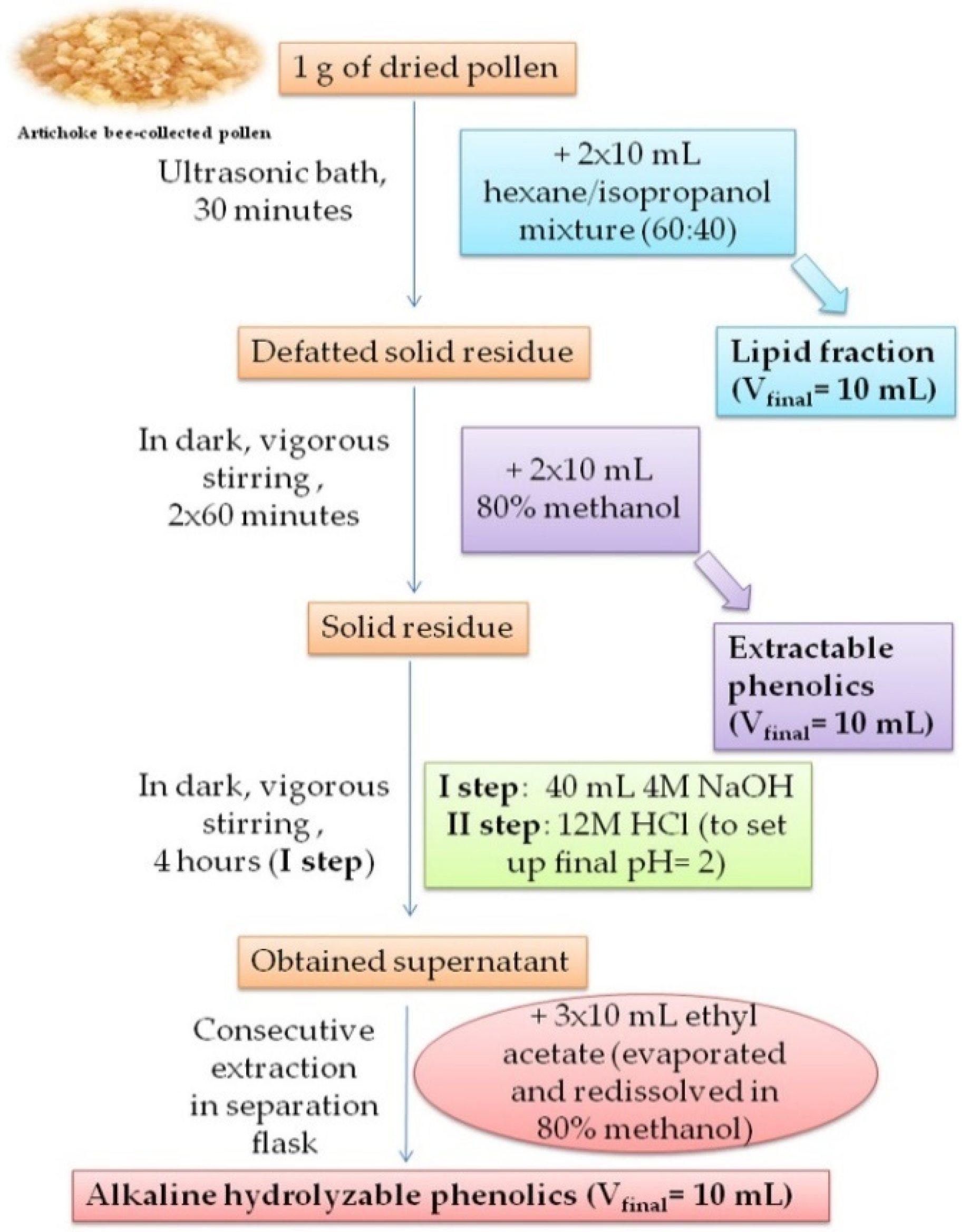
2.2. Preparation of Pollen Extracts
2.3. Determination of Total Phenolic, Flavonoid and Dihydroxycinnamic Derivative Content
2.4. Determination of Total Carotenoid Content
2.5. UHPLC-DAD MS/MS Analysis of Pollen Fractions
2.6. GC-FID Analysis of Fatty Acid Content in Pollen with Nutritional Assessment
2.7. Antioxidant Properties of Bee-Collected Artichoke Pollen
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic, Flavonoid, Dihydroxycinnamic Derivative and Carotenoid Content
3.2. The Phenolic Profile of Bee-Collected Artichoke Pollen
3.3. The Fatty Acid Profile of Bee-Collected Artichoke Pollen with Nutritional Assessment
3.4. Antioxidant Properties of Bee-Collected Artichoke Pollen
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Extraction and determination of bioactive compounds from bee pollen. J. Pharm. Biomed. Anal. 2018, 147, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Ketkar, S.S.; Rathore, A.S.; Lohidasan, S.; Rao, L.; Paradkar, A.R.; Mahadik, K.R. Investigation of the nutraceutical potential of monofloral Indian mustard bee pollen. J. Integr. Med. 2014, 12, 379–389. [Google Scholar] [CrossRef]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; de Carvalho, C.; das Campos, M.G.R. Phytochemical analysis and botanical origin of Apis mellifera bee pollen from the municipality of Canavieiras, Bahia State, Brazil. Brazilian J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. Phenolic profile by HPLC-MS, biological potential, and nutritional value of a promising food: Monofloral bee pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Screening of Indian bee pollen based on antioxidant properties and polyphenolic composition using UHPLC-DAD-MS/MS: A multivariate analysis and ANN based approach. Food Res. Int. 2021, 140, 110041. [Google Scholar] [CrossRef] [PubMed]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.-A.; Argena, N. Mineral content in monofloral bee pollen: Investigation of the effect of the botanical and geographical origin. J. Food Meas. Charact. 2019, 13, 1674–1682. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J.M.F.G. Plant Glycosides and Glycosidases: A Treasure-Trove for Therapeutics. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.; Amino, Y.; Kohmura, M.; Ueda, Y.; Kuroda, M. Human–Environment Interactions—Taste. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 631–671. [Google Scholar]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Serra Bonvehí, J.; Escolà Jordà, R. Nutrient Composition and Microbiological Quality of Honeybee-Collected Pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Serra Bonvehí, J.; Soliva Torrentó, M.; Centelles Lorente, E. Evaluation of Polyphenolic and Flavonoid Compounds in Honeybee-Collected Pollen Produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; da Cunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.Ž.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Pešić, M.B.; Trbović, D.; Petronijević, R.; Dramićanin, A.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. The fatty acid profile of Serbian bee-collected pollen—A chemotaxonomic and nutritional approach. J. Apic. Res. 2017, 56, 533–542. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically active antimicrobial and antioxidant substances in the Helianthus annuus L. bee pollen. J. Environ. Sci. Heal. Part B 2016, 51, 176–181. [Google Scholar] [CrossRef]
- Ecem Bayram, N. Vitamin, mineral, polyphenol, amino acid profile of bee pollen from Rhododendron ponticum (source of “mad honey”): Nutritional and palynological approach. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Martínez-Meza, Y.; Reynoso-Camacho, R.; Pérez-Jiménez, J. Nonextractable Polyphenols: A Relevant Group with Health Effects. In Dietary Polyphenols; Wiley: Hoboken, NJ, USA, 2020; pp. 31–83. [Google Scholar]
- Cheng, Y.; Quan, W.; Qu, T.; He, Y.; Wang, Z.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of 60Co-irradiation and superfine grinding wall disruption pretreatment on phenolic compounds in pine (Pinus yunnanensis) pollen and its antioxidant and α-glucosidase-inhibiting activities. Food Chem. 2021, 345, 128808. [Google Scholar] [CrossRef] [PubMed]
- Dulger Altiner, D.; Sandikci Altunatmaz, S.; Sabuncu, M.; Aksu, F.; Sahan, Y. In-vitro bioaccessibility of antioxidant properties of bee pollen in Turkey. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Mahmoud, A.A.; Roby, M.H.H.; Smetanska, I.; Ramadan, M.F. Phenolic Extract from Propolis and Bee Pollen: Composition, Antioxidant and Antibacterial Activities. J. Food Biochem. 2015, 39, 538–547. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2019, 54, 335–346. [Google Scholar] [CrossRef]
- Mazzeo, G.; Scavo, A.; Lo Monaco, A.; Longo, S.; Mauromicale, G. Insect pollinators improve seed production in globe artichoke (Cynara cardunculus var. scolymus). Ann. Appl. Biol. 2020, 176, 241–248. [Google Scholar] [CrossRef]
- Spiric, A.; Trbovic, D.; Vranic, D.; Djinovic, J.; Petronijevic, R.; Matekalo-Sverak, V. Statistical evaluation of fatty acid profile and cholesterol content in fish (common carp) lipids obtained by different sample preparation procedures. Anal. Chim. Acta 2010, 672, 66–71. [Google Scholar] [CrossRef]
- Harrat, M.; Gourine, N.; Válega, M.; Silva, A.M.S.; Yousfi, M. Seasonal variability of chemical composition and antioxidant activity of lipids (fatty acids and tocopherols) from the leaves of Pistacia lentiscus L. J. Food Meas. Charact. 2020, 14, 1939–1956. [Google Scholar] [CrossRef]
- Kováčová, M.; Malinová, E. Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes. Czech J. Food Sci. 2008, 25, 325–332. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 02, 181–192. [Google Scholar] [CrossRef]
- Kozyra, M.; Korga, A.; Ostrowska, M.; Humeniuk, E.; Adamczuk, G.; Gieroba, R.; Makuch-Kocka, A.; Dudka, J. Cytotoxic activity of methanolic fractions of different Marrubium spp. against melanoma cells is independent of antioxidant activity and total phenolic content. FEBS Open Bio 2020, 10, 86–95. [Google Scholar] [CrossRef]
- Fikselová, M.; Šilhár, S.; Mareček, J.; Frančáková, H. Extraction of carrot (Daucus carota L.) carotenes under different conditions. Czech J. Food Sci. 2008, 26, 268–274. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Vegetables; Springer US: Boston, MA, USA, 1991; ISBN 978-1-4613-5842-8. [Google Scholar]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Milinčić, D.D.; Stanisavljević, N.S.; Gašić, U.M.; Lević, S.; Kojić, M.O.; Tešić, L.Ž.; Nedović, V.; Barać, M.B.; Pešić, M.B. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Mačukanović-Jocić, M.P.; Špirović Trifunović, B.D.; Vukašinović, I.Ž.; Pavlović, V.B.; Pešić, M.B. Fatty acids of maize pollen—Quantification, nutritional and morphological evaluation. J. Cereal Sci. 2017, 77, 180–185. [Google Scholar] [CrossRef]
- Gawron-Gzella, A.; Krolikowska, A.; Pietrzak, M. Antioxidant activity of teas obtained from leaves of Camellia sinensis (L.) Kuntze in course of various production processes available on Polish market. Herba Pol. 2018, 64, 60–67. [Google Scholar] [CrossRef][Green Version]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Tomás-Lorente, F.; Ferreres, F.; Garcia-Viguera, C. Flavonoids as biochemical markers of the plant origin of bee pollen. J. Sci. Food Agric. 1989, 47, 337–340. [Google Scholar] [CrossRef]
- Mihajlovic, L.; Radosavljevic, J.; Burazer, L.; Smiljanic, K.; Cirkovic Velickovic, T. Composition of polyphenol and polyamide compounds in common ragweed (Ambrosia artemisiifolia L.) pollen and sub-pollen particles. Phytochemistry 2015, 109, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Viskupičová, J.; Ondrejovič, M.; Šturdík, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Państwowego Zakładu Hig. 2013, 64, 79–84. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Ischebeck, T. Lipids in pollen—They are different. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1315–1328. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Assessment of physico-chemical properties, fatty acid, amino acid and mineral profile of bee pollen from India with a multivariate perspective. J. Food Nutr. Res. 2018, 57, 328–340. [Google Scholar]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Saa-Otero, M.P.; Díaz-Losada, E.; Fernández-Gómez, E. Analysis of fatty acids, proteins and ethereal extract in honeybee pollen—Considerations of their floral origin. Grana 2000, 39, 175–181. [Google Scholar] [CrossRef]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Rebiai, A.; Lanez, T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from Northwest Algeria. J. Fundam. Appl. Sci. 2012, 4, 26–35. [Google Scholar] [CrossRef]
- Asmae, E.G.; Nawal, E.M.; Bakour, M.; Lyoussi, B. Moroccan Monofloral Bee Pollen: Botanical Origin, Physicochemical Characterization, and Antioxidant Activities. J. Food Qual. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Widyawati, P.S.; Budianta, T.D.W.; Kusuma, F.A.; Wijaya, E.L. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia Less leaves extracts. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 850–855. [Google Scholar]
- Singh, D.P.; Verma, S.; Prabha, R. Investigations on Antioxidant Potential of Phenolic Acids and Flavonoids: The Common Phytochemical Ingredients in Plants. J. Plant Biochem. Physiol. 2018, 6. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Ayebakuro, A.D.; Awakan, O.J.; Atolani, O.; Adejumo, O.; Ibrahim, A.; Rotimi, D.; El-Saber Batiha, G.; Ojediran, J.O. Comparative evaluation of the antioxidant capacity of ferulic acid and synthesized propionyl ferulate. J. Appl. Pharm. Sci. 2020, 10, 97–103. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Pokorná, J.; Venskutonis, P.R.; Kraujalyte, V.; Kraujalis, P.; Dvořák, P.; Tremlová, B.; Kopřiva, V.; Ošťádalová, M. Comparison of different methods of antioxidant activity evaluation of green and roast C. Arabica and C. Robusta coffee beans. Acta Aliment. 2015, 44, 454–460. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
| Compound | Extractable Fraction | Alkaline Hydrolyzable Fraction |
|---|---|---|
| TPC * (mg/kg GAE 1 DW) | 5314.2 ± 60.9 a,A | 497.9 ± 3.8 b,A |
| TFC * (mg/kg QE 1 DW) | 812.0 ± 82.9 a,B | 957.0 ± 145.0 a,B |
| TCD * (mg/kg CGAE 1 DW) | 1064.7 ± 80.3 C | n.d. 1 |
| TC (mg/kg DW) | 5.00 ± 0.20 | |
| Compound | Extractable Fraction | Alkaline Hydrolyzable Fraction |
|---|---|---|
| Phenolic acids | ||
| Caffeic acid | 0.416 ± 0.019 | n.d. 1 |
| Ferulic acid | n.d. | 30.393 ± 1.586 |
| Σ | 0.416 (0.66) * | 30.393 (83.28) |
| Flavonols | ||
| Quercetin 3-O-glucoside (Isoquercetin) | 1.836 ± 0.064 | n.d. |
| Rutin | 3.662 ± 0.158 a | 0.655 ± 0.019 b |
| Isorhamnetin 3-O-glucoside | 49.171 ± 1.679 a | 4.613 ± 0.128 b |
| Kaempferol | 1.527 ± 0.058 | n.d. |
| Astragalin (Kaempferol-3-O-glucoside) | 2.508 ± 0.032 a | 0.189 ± 0.005 b |
| Σ | 58.704 (92.41) | 5.457 (14.95) |
| Other detected phenolics | ||
| Apigenin | 2.633 ± 0.060 a | 0.373 ± 0.014 b |
| Apigetrin (Apigenin-7-O-glucoside) | 1.332 ± 0.084 a | 0.274 ± 0.019 b |
| Aesculetin | 0.438 ± 0.025 | n.d. |
| Σ | 4.403 (6.93) | 0.647 (1.77) |
| Total sum | 63.523 | 36.497 |
| Compound/Parameter | The Content |
|---|---|
| C12:0 | 10.50 ± 0.60 a |
| C14:0 | 1.20 ± 0.08 b |
| C14:1 | 8.95 ± 0.53 c |
| C15:0 | 11.46 ± 0.45 a |
| C16:0 | 0.50 ± 0.03 d |
| C16:1 | 2.57 ± 0.18 e |
| C17:1 | 1.30 ± 0.09 b |
| C18:1n9c | 24.90 ± 1.45 f |
| C18:2n6c | 6.60 ± 0.23 g |
| C20:2 | 3.60 ± 0.02 h |
| C20:5n3 | 28.40 ± 1.1 i |
| nutritional parameters | |
| UFA 1 | 76.32 |
| MUFA | 37.72 |
| PUFA | 38.60 |
| SFA | 23.66 |
| ω-6 | 10.20 |
| ω-3 | 28.40 |
| UFA/SFA ratio | 3.23 |
| ω-6/ω-3 ratio | 0.36 |
| Assay | Extractable Fraction | Alkaline Hydrolyzable Fraction | Lipid Fraction |
|---|---|---|---|
| * TAC (mg/kg AAE 1 DW) | 21,912.4 ± 1118.1 a | 813.0 ± 20.8 c | 7830.9 ± 425.6 b |
| * FRP (mg/kg AAE 1 DW) | 468.6 ± 12.3 a | 115.1 ± 1.6 b | n.d. |
| * ABTS∙+ (% of inhibition) | 81.41 ± 0.88 a | 14.74 ± 0.11 b | 4.80 ± 2.18 c |
| * DPPH∙ (% of inhibition) | 38.30 ± 0.77 b | 82.23 ± 0.33 a | 22.32 ± 0.39 c |
| * FCC (% of chelating ability) | 13.31 ± 0.55 a | 14.25 ± 0.72 a | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, A.Ž.; Milinčić, D.D.; Nedić, N.; Gašić, U.M.; Špirović Trifunović, B.; Vojt, D.; Tešić, Ž.L.; Pešić, M.B. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants 2021, 10, 1091. https://doi.org/10.3390/antiox10071091
Kostić AŽ, Milinčić DD, Nedić N, Gašić UM, Špirović Trifunović B, Vojt D, Tešić ŽL, Pešić MB. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants. 2021; 10(7):1091. https://doi.org/10.3390/antiox10071091
Chicago/Turabian StyleKostić, Aleksandar Ž., Danijel D. Milinčić, Nebojša Nedić, Uroš M. Gašić, Bojana Špirović Trifunović, Denis Vojt, Živoslav Lj. Tešić, and Mirjana B. Pešić. 2021. "Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen" Antioxidants 10, no. 7: 1091. https://doi.org/10.3390/antiox10071091
APA StyleKostić, A. Ž., Milinčić, D. D., Nedić, N., Gašić, U. M., Špirović Trifunović, B., Vojt, D., Tešić, Ž. L., & Pešić, M. B. (2021). Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants, 10(7), 1091. https://doi.org/10.3390/antiox10071091










