Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemicals
2.3. Preparation of Microcapsules by Spray Drying and Study of Storage Stability
2.4. Characterization of Phenolic Compounds and Antioxidant Properties from Encapsulated Calafate
2.5. Neuroprotective Properties of Calafate Microcapsules
2.5.1. PC-12 Cells
2.5.2. Soluble Oligomers of Aβ (SO-Aβ) Preparation
2.5.3. Cell Viability Assay
2.6. Scanning Electron Microscopy of Microcapsules
2.7. Statistical Analysis
3. Results
3.1. Polyphenols Recovery from Calafate Formulations
3.2. Antioxidant Activity and Calafate Anthocyanin Stability at Different Temperatures of Storage
3.3. In-Vitro Study of Neuroprotective Properties of Calafate Microcapsules in Neurodegenerative Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López, M.D.; Baenas, N.; Retamal-Salgado, J.; Zapata, N.; Moreno, D.A. Underutilized Native Biobío Berries: Opportunities for Foods and Trade. Nat. Prod. Commun. 2018, 13, 1934578x1801301226. [Google Scholar] [CrossRef] [Green Version]
- Romero-Román, M.E.; Schoebitz, M.; Bastías, R.M.; Fernández, P.S.; García-Viguera, C.; López-Belchi, M.D. Native Species Facing Climate Changes: Response of Calafate Berries to Low Temperature and UV Radiation. Foods 2021, 10, 196. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis Microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Morais-Braga, F.B. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Jimenez, P.; Garcia-Diaz, D.F. Chilean Native Fruit Extracts Inhibit Inflammation Linked to the Pathogenic Interaction Between Adipocytes and Macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; De Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and Antioxidant Activity of Proteins from Feijoa Sellowiana Berg. Fruit before and after in Vitro Gastrointestinal Digestion. Nat. Prod. Res. 2020, 34, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [Green Version]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and Nano Encapsulation, Retention and Controlled Release of Flavor and Aroma Compounds: A Critical Review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Righi da Rosa, J.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of Anthocyanin Compounds Extracted from Blueberry (Vaccinium Spp.) by Spray Drying: Characterization, Stability and Simulated Gastrointestinal Conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation Optimization of Natural Anthocyanins with Maltodextrin, Gum Arabic and Gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The Microencapsulation of Maqui (Aristotelia Chilensis (Mol.) Stuntz) Juice by Spray-Drying and Freeze-Drying Produces Powders with Similar Anthocyanin Stability and Bioaccessibility. Molecules 2018, 23, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezem, M.T.; Johannessen, F.G.; Jung-KC, K.; Gundersen, E.T.; Jorge-Finnigan, A.; Ying, M.; Betbeder, D.; Herfindal, L.; Martinez, A. Stabilization of Human Tyrosine Hydroxylase in Maltodextrin Nanoparticles for Delivery to Neuronal Cells and Tissue. Bioconjug. Chem. 2018, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Thibado, S.P.; Thornthwaite, J.T.; Ballard, T.K.; Goodman, B.T. Anticancer Effects of Bilberry Anthocyanins Compared with NutraNanoSphere Encapsulated Bilberry Anthocyanins. Mol. Clin. Oncol. 2018, 8, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. BioMed Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Lu, S.; Yu, X.-L.; Yang, S.-G.; Liu, W.; Liu, X.-M.; Wang, S.-W.; Zhu, J.; Ji, M.; Liu, D.-Q.; et al. Fruitless Wolfberry-Sprout Extract Rescued Cognitive Deficits and Attenuated Neuropathology in Alzheimer’s Disease Transgenic Mice. Curr. Alzheimer Res. 2018, 15, 856–868. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS Profiling of Phenolic and Carotenoid Compounds and Their Influence on Anticholinergic Potential for AChE and BuChE Inhibition and on-Line Antioxidant Activity of Selected Hippophaë Rhamnoides L. Cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef]
- Agulló, V.; Villaño, D.; García-Viguera, C.; Domínguez-Perles, R. Anthocyanin Metabolites in Human Urine after the Intake of New Functional Beverages. Molecules 2020, 25, 371. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical Characterisation for Industrial Use of Pomegranate (Punica Granatum L.) Cultivars Grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Migues, I.; Baenas, N.; Gironés-Vilaplana, A.; Cesio, M.V.; Heinzen, H.; Moreno, D.A. Phenolic Profiling and Antioxidant Capacity of Eugenia Uniflora L. (Pitanga) Samples Collected in Different Uruguayan Locations. Foods 2018, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sáez-Orellana, F.; Godoy, P.A.; Bastidas, C.Y.; Silva-Grecchi, T.; Guzmán, L.; Aguayo, L.G.; Fuentealba, J. ATP Leakage Induces P2XR Activation and Contributes to Acute Synaptic Excitotoxicity Induced by Soluble Oligomers of β-Amyloid Peptide in Hippocampal Neurons. Neuropharmacology 2016, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Orellana, F.; Fuentes-Fuentes, M.C.; Godoy, P.A.; Silva-Grecchi, T.; Panes, J.D.; Guzmán, L.; Yévenes, G.E.; Gavilán, J.; Egan, T.M.; Aguayo, L.G.; et al. P2X Receptor Overexpression Induced by Soluble Oligomers of Amyloid Beta Peptide Potentiates Synaptic Failure and Neuronal Dyshomeostasis in Cellular Models of Alzheimer’s Disease. Neuropharmacology 2018, 128, 366–378. [Google Scholar] [CrossRef] [PubMed]
- López-Belchí, M.D.; Caamaño, E.F.; Pascual, G.; Noriega, F.; Fierro-Morales, P.; Romero-Román, M.E.; Jara, P.; Schoebitz, M.; Serra, I.; Moreno, D.A. Spray-Dried Formulations Rich in Malvidin from Tintorera Grape Wastes: Characterization, Stability, and Storage. Processes 2021, 9, 518. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015; Volume 42, p. 14. Available online: http://www.rstudio.com (accessed on 10 September 2021).
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of Anthocyanin Extracted from Purple Flesh Cultivated Potatoes by Spray Drying and Its Effects on In Vitro Gastrointestinal Digestion. Molecules 2020, 25, 722. [Google Scholar] [CrossRef] [Green Version]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of Anthocyanins in Novel Chilean Maqui Berry Clones by HPLC–DAD–ESI/MSn and NMR-Spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin Profiles in South Patagonian Wild Berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Thomas-Valdés, S.; Schulz, A.; Ladio, A.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant Activity and Phenolic Profiles of the Wild Currant Ribes Magellanicum from Chilean and Argentinean Patagonia. Food Sci. Nutr. 2016, 4, 595–610. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A Review with Examples from the Development of Monoamine Oxidase and Cholinesterase Inhibitory Drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [Green Version]
- Noriega, F.; Mardones, C.; Fischer, S.; García-Viguera, C.; Moreno, D.A.; Dolores López, M. Seasonal Changes in White Strawberry: Effect on Aroma, Phenolic Compounds and Its Biological Activity. J. Berry Res. 2021, 11, 103–118. [Google Scholar] [CrossRef]
- Fuentealba, J.; Dibarrart, A.; Saez-Orellana, F.; Fuentes-Fuentes, M.C.; Oyanedel, C.N.; Guzmán, J.; Perez, C.; Becerra, J.; Aguayo, L.G. Synaptic Silencing and Plasma Membrane Dyshomeostasis Induced by Amyloid-β Peptide Are Prevented by Aristotelia Chilensis Enriched Extract. J. Alzheimer Dis. 2012, 31, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa Sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Lingua, M.S.; Salomón, V.; Baroni, M.V.; Blajman, J.E.; Maldonado, L.M.; Páez, R. Effect of Spray Drying on the Microencapsulation of Blueberry Natural Antioxidants. Proceedings 2020, 70, 26. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Yang, X.; Luo, E.; Liu, X.; Han, B.; Yu, X.; Peng, X. Delphinidin-3-Glucoside Suppresses Breast Carcinogenesis by Inactivating the Akt/HOTAIR Signaling Pathway. BMC Cancer 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, J.; Park, K.-C.; Awasthi, A.; Prasad, B. Silymarin Extends Lifespan and Reduces Proteotoxicity in C. elegans Alzheimer’s Model. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2015, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Parra, D.; Chirife, J.; Zamora, C.; de Pascual-Teresa, S. Chemical Characterization of an Encapsulated Red Wine Powder and Its Effects on Neuronal Cells. Molecules 2018, 23, 842. [Google Scholar] [CrossRef] [Green Version]

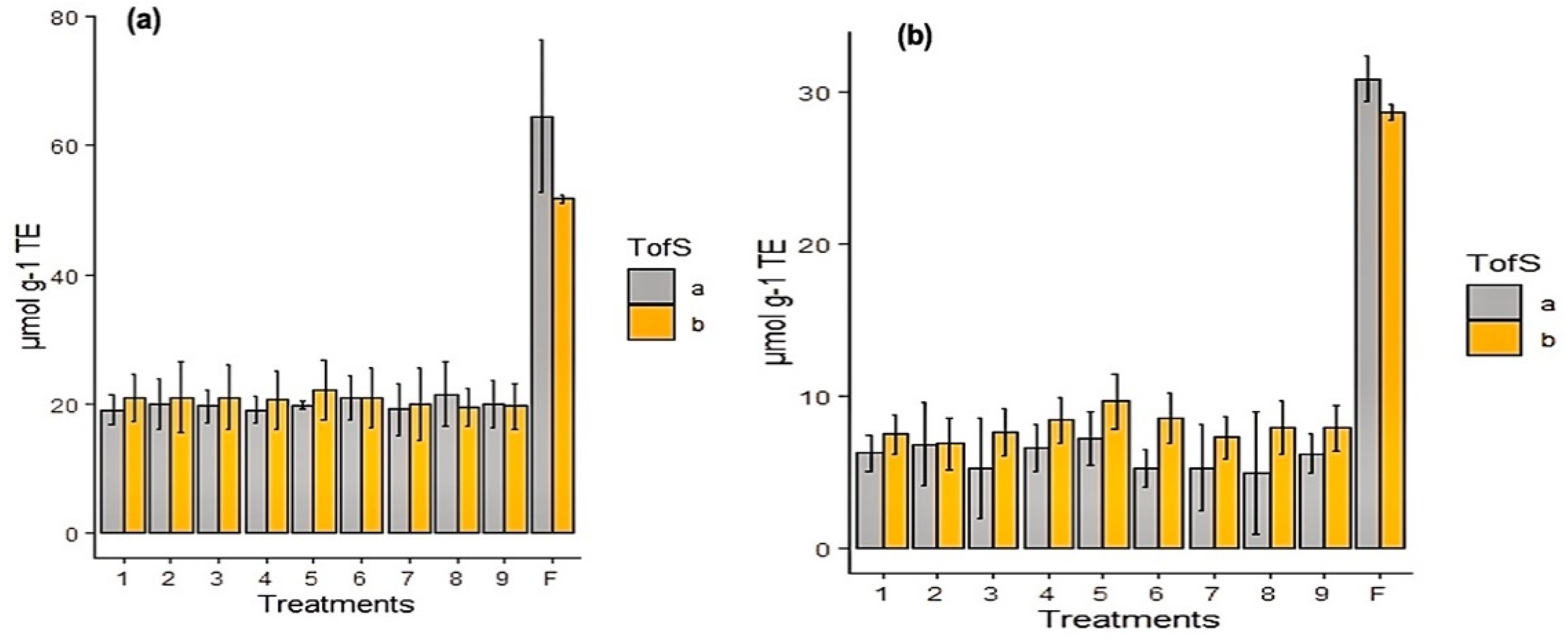
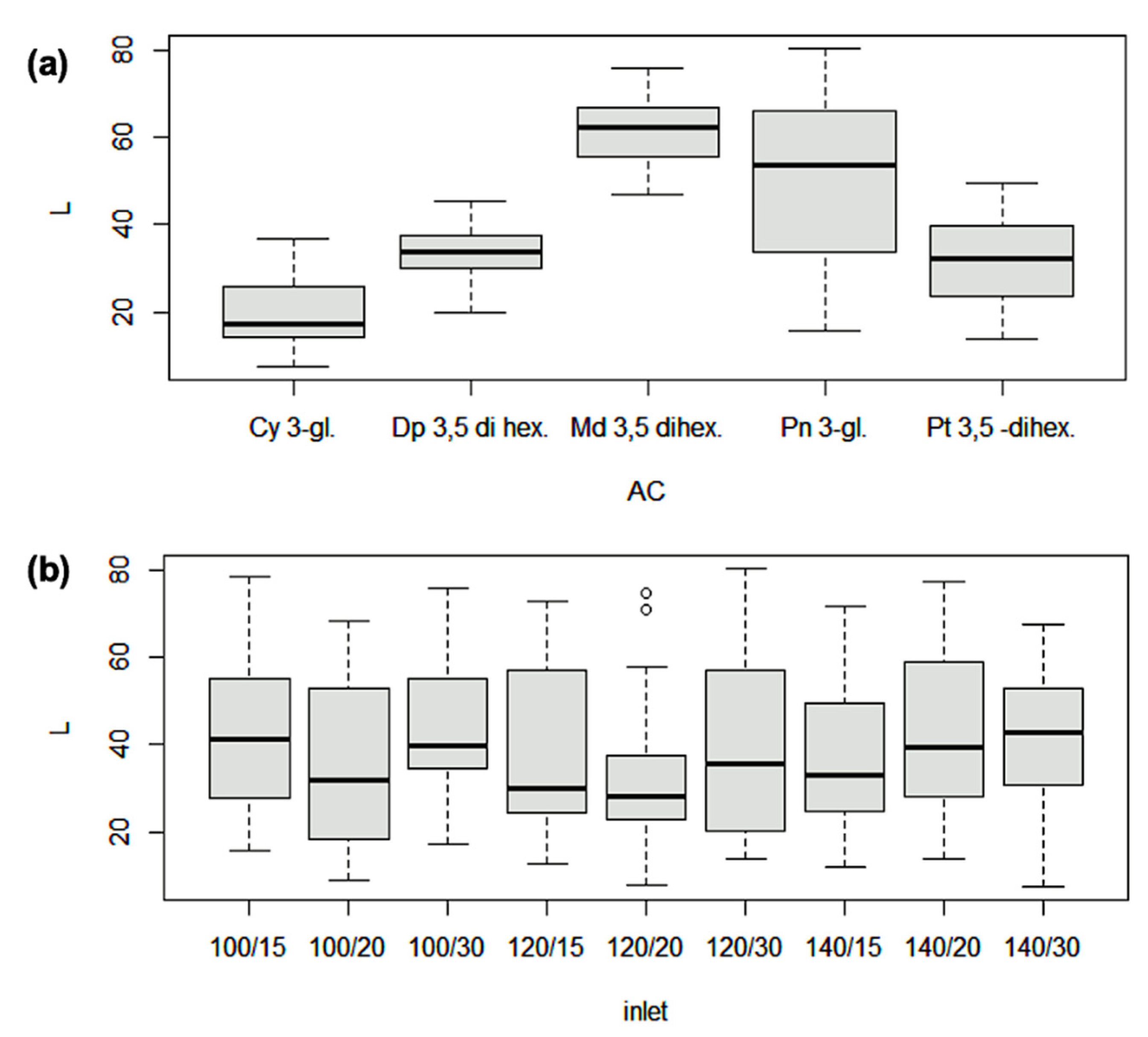
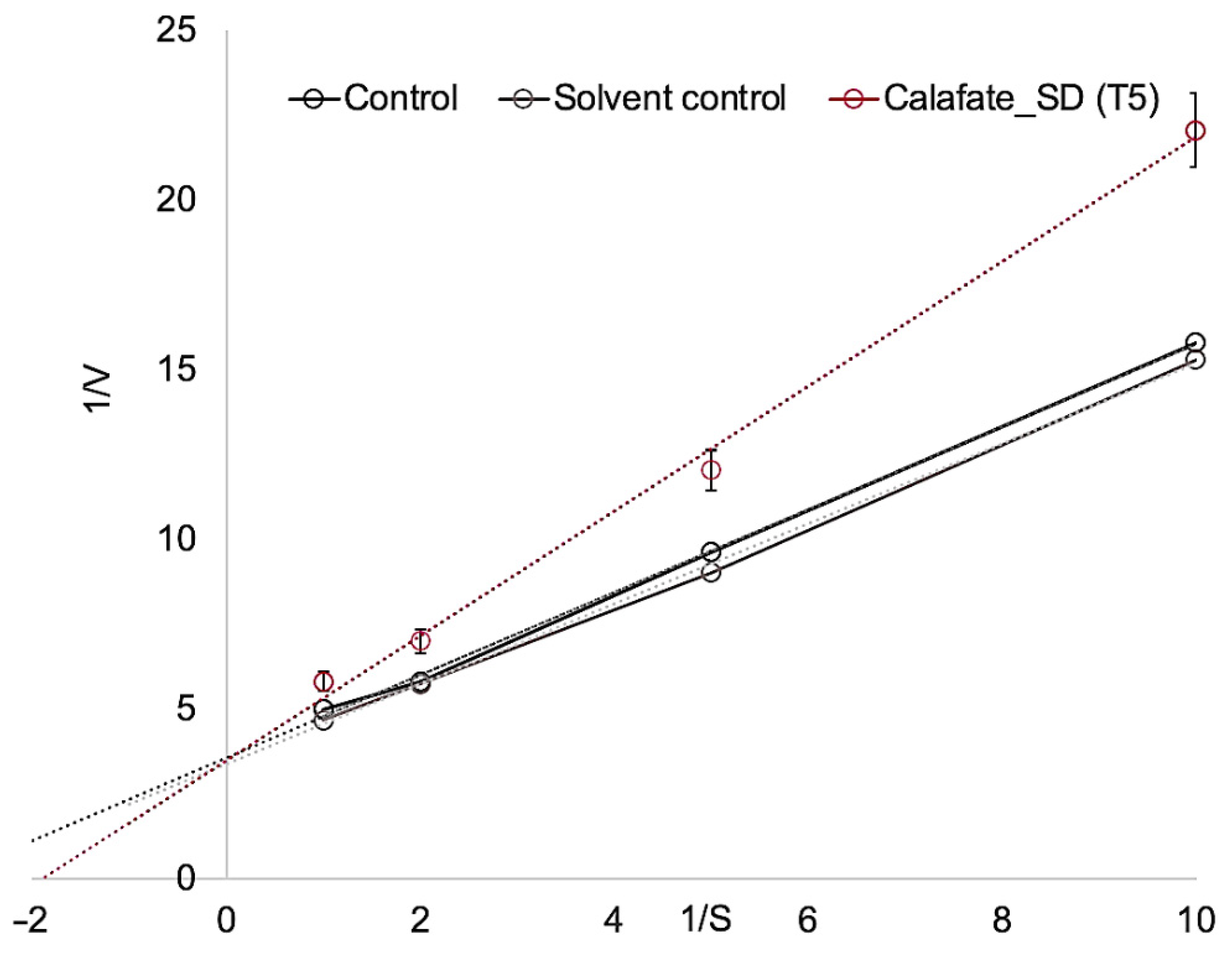
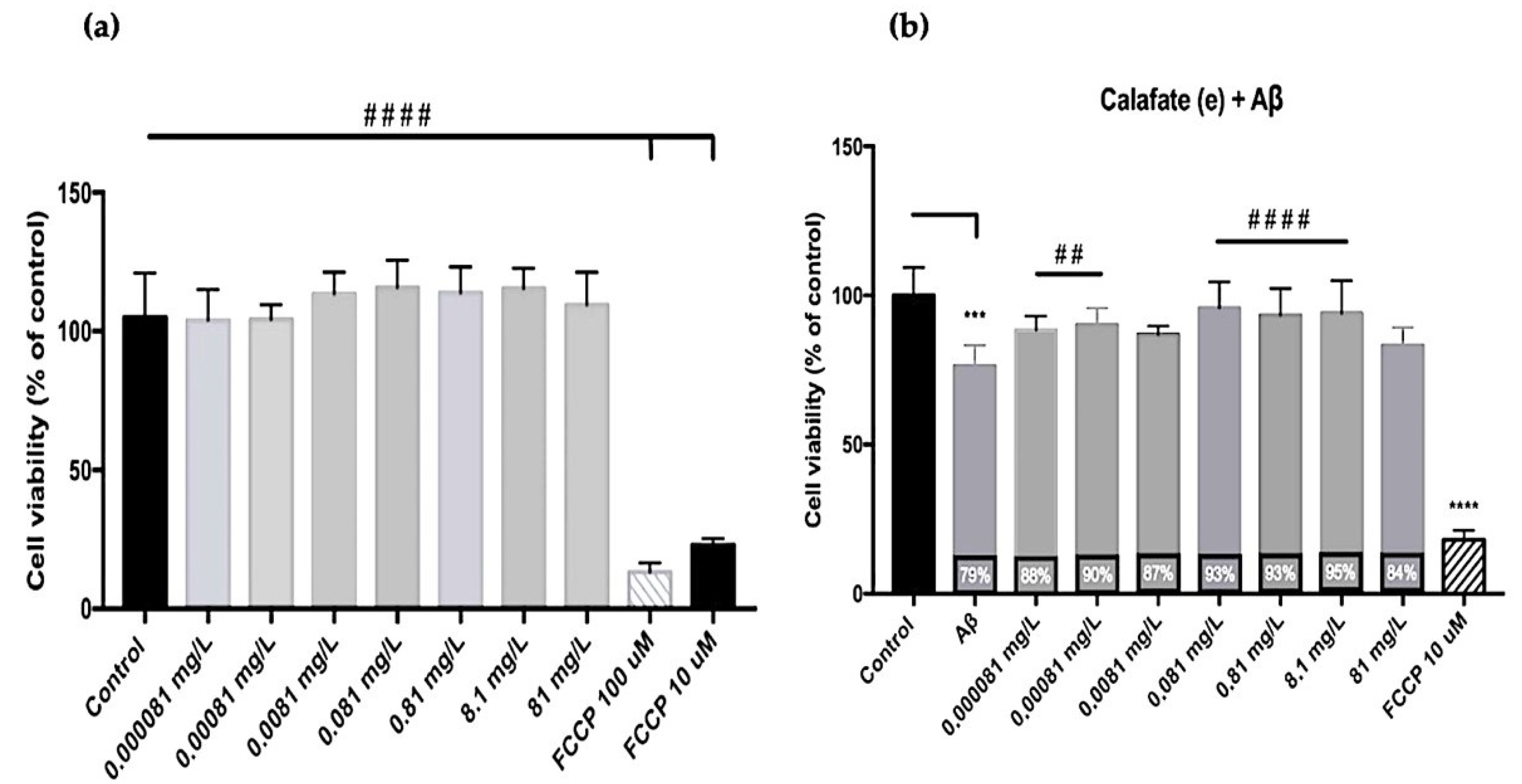
| Code | Treatment | EE (%) | Recovery (%) | Size (µm) | TACs (mg g−1) | TPCs (mg g−1) |
|---|---|---|---|---|---|---|
| (MD-Temp) | ||||||
| T1 | 15–100 | 43 ± 0.48 a | 56.9 ± 1.26 a | 7.51 ± 2.9 a | 12.6 ± 0.87 a | 16.6 ± 0.7 a |
| T2 | 15–120 | 50.4 ± 0.83 a | 59.3 ± 3.4 a | 8.05 ± 5.85 a | 12.2 ± 1.23 a | 15.4 ± 1 a |
| T3 | 15–140 | 49.2 ± 1.2 a | 57 ± 2.5 a | 7.07 ± 4.78 a | 12.2 ± 1.15 a | 15.4 ± 0.9 a |
| T4 | 20–100 | 41.1 ± 1.99 a | 58 ± 3.2 a | 6.69 ± 3.32 a | 13 ± 2.4 a | 16.5 ± 1.5 a |
| T5 | 20–120 | 46.3 ± 2.27 a | 58.3 ± 1.8 a | 7.64 ± 4.63 a | 14.8 ± 1.02 a | 19 ± 0.8 a |
| T6 | 20–140 | 42.4 ± 2.16 a | 60.1 ± 4.2 a | 6.63 ± 3.66 a | 12.5 ± 0.67 a | 15.9 ± 0.6 a |
| T7 | 30–100 | 46.3 ± 2.43 a | 59.5 ± 1.87 a | 6.89 ± 3.29 a | 13.2 ± 0.34 a | 16.5 ± 0.3 a |
| T8 | 30–120 | 56.7 ± 0.5 a | 58 ± 2.21 a | 6.5 ± 3.19 a | 13.1 ± 1.02 a | 16.5 ± 0.8 a |
| T9 | 30–140 | 47.2 ± 0.56 a | 61.4 ± 3.87 a | 6.61 ± 3.7 a | 11.9 ± 2.1 a | 14.9 ± 1.2 a |
| F | Freeze-Drying | 82.5 ± 5.6 b | 93.7 ± 2.5 b | - | 20 ± 3.8 b | 24.8 ± 2 b |
| Retention Time | λ (nm) | M+ or M− | Ion | MSn | Main Phenolic Compounds | Concentration |
|---|---|---|---|---|---|---|
| 11.58 | 278, 524 | 627 | + | 303 | delphinidin 3,5-dihexoside | 9.06 |
| 16.8 | 280, 524 | 448 | + | 287 | cyanidin 3-glucoside | 0.97 |
| 20.6 | 278, 524 | 640 | + | 317 | petunidin 3,5-dihexoside | 0.82 |
| 27.8 | 276, 524 | 462 | + | 301 | peonidin 3-glucoside | 0.14 |
| 30.3 | 280, 524 | 654 | + | 331 | malvidin 3,5-dihexoside | 3.26 |
| 31.7 | 278, 524 | 464 | + | 303 | delphinidin 3-glucoside | 3.26 |
| 34.5 | 280, 525 | 611 | + | 303 | delphinidin 3-rutinoside | 2.87 |
| 39.3 | 274, 530 | 492 | + | 331 | malvidin 3-glucoside | 1.46 |
| 30.1 | 298, 355 | 481/479 | − | 319/317 | myricetin 3-glucoside | 0.16 |
| 34 | 296, 356 | 627/625 | − | 481/319 | myricetin 3-rutinoside | 0.23 |
| 36.8 | 298, 350 | 609/610 | − | 301 | quercetin 3-rutinoside | 1.17 |
| 39.8 | 296, 352 | 463/464 | − | 301 | quercetin 3-glucoside | 1.47 |
| 40.5 | 300, 324 | 515 | − | 353.19 | quercetin 3-galactoside | 0.34 |
| 46.3 | 284, 352 | 447 | − | 301 | quercetin 3-o-rhamnoside | 1.02 |
| 46.9 | 264, 350 | 477 | − | 315 | isorhamnetin 3-o-hexoside | 0.74 |
| 48.1 | 266, 354 | 623 | − | 315 | isorhamnetin3-o-hexoside-derivative | 0.78 |
| Total | 27.74 |
| Anthocyanin | Time of Storage | (mg g−1) |
|---|---|---|
| peonidin 3-glucoside | 0 | 0.269 a |
| petunidin 3,5-dihexoside | 0 | 0.877 abc |
| malvidin 3,5-dihexoside | 0 | 0.987 abc |
| cyanidin 3-glucoside | 0 | 1.322 abc |
| delphinidin 3,5-dihexoside | 0 | 9.467 e |
| peonidin 3-glucoside | 24 | 0.316 a |
| petunidin 3,5-dihexoside | 24 | 0.895 abc |
| cyanidin 3-glucoside | 24 | 1.279 abc |
| malvidin 3,5-dihexoside | 24 | 1.550 bc |
| delphinidin 3,5-dihexoside | 24 | 9.600 e |
| peonidin 3-glucoside | 48 | 0.332 ab |
| petunidin 3,5-dihexoside | 48 | 0.791 abc |
| malvidin 3,5-dihexoside | 48 | 0.802 abc |
| cyanidin 3-glucoside | 48 | 1.800 c |
| delphinidin 3,5-dihexoside | 48 | 8.366 de |
| peonidin 3-glucoside | 168 | 0.302 ab |
| cyanidin 3-glucoside | 168 | 1.016 abc |
| malvidin 3,5-dihexoside | 168 | 1.111 abc |
| petunidin 3,5-dihexoside | 168 | 1.123 abc |
| delphinidin 3,5-dihexoside | 168 | 7.911 d |
| peonidin 3-glucoside | 336 | 0.151 a |
| malvidin 3,5-dihexoside | 336 | 0.569 abc |
| cyanidin 3-glucoside | 336 | 0.731 abc |
| petunidin 3,5-dihexoside | 336 | 0.941 abc |
| delphinidin 3,5-dihexoside | 336 | 7.439 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Román, M.E.; Schoebitz, M.; Fuentealba, J.; García-Viguera, C.; Belchí, M.D.L. Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient. Antioxidants 2021, 10, 1830. https://doi.org/10.3390/antiox10111830
Romero-Román ME, Schoebitz M, Fuentealba J, García-Viguera C, Belchí MDL. Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient. Antioxidants. 2021; 10(11):1830. https://doi.org/10.3390/antiox10111830
Chicago/Turabian StyleRomero-Román, María E., Mauricio Schoebitz, Jorge Fuentealba, Cristina García-Viguera, and María D. López Belchí. 2021. "Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient" Antioxidants 10, no. 11: 1830. https://doi.org/10.3390/antiox10111830
APA StyleRomero-Román, M. E., Schoebitz, M., Fuentealba, J., García-Viguera, C., & Belchí, M. D. L. (2021). Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient. Antioxidants, 10(11), 1830. https://doi.org/10.3390/antiox10111830









