Antioxidant Properties of Alpha-Lipoic (Thioctic) Acid Treatment on Renal and Heart Parenchyma in a Rat Model of Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Treatment
2.2. Histological Analysis, Immunohistochemistry and Immunofluorescence
2.3. Protein Extraction and Western Blot Analysis
2.4. Statistical Analysis
3. Results
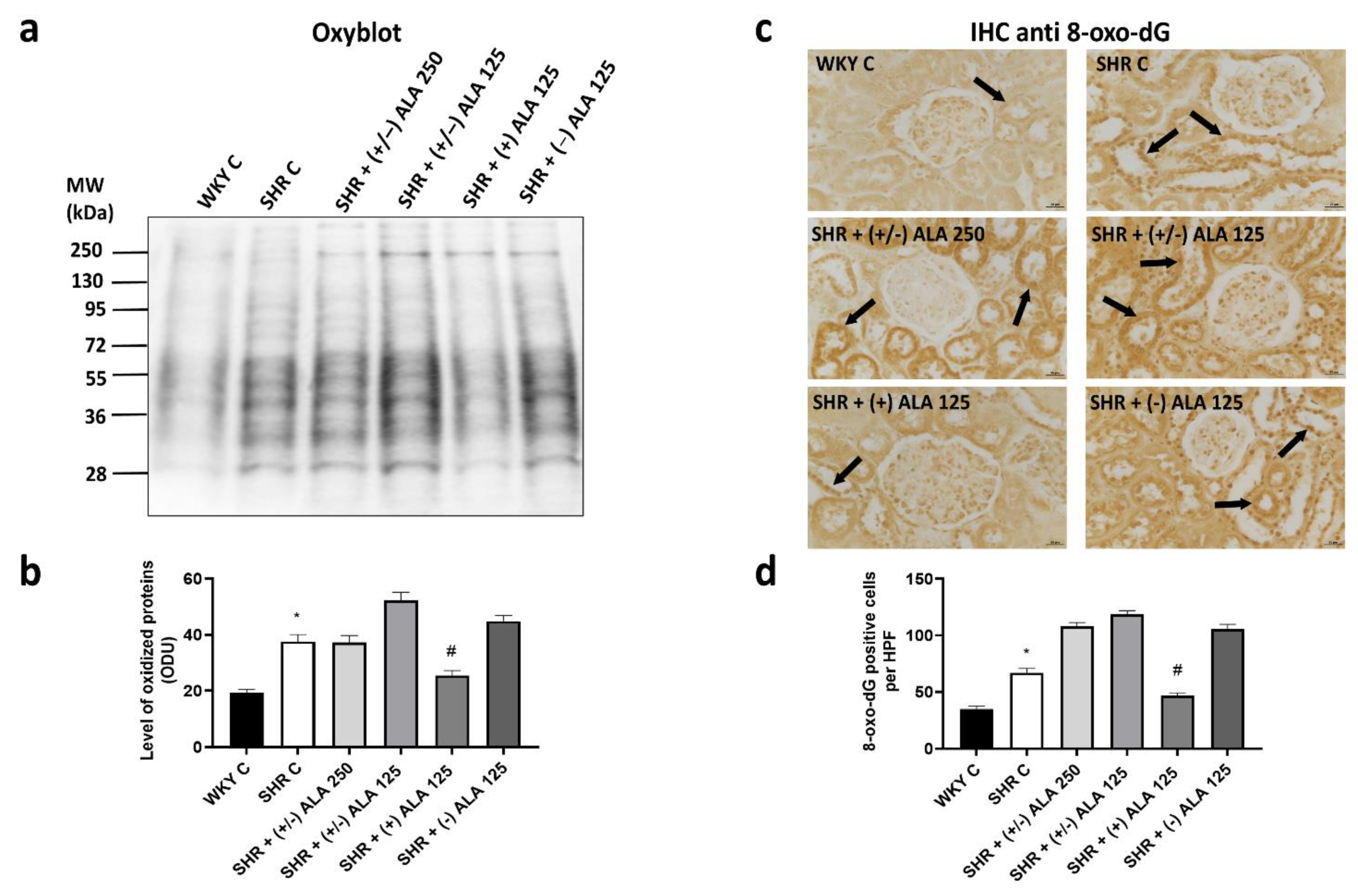
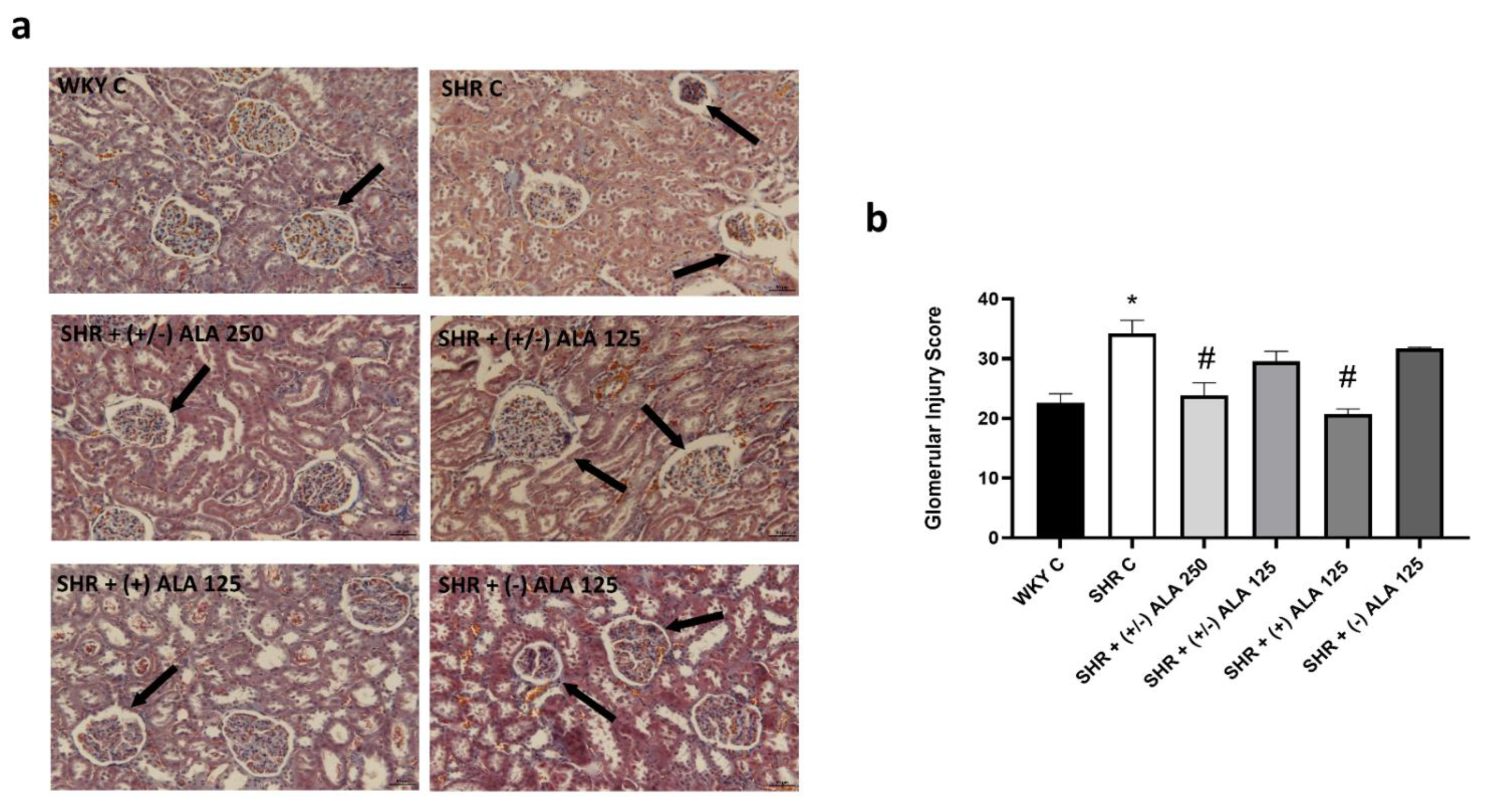
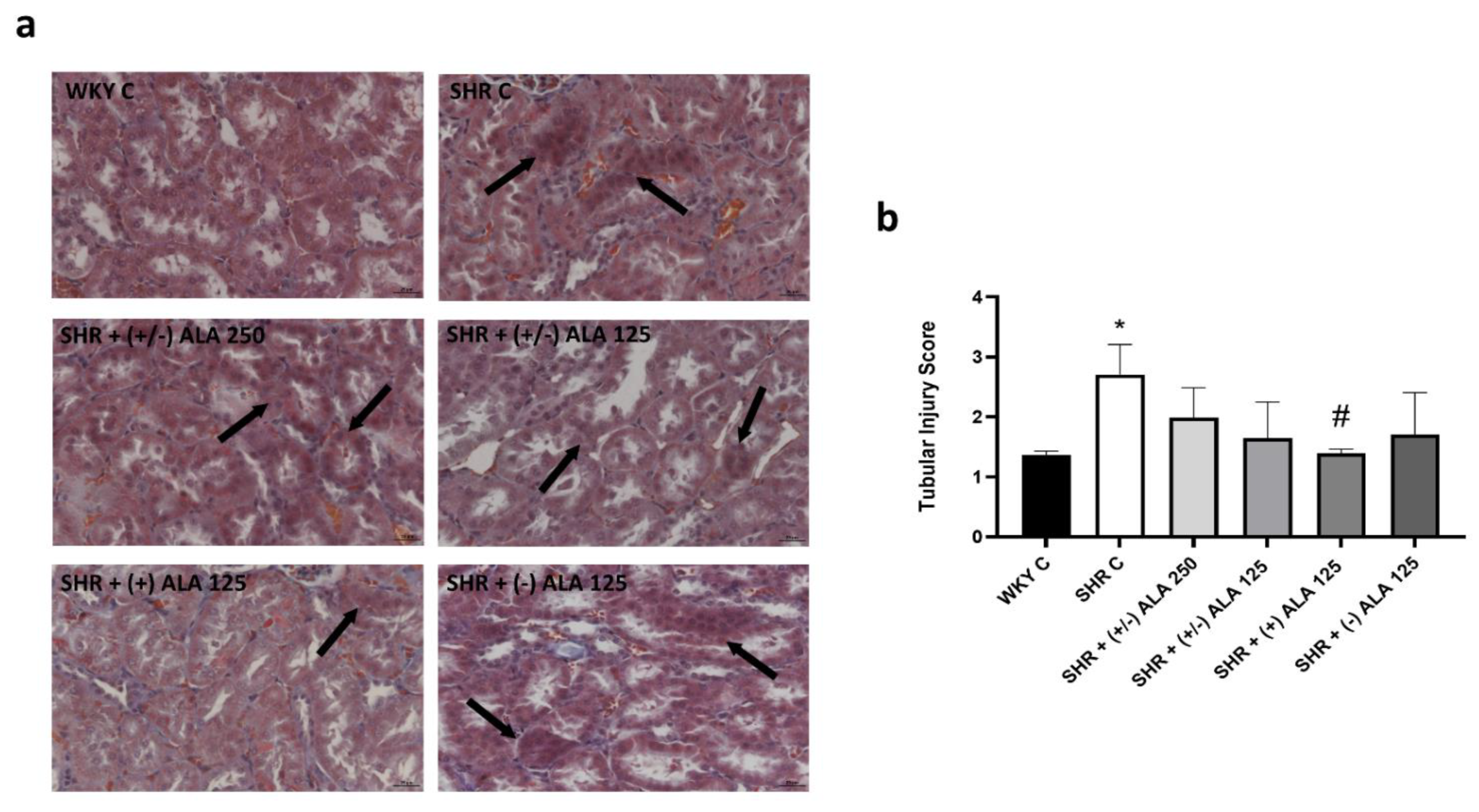
3.1. Kidney
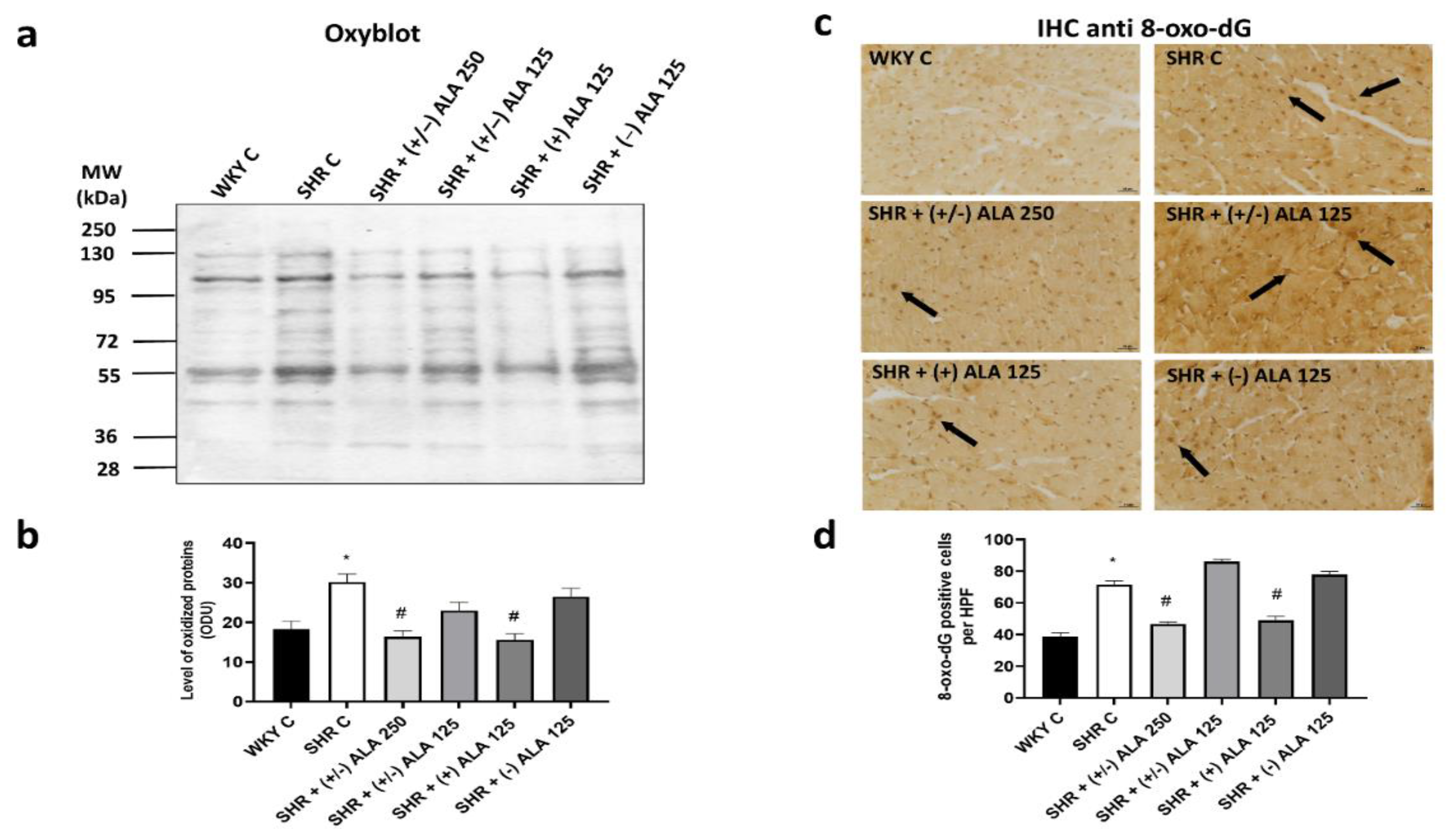
3.2. Heart
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sievers, L.K.; Eckardt, K.U. Molecular Mechanisms of Kidney Injury and Repair in Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 2138. [Google Scholar] [CrossRef]
- Sliwa, K.; Stewart, S.; Gersh, B.J. Hypertension: A global perspective. Circulation 2011, 123, 2892–2896. [Google Scholar] [CrossRef]
- Messerli, F.H.; Rimoldi, S.F.; Bangalore, S. The Transition from Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail. 2017, 5, 543–551. [Google Scholar] [CrossRef]
- Bock, J.S.; Gottlieb, S.S. Cardiorenal syndrome: New perspectives. Circulation 2010, 121, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.S. Hypertensive nephrosclerosis. Curr. Opin. Nephrol. Hypertens. 2008, 17, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Iliev, A.; Kotov, G.; Dimitrova, I.N.; Landzhov, B. Hypertension-induced changes in the rat myocardium during the development of cardiac hypertrophy—A comparison between the left and the right ventricle. Acta Histochem. 2019, 121, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Shah, A.M. Alterations in cardiac structure and function in hypertension. Curr. Hypertens. Rep. 2014, 16, 428. [Google Scholar] [CrossRef]
- Mennuni, S.; Rubattu, S.; Pierelli, G.; Tocci, G.; Fofi, C.; Volpe, M. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 2014, 28, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Lerman, A.; Lerman, L.O. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur. Heart J. 2014, 35, 3258–3266. [Google Scholar] [CrossRef]
- Rubattu, S.; Pagliaro, B.; Pierelli, G.; Santolamazza, C.; Di Castro, S.; Mennuni, S.; Volpe, M. Pathogenesis of target organ damage in hypertension: Role of mitochondrial oxidative stress. Int. J. Mol. Sci. 2014, 16, 823–839. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Valls, N.; Brito, R.; Rodrigo, R. Essential hypertension and oxidative stress: New insights. World J. Cardiol. 2014, 6, 353–366. [Google Scholar] [CrossRef]
- Brito, R.; Castillo, G.; González, J.; Valls, N.; Rodrigo, R. Oxidative stress in hypertension: Mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes 2015, 123, 325–335. [Google Scholar] [CrossRef]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: Vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Manning, R.D., Jr.; Tian, N.; Meng, S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am. J. Nephrol. 2005, 25, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Nishiyama, A. Is antioxidant therapy effective for advanced hypertension and renal injury? J. Hypertens. 2014, 32, 475–476. [Google Scholar] [CrossRef]
- Zanchetti, A. Challenges in hypertension: Prevalence, definition, mechanisms and management. J. Hypertens. 2014, 32, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Tomassoni, D.; Di Cesare Mannelli, L.; Amenta, F. Effect of treatment with the antioxidant alpha-lipoic (thioctic) acid on heart and kidney microvasculature in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2016, 38, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Tabei, R.; Yamori, Y.; Ooshima, A. Spontaneously hypertensive rat as a useful model for hypertension research. Jikken Dobutsu. 1973, 22, 289–298. [Google Scholar]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fallahzadeh, M.K.; McCullough, P.A. Aging Male Spontaneously Hypertensive Rat as an Animal Model for the Evaluation of the Interplay between Contrast-Induced Acute Kidney Injury and Cardiorenal Syndrome in Humans. Cardiorenal. Med. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Tomassoni, D.; Amenta, F.; Di Cesare Mannelli, L.; Ghelardini, C.; Nwankwo, I.E.; Pacini, A.; Tayebati, S.K. Neuroprotective activity of thioctic acid in central nervous system lesions consequent to peripheral nerve injury. Biomed. Res. Int. 2013, 2013, 985093. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Tomassoni, D.; Trallori, E.; Micheli, L.; Amenta, F.; Ghelardini, C.; Di Cesare Mannelli, L.; Traini, E. Comparative Assessment of the Activity of Racemic and Dextrorotatory Forms of Thioctic (Alpha-Lipoic) Acid in Low Back Pain: Preclinical Results and Clinical Evidences from an Open Randomized Trial. Front. Pharmacol. 2021, 12, 607572. [Google Scholar] [CrossRef]
- Salehi, B.; Yılmaz, Y.B.; Antika, G.; Tumer, T.B.; Mahomoodally, M.F.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Tomassoni, D.; Amenta, F. Spontaneously hypertensive rat as a model of vascular brain disorder: Microanatomy, neurochemistry and behavior. J. Neurol. Sci. 2012, 322, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Raij, L.; Azar, S.; Keane, W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984, 26, 137–143. [Google Scholar] [CrossRef]
- Komatsu, K.; Frohlich, E.D.; Ono, H.; Ono, Y.; Numabe, A.; Willis, G.W. Glomerular dynamics and morphology of aged spontaneously hypertensive rats. Effect of angiotesin-converting enzyme inhibition. Hypertension 1995, 25, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Leonardi, A.; Testa, R.; Vitaioli, L.; Amenta, F. Effect of calcium antagonists on glomerular arterioles in spontaneously hypertensive rats. Hypertension 2000, 35, 775–779. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Conway, E.M.; Harris, R.C. Survivin mediates renal proximal tubule recovery from AKI. J. Am. Soc. Nephrol. 2013, 24, 2023–2033. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. Int. J. Mol. Sci. 2020, 21, 2035. [Google Scholar] [CrossRef]
- Muñoz-Durango, N.; Fuentes, C.A.; Castillo, A.E.; González-Gómez, L.M.; Vecchiola, A.; Fardella, C.E.; Kalergis, A.M. Role of the Renin-Angiotensin-Aldosterone System beyond Blood Pressure Regulation: Molecular and Cellular Mechanisms Involved in End-Organ Damage during Arterial Hypertension. Int. J. Mol. Sci. 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Paul, M.; Ganten, D. Lessons from rat models of hypertension: From Goldblatt to genetic engineering. Cardiovasc. Res. 1998, 39, 77–88. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement from the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Vitaioli, L.; Baldoni, E.; Amenta, F. Nephroprotective effect of treatment with calcium channel blockers in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 2000, 294, 948–954. [Google Scholar] [PubMed]
- Sabbatini, M.; Leonardi, A.; Testa, R.; Tomassoni, D.; Vitaioli, L.; Amenta, F. Effects of dihydropyridine-type Ca2+ antagonists on the renal arterial tree in spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2002, 39, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Peleg, E.; Tomassoni, D.; Sabbatini, M.; Rosenthal, T. Effect of treatment with lercanidipine on heart of Cohen-Rosenthal diabetic hypertensive rats. Hypertension 2003, 41, 330–335. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Deng, X.U.; Xia, K.E.; Chen, P.O.; Sheikh, M.S.A.; Yang, D.F.; Li, S.M.; Yang, T.L. Reversion of left ventricle remodeling in spontaneously hypertensive rats by valsartan is associated with the inhibition of caspase-3, -8 and -9 activities. Biomed. Rep. 2015, 3, 533–536. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Cui, Y.; Lin, N.; Pang, S. Correlation of cardiomyocyte apoptosis with duration of hypertension, severity of hypertension and caspase-3 expression in hypertensive rats. Exp. Ther. Med. 2019, 17, 2741–2745. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.M.; Nadar, A. A subpressor dose of angiotensin II elevates blood pressure in a normotensive rat model by oxidative stress. Physiol. Res. 2015, 64, 153–159. [Google Scholar] [CrossRef]
- Lassègue, B.; Griendling, K.K. Reactive oxygen species in hypertension; An update. Am. J. Hypertens. 2004, 17, 852–860. [Google Scholar] [CrossRef]
- Lopez-Ruiz, A.; Sartori-Valinotti, J.; Yanes, L.L.; Iliescu, R.; Reckelhoff, J.F. Sex differences in control of blood pressure: Role of oxidative stress in hypertension in females. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H466–H474. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Lipsitz, S.R.; Natarajan, S. Association between a DASH-like diet and mortality in adults with hypertension: Findings from a population-based follow-up study. Am. J. Hypertens. 2009, 22, 9–16. [Google Scholar] [CrossRef]
- Mullan, B.A.; Young, I.S.; Fee, H.; McCance, D.R. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 2002, 40, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Bertrais, S.; Blacher, J.; Galan, P.; Briançon, S.; Favier, A.; Safar, M.; Hercberg, S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI.MAX study: Association with plasma antioxidant levels. J. Hypertens. 2005, 23, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. α-Lipoic Acid Reduces Iron-induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef]
- Zhang, W.J.; Frei, B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001, 15, 2423–2432. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Zhang, J.; McCullough, P.A. Lipoic Acid in the Prevention of Acute Kidney Injury. Nephron 2016, 134, 133–140. [Google Scholar] [CrossRef]
- Takaoka, M.; Ohkita, M.; Kobayashi, Y.; Yuba, M.; Matsumura, Y. Protective effect of alpha-lipoic acid against ischaemic acute renal failure in rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Zaher, A.O.; Abdel-Hady, R.H.; Mahmoud, M.M.; Farrag, M.M.Y. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2008, 243, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Bae, S.Y.; Won, N.H.; Pyo, H.J.; Kwon, Y.J. Alpha-lipoic acid attenuates cisplatin-induced tubulointerstitial injuries through inhibition of mitochondrial bax translocation in rats. Nephron. Exp. Nephrol. 2009, 113, e104–e112. [Google Scholar] [CrossRef]
- Armagan, I.; Bayram, D.; Candan, I.A.; Yigit, A.; Celik, E.; Armagan, H.H.; Uğuz, A.C. Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ. Toxicol. Pharmacol. 2015, 39, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Kim, J.H.; Jang, H.N.; Lee, T.W.; Jung, M.H.; Kim, T.H.; Chang, S.H.; Park, D.J. Alpha-lipoic acid ameliorates the epithelial mesenchymal transition induced by unilateral ureteral obstruction in mice. Sci. Rep. 2017, 7, 46065. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, C.; Sirajudeen, K.N.S. Effect of alpha-lipoic acid supplementation on blood pressure, renal oxidant-antioxidant status and renal damage in spontaneously hypertensive rats. Asian Pac. J. Trop. Biomed. 2019, 9, 415–423. [Google Scholar] [CrossRef]
- Lai, S.; Petramala, L.; Muscaritoli, M.; Cianci, R.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; D’Ambrosio, V.; Carta, M.; Ansuini, M.; et al. α-lipoic acid in patients with autosomal dominant polycystic kidney disease. Nutrition 2020, 71, 110594. [Google Scholar] [CrossRef]
- Cheserek, M.J.; Wu, G.; Li, L.; Li, L.; Karangwa, E.; Shi, Y.; Le, G. Cardioprotective effects of lipoic acid, quercetin and resveratrol on oxidative stress related to thyroid hormone alterations in long-term obesity. J. Nutr. Biochem. 2016, 33, 36–44. [Google Scholar] [CrossRef]
- Pop, C.; Ștefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef]
- Vasdev, S.; Ford, C.A.; Parai, S.; Longerich, L.; Gadag, V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J. Hypertens. 2000, 18, 567–573. [Google Scholar] [CrossRef]
- Koçak, G.; Aktan, F.; Canbolat, O.; Ozoğul, C.; Elbeğ, S.; Yildizoglu-Ari, N.; Karasu, C.; ADIC Study Group—Antioxidants in Diabetes-Induced Complications. Alpha-lipoic acid treatment ameliorates metabolic parameters, blood pressure, vascular reactivity and morphology of vessels already damaged by streptozotocin-diabetes. Diabetes Nutr. Metab. 2000, 13, 308–318. [Google Scholar]
- Takaoka, M.; Kobayashi, Y.; Yuba, M.; Ohkita, M.; Matsumura, Y. Effects of alpha-lipoic acid on deoxycorticosterone acetate-salt-induced hypertension in rats. Eur. J. Pharmacol. 2001, 424, 121–129. [Google Scholar] [CrossRef]
- Dudek, M.; Razny, K.; Bilska-Wilkosz, A.; Iciek, M.; Sapa, J.; Wlodek, L.; Filipek, B. Hypotensive effect of alpha-lipoic acid after a single administration in rats. Anatol. J. Cardiol. 2016, 16, 306–309. [Google Scholar] [CrossRef]
- Queiroz, T.M.; Guimarães, D.D.; Mendes-Junior, L.G.; Braga, V.A. α-lipoic acid reduces hypertension and increases baroreflex sensitivity in renovascular hypertensive rats. Molecules 2012, 17, 13357–13367. [Google Scholar] [CrossRef]
- Midaoui, A.E.; de Champlain, J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension 2002, 39, 303–307. [Google Scholar] [CrossRef]
- Cure, E.; Cure, M.C. Alpha-lipoic acid may protect patients with diabetes against COVID-19 infection. Med. Hypotheses. 2020, 143, 110185. [Google Scholar] [CrossRef]
- Teichert, J.; Kern, J.; Tritschler, H.J.; Ulrich, H.; Preiss, R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int. J. Clin. Pharmacol. Ther. 1998, 36, 625–628. [Google Scholar]
- Brufani, M.; Figliola, R. (R)-α-lipoic acid oral liquid formulation: Pharmacokinetic parameters and therapeutic efficacy. Acta Biomed. 2014, 85, 108–115. [Google Scholar] [PubMed]
- Maglione, E.; Marrese, C.; Migliaro, E.; Marcuccio, F.; Panico, C.; Salvati, C.; Citro, G.; Quercio, M.; Roncagliolo, F.; Torello, C.; et al. Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain. Acta Biomed. 2015, 86, 226–233. [Google Scholar]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid. Med. Cell. Longev. 2018, 2018, 1767265. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Rizzo, M.; Krogager, C.; Kennedy, C.; Georges, C.M.G.; Knežević, T.; Liberopoulos, E.; Vallée, A.; Pérez-Martínez, P.; Wenstedt, E.F.E.; et al. Safety Evaluation of α-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Lucarini, E.; Trallori, E.; Tomassoni, D.; Amenta, F.; Ghelardini, C.; Pacini, A.; Di Cesare Mannelli, L. Toxicological Profile of the Pain-Relieving Antioxidant Compound Thioctic Acid in Its Racemic and Enantiomeric Forms. Antioxidants 2020, 9, 749. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, I.; Tomassoni, D.; Roy, P.; Di Cesare Mannelli, L.; Amenta, F.; Tayebati, S.K. Antioxidant Properties of Alpha-Lipoic (Thioctic) Acid Treatment on Renal and Heart Parenchyma in a Rat Model of Hypertension. Antioxidants 2021, 10, 1006. https://doi.org/10.3390/antiox10071006
Martinelli I, Tomassoni D, Roy P, Di Cesare Mannelli L, Amenta F, Tayebati SK. Antioxidant Properties of Alpha-Lipoic (Thioctic) Acid Treatment on Renal and Heart Parenchyma in a Rat Model of Hypertension. Antioxidants. 2021; 10(7):1006. https://doi.org/10.3390/antiox10071006
Chicago/Turabian StyleMartinelli, Ilenia, Daniele Tomassoni, Proshanta Roy, Lorenzo Di Cesare Mannelli, Francesco Amenta, and Seyed Khosrow Tayebati. 2021. "Antioxidant Properties of Alpha-Lipoic (Thioctic) Acid Treatment on Renal and Heart Parenchyma in a Rat Model of Hypertension" Antioxidants 10, no. 7: 1006. https://doi.org/10.3390/antiox10071006
APA StyleMartinelli, I., Tomassoni, D., Roy, P., Di Cesare Mannelli, L., Amenta, F., & Tayebati, S. K. (2021). Antioxidant Properties of Alpha-Lipoic (Thioctic) Acid Treatment on Renal and Heart Parenchyma in a Rat Model of Hypertension. Antioxidants, 10(7), 1006. https://doi.org/10.3390/antiox10071006








