Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts
Abstract
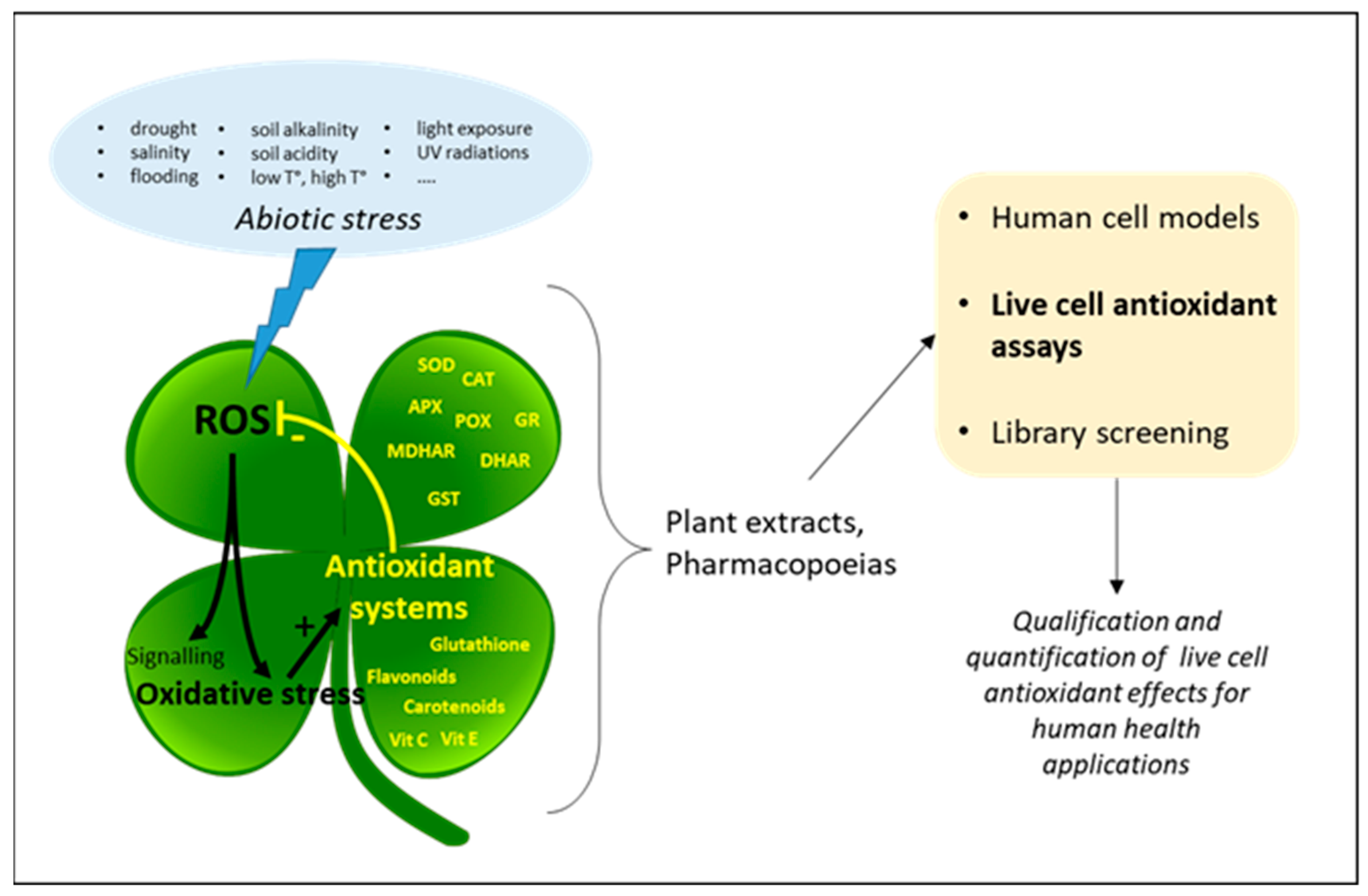
1. Introduction
2. Cell Models
3. Antioxidant Effects in Live Cells
4. Live Cell Antioxidant Assays
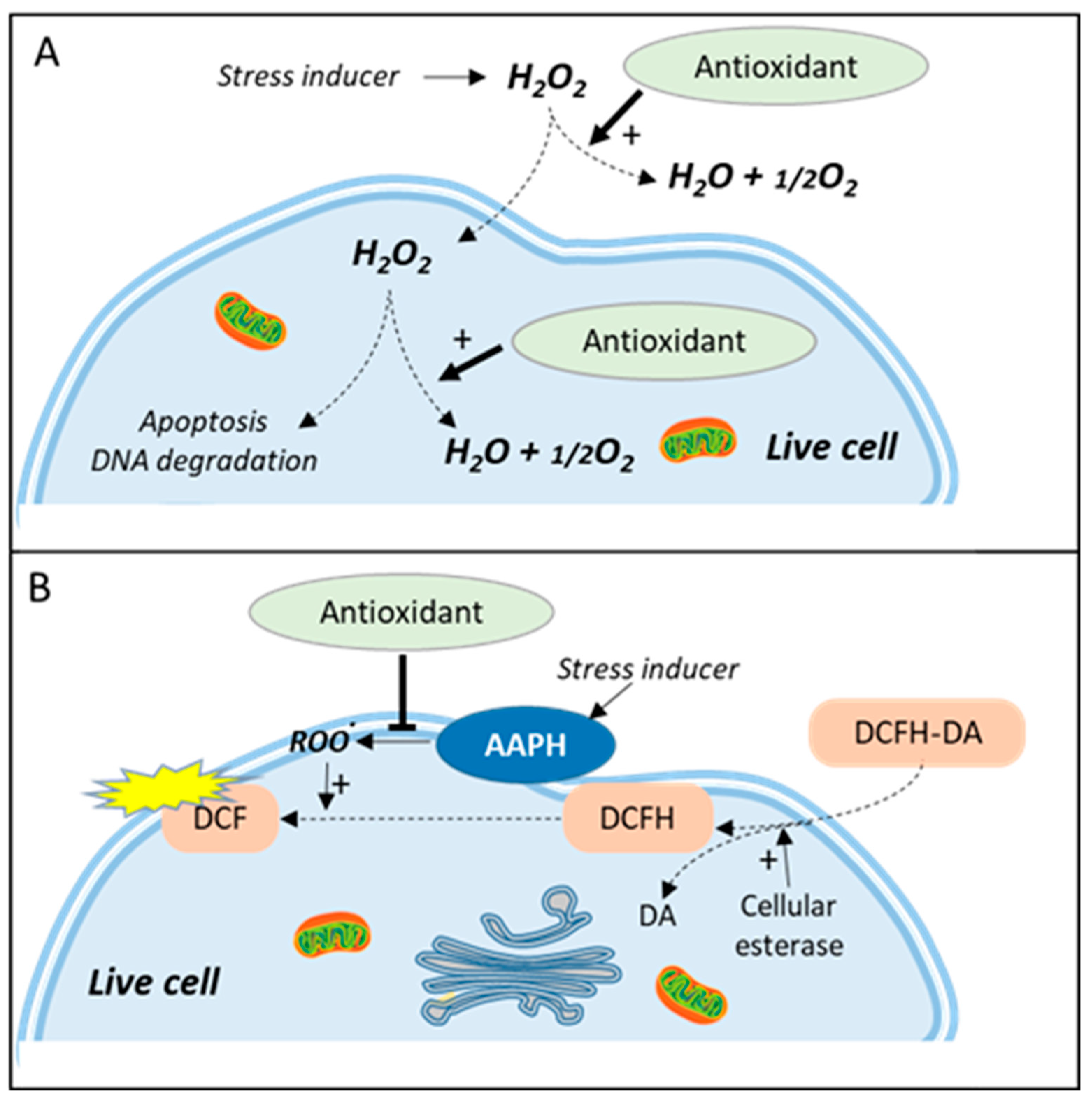
5. Catalase-Like Assay
6. Cell Antioxidant Assay (CAA)
7. Toward a Better Monitoring of ROS Production in the Cell
8. AOP1, a New Antioxidant Live Cell Approach Based on Photoinduction
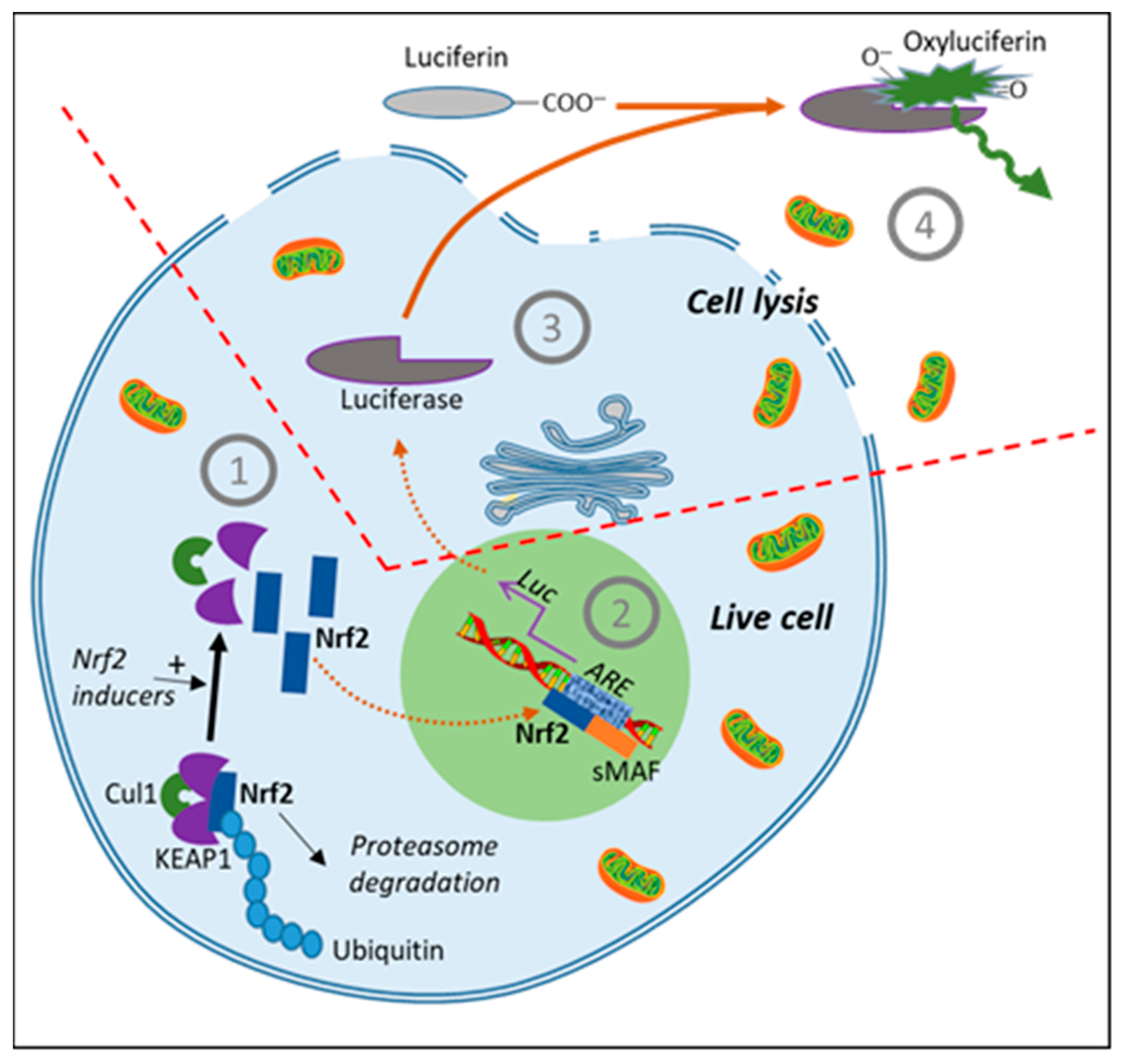
9. Measure of Indirect Antioxidant Effects by KEAP1/Nrf2/ARE Activation
10. Outlook—Toward an Industrial Standard of Cell-Based Assays
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants Facing Oxidative Challenges—A Little Help from the Antioxidant Networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant. Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS Systems Are a New Integrated Network for Sensing Homeostasis and Alarming Stresses in Organelle Metabolic Processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant. Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic Carotenoid Oxidation and Photooxidative Stress Signalling in Plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How Relevant Are Flavonoids as Antioxidants in Plants? Trends Plant. Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant. Sci. 2020, 11, 552969. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The Use of Total Antioxidant Capacity as Surrogate Marker for Food Quality and its Effect on Health is to be Discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Pastore, D. The Soybean Lipoxygenase-Fluorescein Reaction may be used to Assess Antioxidant Capacity of Phytochemicals and Serum. Anal. Methods 2016, 8, 4354–4362. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Alfarano, M.; Dalfino, G.; Panunzio, M.F.; Pastore, D. Antioxidant/Oxidant Balance as a novel approach to evaluate the effect on serum of long-term intake of plant antioxidant-rich foods. J. Funct. Foods 2018, 40, 778–784. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative Stress in Cell Culture: An under-Appreciated Problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Halliwell, B. Cell Culture, Oxidative Stress, and Antioxidants: Avoiding Pitfalls. Biomed. J. 2014, 37, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. The Effects of Oxaloacetate on Hydrogen Peroxide Generation from Ascorbate and Epigallocatechin Gallate in Cell Culture Media: Potential for Altering Cell Metabolism. Biochem. Biophys. Res. Commun. 2012, 417, 446–450. [Google Scholar] [CrossRef]
- Liu, R.H.; Finley, J. Potential Cell Culture Models for Antioxidant Research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Baldi, A. Nutrition-Based Health: Cell-Based Bioassays for Food Antioxidant Activity Evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef] [PubMed]
- Furger, C. Live Cell Assays. From Research to Health and Regulatory Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-84821-858-1. [Google Scholar]
- Allen, D.D.; Caviedes, R.; Cárdenas, A.M.; Shimahara, T.; Segura-Aguilar, J.; Caviedes, P.A. Cell Lines as in Vitro Models for Drug Screening and Toxicity Studies. Drug Dev. Ind. Pharm. 2005, 31, 757–768. [Google Scholar] [CrossRef]
- Goya, L.; Martin, M.; Ramos, S.; Mateos, R.; Bravo, L. A Cell Culture Model for the Assessment of the Chemopreventive Potential of Dietary Compounds. Curr. Nutr. Food Sci. 2009, 5, 56–64. [Google Scholar] [CrossRef][Green Version]
- Scott, C.W.; Peters, M.F.; Dragan, Y.P. Human Induced Pluripotent Stem Cells and Their Use in Drug Discovery for Toxicity Testing. Toxicol. Lett. 2013, 219, 49–58. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In Vitro Measurements and Interpretation of Total Antioxidant Capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the Antioxidant Capacity of Natural Products: A Review on Chemical and Cellular-Based Assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and Limitations of Common Testing Methods for Antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of Chemical and Cell-Based Antioxidant Methods for Evaluation of Foods and Natural Products: Generating Multifaceted Data by Parallel Testing Using Erythrocytes and Polymorphonuclear Cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Mello, L.D.; Kisner, A.; Goulart, M.O.F.; Kubota, L.T. Biosensors for Antioxidant Evaluation in Biological Systems. Comb. Chem. High. Throughput Screen. 2013, 16, 109–120. [Google Scholar] [CrossRef]
- Mason, R.P. Imaging Free Radicals in Organelles, Cells, Tissue, and in Vivo with Immuno-Spin Trapping. Redox Biol. 2016, 8, 422–429. [Google Scholar] [CrossRef]
- Andina, D.; Leroux, J.-C.; Luciani, P. Ratiometric Fluorescent Probes for the Detection of Reactive Oxygen Species. Chemistry 2017, 23, 13549–13573. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The Challenges of Using Fluorescent Probes to Detect and Quantify Specific Reactive Oxygen Species in Living Cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Debowska, K.; Debski, D.; Hardy, M.; Jakubowska, M.; Kalyanaraman, B.; Marcinek, A.; Michalski, R.; Michalowski, B.; Ouari, O.; Sikora, A.; et al. Toward Selective Detection of Reactive Oxygen and Nitrogen Species with the Use of Fluorogenic Probes--Limitations, Progress, and Perspectives. Pharm. Rep. 2015, 67, 756–764. [Google Scholar] [CrossRef]
- Gough, D.R.; Cotter, T.G. Hydrogen Peroxide: A Jekyll and Hyde Signalling Molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef]
- Rincheval, V.; Bergeaud, M.; Mathieu, L.; Leroy, J.; Guillaume, A.; Mignotte, B.; Le Floch, N.; Vayssière, J.-L. Differential Effects of Bcl-2 and Caspases on Mitochondrial Permeabilization during Endogenous or Exogenous Reactive Oxygen Species-Induced Cell Death: A Comparative Study of H2O2, Paraquat, t-BHP, Etoposide and TNF-α-Induced Cell Death. Cell Biol. Toxicol. 2012, 28, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Nascimento da Silva, L.C.; Bezerra Filho, C.M.; de Paula, R.A.; Silva e Silva, C.S.; Oliveira de Souza, L.I.; da Silva, M.V.; Correia, M.T.D.S.; de Figueiredo, R.C.B.Q. In Vitro Cell-Based Assays for Evaluation of Antioxidant Potential of Plant-Derived Products. Free Radic. Res. 2016, 50, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Chen, Z. Protective Effects of Rice Dreg Protein Hydrolysates against Hydrogen Peroxide-Induced Oxidative Stress in HepG-2 Cells. Food Funct. 2016, 7, 1429–1437. [Google Scholar] [CrossRef]
- Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells. Molecules 2018, 24, 124. [Google Scholar] [CrossRef]
- Somanah, J.; Bourdon, E.; Bahorun, T. Extracts of Mauritian Carica Papaya (Var. Solo) Protect SW872 and HepG2 Cells against Hydrogen Peroxide Induced Oxidative Stress. J. Food Sci. Technol. 2017, 54, 1917–1927. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Zarrabi, A.; Rahgozar, S. The Antitoxic Effects of Quercetin and Quercetin-Conjugated Iron Oxide Nanoparticles (QNPs) against H2O2-Induced Toxicity in PC12 Cells. Int. J. Nanomed. 2019, 14, 6813–6830. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.O.; Branco, C.S.; Sene, J.; DallAgnol, R.; Agostini, F.; Moura, S.; Salvador, M. Antioxidant and Antigenotoxic Activities of the Brazilian Pine Araucaria Angustifolia (Bert.) O. Kuntze. Antioxidants 2014, 3, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.D.; Acosta, D. Failure of Gentamicin to Elevate Cellular Malondialdehyde Content or Increase Generation of Intracellular Reactive Oxygen Species in Primary Cultures of Renal Cortical Epithelial Cells. Biochem. Pharm. 1990, 40, 1523–1526. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A Microplate Assay for the Detection of Oxidative Products Using 2′,7′-Dichlorofluorescin-Diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying Cellular Oxidative Stress by Dichlorofluorescein Assay Using Microplate Reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular Antioxidant Activity of Common Fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- De la Haba, C.; Palacio, J.R.; Martínez, P.; Morros, A. Effect of Oxidative Stress on Plasma Membrane Fluidity of THP-1 Induced Macrophages. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Yang, J.-H.; Yoon, S.J.; Lee, J.-H.; Yang, E.S.; Park, J.-W. Lipid Peroxidation-Mediated Cytotoxicity and DNA Damage in U937 Cells. Biochimie 2002, 84, 1199–1205. [Google Scholar] [CrossRef]
- Sunitha, D. A Review on Antioxidant Methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the Cellular Antioxidant Activity (CAA) Assay to Study Phenolic Antioxidants in a Caco-2 Cell Line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.M.; Sarvazyan, N. Localization of Dichlorofluorescin in Cardiac Myocytes: Implications for Assessment of Oxidative Stress. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H982–H990. [Google Scholar] [CrossRef]
- Afri, M.; Frimer, A.A.; Cohen, Y. Active Oxygen Chemistry within the Liposomal Bilayer. Part IV: Locating 2′,7′-Dichlorofluorescein (DCF), 2′,7′-Dichlorodihydrofluorescein (DCFH) and 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA) in the Lipid Bilayer. Chem. Phys. Lipids 2004, 131, 123–133. [Google Scholar] [CrossRef]
- González, E.; Vaillant, F.; Rojas, G.; Pérez, A. Novel Semiautomated Method for Assessing in Vitro Cellular Antioxidant Activity Using the Light-Scattering Properties of Human Erythrocytes. J. Agric. Food Chem. 2010, 58, 1455–1461. [Google Scholar] [CrossRef]
- González, E.; Vaillant, F.; Pérez, A. In Vitro Cell-Mediated Antioxidant Protection of Human Erythrocytes by Some Common Tropical Fruits. J. Nutr. Food Sci. 2012, 2. [Google Scholar] [CrossRef]
- Dobiasová, S.; Řehořová, K.; Kučerová, D.; Biedermann, D.; Káňová, K.; Petrásková, L.; Koucká, K.; Václavíková, R.; Valentová, K.; Ruml, T.; et al. Multidrug Resistance Modulation Activity of Silybin Derivatives and Their Anti-Inflammatory Potential. Antioxidants 2020, 9, 455. [Google Scholar] [CrossRef]
- Gong, E.S.; Liu, C.; Li, B.; Zhou, W.; Chen, H.; Li, T.; Wu, J.; Zeng, Z.; Wang, Y.; Si, X.; et al. Phytochemical Profiles of Rice and Their Cellular Antioxidant Activity against ABAP Induced Oxidative Stress in Human Hepatocellular Carcinoma HepG2 Cells. Food Chem. 2020, 318, 126484. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant Activity and Cytotoxicity of Jerusalem Artichoke Tubers and Leaves Extract on HaCaT and BJ Fibroblast Cells. Lipids Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2′,7′-Dichlorofluorescin and Dihydrorhodamine 123 as Fluorescent Probes for Intracellular H2O2 in Cultured Endothelial Cells. Arch. Biochem. Biophys. 1993, 302, 348–355. [Google Scholar] [CrossRef]
- Yazdani, M. Concerns in the Application of Fluorescent Probes DCDHF-DA, DHR 123 and DHE to Measure Reactive Oxygen Species in Vitro. Toxicol. Vitr. 2015, 30, 578–582. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, L.; Wei, Y.; Liu, Y.; Jia, G.; Zhou, F.; Ji, B. Development of a Cell-Based Antioxidant Activity Assay Using Dietary Fatty Acid as Oxidative Stressor. Food Chem. 2013, 141, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a Fluorescent Probe for Reactive Oxygen Species Measurement: Forty Years of Application and Controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Escorza, M.A.; González-Martínez, M.T.; del Pilar, I.-O.M.; Calderón-Salinas, J.V. Oxidative Damage Increases Intracellular Free Calcium [Ca2+]i Concentration in Human Erythrocytes Incubated with Lead. Toxicol. In Vitro 2010, 24, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, G.; Zhang, S.; Wang, H.; Ke, L.; Zhou, J.; Rao, P.; Wang, Q.; Li, J. Influences of Calcium and Magnesium Ions on Cellular Antioxidant Activity (CAA) Determination. Food Chem. 2020, 320, 126625. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Foster, T.H. Optogenetic Control of ROS Production. Redox Biol. 2014, 2, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A Genetically Encoded Photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; van Vroonhoven, E.; Ter Beest, M.; van den Bogaart, G. Radical Stress Is More Cytotoxic in the Nucleus than in Other Organelles. Int. J. Mol. Sci. 2019, 20, 4147. [Google Scholar] [CrossRef]
- Vitriol, E.A.; Uetrecht, A.C.; Shen, F.; Jacobson, K.; Bear, J.E. Enhanced EGFP-Chromophore-Assisted Laser Inactivation Using Deficient Cells Rescued with Functional EGFP-Fusion Proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 6702–6707. [Google Scholar] [CrossRef]
- Trewin, A.J.; Berry, B.J.; Wei, A.Y.; Bahr, L.L.; Foster, T.H.; Wojtovich, A.P. Light-Induced Oxidant Production by Fluorescent Proteins. Free Radic. Biol. Med. 2018, 128, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Matsuda, T.; Sakai, N.; Fu, D.; Noda, M.; Uchiyama, S.; Kotera, I.; Arai, Y.; Horiuchi, M.; Fukui, K.; et al. SuperNova, a Monomeric Photosensitizing Fluorescent Protein for Chromophore-Assisted Light Inactivation. Sci. Rep. 2013, 3, 2629. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Lev-Ram, V.; Deerinck, T.J.; Qi, Y.; Ramko, E.B.; Davidson, M.W.; Jin, Y.; Ellisman, M.H.; Tsien, R.Y. A Genetically Encoded Tag for Correlated Light and Electron Microscopy of Intact Cells, Tissues, and Organisms. PLoS Biol. 2011, 9, e1001041. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.K. Photodynamic Therapy: Current Role in the Treatment of Chorioretinal Conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Lubitz, I.; Zikich, D.; Kotlyar, A. Specific High-Affinity Binding of Thiazole Orange to Triplex and G-Quadruplex DNA. Biochemistry 2010, 49, 3567–3574. [Google Scholar] [CrossRef]
- Karunakaran, V.; Pérez Lustres, J.L.; Zhao, L.; Ernsting, N.P.; Seitz, O. Large Dynamic Stokes Shift of DNA Intercalation Dye Thiazole Orange Has Contribution from a High-Frequency Mode. J. Am. Chem. Soc. 2006, 128, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.; Svanvik, N.; Kubista, M. The Interactions between the Fluorescent Dye Thiazole Orange and DNA. Biopolymers 1998, 46, 39–51. [Google Scholar] [CrossRef]
- Armitage, B.A. Cyanine Dye–Nucleic Acid Interactions. In Heterocyclic Polymethine Dyes; Strekowski, L., Ed.; Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; Volume 14, pp. 11–29. ISBN 978-3-540-79063-1. [Google Scholar]
- Derick, S.; Gironde, C.; Perio, P.; Reybier, K.; Nepveu, F.; Jauneau, A.; Furger, C. LUCS (Light-Up Cell System), a Universal High Throughput Assay for Homeostasis Evaluation in Live Cells. Sci. Rep. 2017, 7, 18069. [Google Scholar] [CrossRef] [PubMed]
- Gironde, C.; Rigal, M.; Dufour, C.; Furger, C. AOP1, a New Live Cell Assay for the Direct and Quantitative Measure of Intracellular Antioxidant Effects. Antioxidants 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a Phytocomplex of Bilberry (Vaccinium Myrtillus) and Spirulina (Spirulina Platensis), Exerts Both Direct Antioxidant Activity and Modulation of ARE/Nrf2 Pathway in HepG2 Cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Bugaj, L.J.; Lim, W.A. High-Throughput Multicolor Optogenetics in Microwell Plates. Nat. Protoc. 2019, 14, 2205–2228. [Google Scholar] [CrossRef]
- Thomas, O.S.; Hörner, M.; Weber, W. A Graphical User Interface to Design High-Throughput Optogenetic Experiments with the OptoPlate-96. Nat. Protoc. 2020, 15, 2785–2787. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an Update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Mol. Cell. Biol. 2004, 24, 10941–10953. [Google Scholar] [CrossRef]
- Levonen, A.-L.; Landar, A.; Ramachandran, A.; Ceaser, E.K.; Dickinson, D.A.; Zanoni, G.; Morrow, J.D.; Darley-Usmar, V.M. Cellular Mechanisms of Redox Cell Signalling: Role of Cysteine Modification in Controlling Antioxidant Defences in Response to Electrophilic Lipid Oxidation Products. Biochem. J. 2004, 378, 373–382. [Google Scholar] [CrossRef]
- Unoki, T.; Akiyama, M.; Kumagai, Y. Nrf2 Activation and Its Coordination with the Protective Defense Systems in Response to Electrophilic Stress. Int. J. Mol. Sci. 2020, 21, 545. [Google Scholar] [CrossRef]
- Baird, L.; Llères, D.; Swift, S.; Dinkova-Kostova, A.T. Regulatory Flexibility in the Nrf2-Mediated Stress Response Is Conferred by Conformational Cycling of the Keap1-Nrf2 Protein Complex. Proc. Natl. Acad. Sci. USA 2013, 110, 15259–15264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. P62 Links Autophagy and Nrf2 Signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lv, Y.-F.; Zhao, J.-L.; You, Q.-D.; Jiang, Z.-Y. Regulation of Nrf2 by Phosphorylation: Consequences for Biological Function and Therapeutic Implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2–ARE Signaling in Cellular Protection: Mechanism of Action and the Regulatory Mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant Response Elements: Discovery, Classes, Regulation and Potential Applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Motahari, P.; Sadeghizadeh, M.; Behmanesh, M.; Sabri, S.; Zolghadr, F. Generation of Stable ARE-Driven Reporter System for Monitoring Oxidative Stress. J. Pharm. Sci. 2015, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Hou, D.-X. Multiple Regulations of Keap1/Nrf2 System by Dietary Phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for Healthy Skin: The Emerging Role of Aryl Hydrocarbon Receptors and Nuclear Factor-Erythroid 2-Related Factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Balstad, T.R.; Carlsen, H.; Myhrstad, M.C.W.; Kolberg, M.; Reiersen, H.; Gilen, L.; Ebihara, K.; Paur, I.; Blomhoff, R. Coffee, Broccoli and Spices Are Strong Inducers of Electrophile Response Element-Dependent Transcription in Vitro and in Vivo—Studies in Electrophile Response Element Transgenic Mice. Mol. Nutr. Food Res. 2011, 55, 185–197. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Health Effects of Coffee: Mechanism Unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef]
- Roy, A.; McDonald, P.; Timmermann, B.N.; Gupta, M.; Chaguturu, R. Bioactivity Profiling of Plant Biodiversity of Panama by High Throughput Screening. Nat. Prod. Commun. 2019, 14, 71–74. [Google Scholar] [CrossRef]
- Zielonka, J.; Zielonka, M.; VerPlank, L.; Cheng, G.; Hardy, M.; Ouari, O.; Ayhan, M.M.; Podsiadły, R.; Sikora, A.; Lambeth, J.D.; et al. Mitigation of NADPH Oxidase 2 Activity as a Strategy to Inhibit Peroxynitrite Formation. J. Biol. Chem. 2016, 291, 7029–7044. [Google Scholar] [CrossRef]

| CAA Assay | AOP1 Assay |
|---|---|
| Based on the production of AAPH-induced peroxyl radicals | Based on the controlled production of 1O2 and free radicals by photoinduction |
| Measures effects of plasma membrane-based antioxidants | Measures effects of intracellular-based antioxidants |
| No control of ROS production | Easy control of ROS production by light intensity; allows monitoring ROS production at a sublethal level (i.e., more physiological concentrations) |
| Interpretation limited to AAPH effects | |
| Does not differentiate between antioxidant and cytotoxic effects | Can easily discriminate between antioxidant and cytotoxic effects |
| Results need to be confirmed by performing a cytotoxicity assay (e.g., MTT) | No other assay needed |
| DCFH-DA subject to auto-oxidation | Sensor not directly involved in the oxidation process |
| Subject to cell leakage | No cell leakage |
| Fluorescence levels vary according to cell density | No effect of cell density (measure on a ratio mode) |
| Needs culture medium washes that disrupt cell culture | No washes required |
| Difficult to standardize | Easy to standardize |
| Detection by fluorescence readers | Detection by fluorescence readers + illuminator |
| Limited to adherent cells | Works for adherent and suspension cells, and organotypic models |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. https://doi.org/10.3390/antiox10060944
Furger C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants. 2021; 10(6):944. https://doi.org/10.3390/antiox10060944
Chicago/Turabian StyleFurger, Christophe. 2021. "Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts" Antioxidants 10, no. 6: 944. https://doi.org/10.3390/antiox10060944
APA StyleFurger, C. (2021). Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants, 10(6), 944. https://doi.org/10.3390/antiox10060944






