Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter pylori Infection in Human Gastric Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Helicobacter pylori Strains, Growth Media, and Culture Conditions
2.2. Determination of Antibiotic Susceptibility of H. pylori Strains
2.3. Identification of Virulence Markers in H. pylori Strains
2.4. Preparation of the GSE and Its OPC-Rich and PPC-Rich Fractions
2.5. Human Gastric Epithelial Cell Cultures
2.6. Evaluation of GSE Cytotoxicity
2.7. Study of the Effect of GSE and Its OPC-Rich and PPC-Rich Fractions on the Inflammatory Response Induced by H. pylori Strains in AGS Cells
2.8. Determination of Antioxidant Activity of GSE and Its OPC-rich and PPC-rich Fractions against Intracellular Reactive Oxygen Species (ROS) Production in AGS Cells
2.9. Determination of Antibacterial Activity of GSE and Its OPC-Rich and PPC-Rich Fractions against H. pylori Strains
2.10. Chemical Characterization of GSE and Its OPC-Rich and PPC-Rich Fractions
2.11. Statistical Analysis
3. Results
3.1. Strains Characterization: Antibiotic Susceptibility
3.2. Characterization of Strains: Virulence Markers
3.3. Study of the Effect of GSE and Its OPC-Rich and PPC-Rich Fractions on the Inflammatory Response Induced by H. pylori in AGS Cells
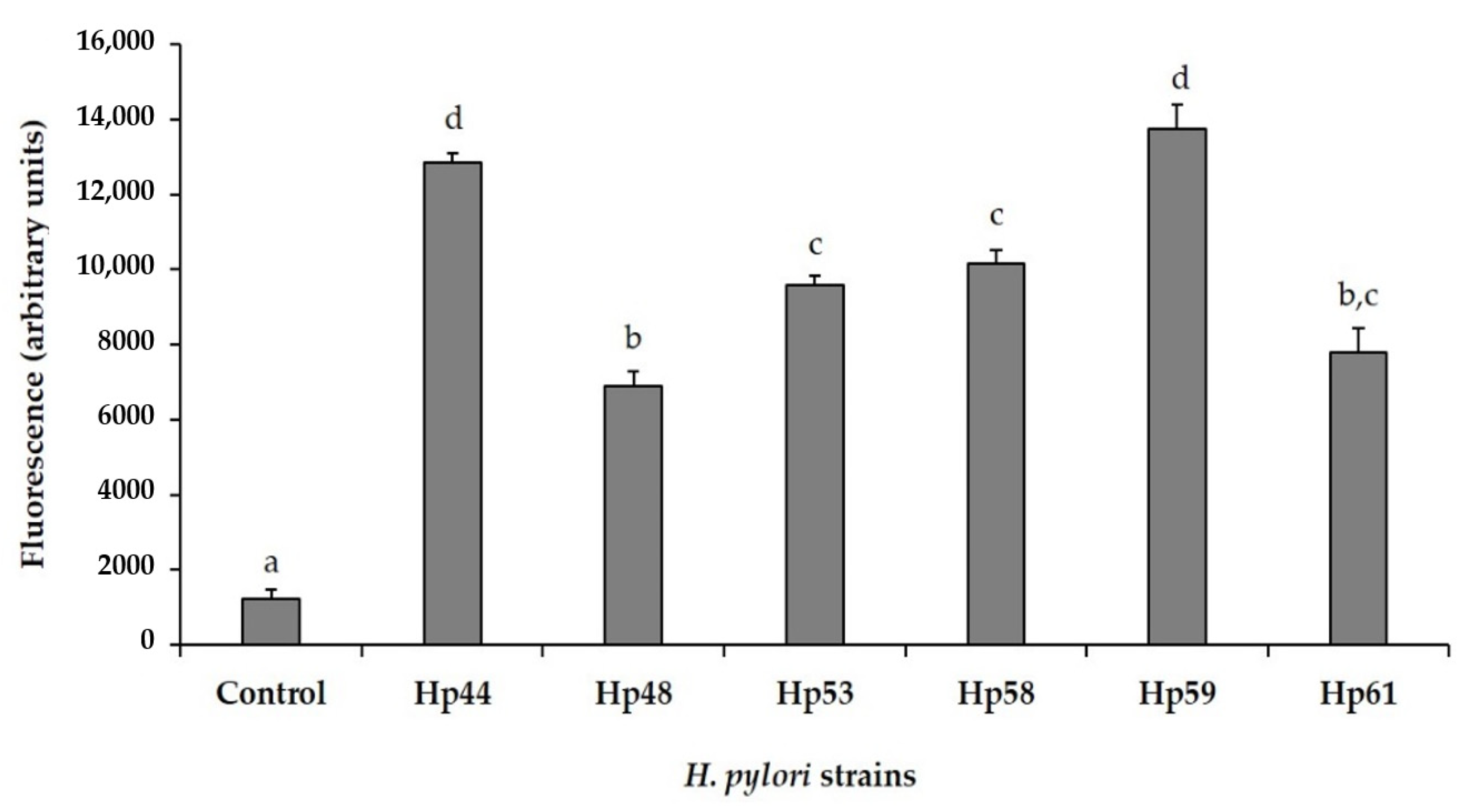
3.4. Antioxidant Activity against Intracellular Reactive Oxygen Species (ROS) Production in AGS Cells
3.5. Antibacterial Activity
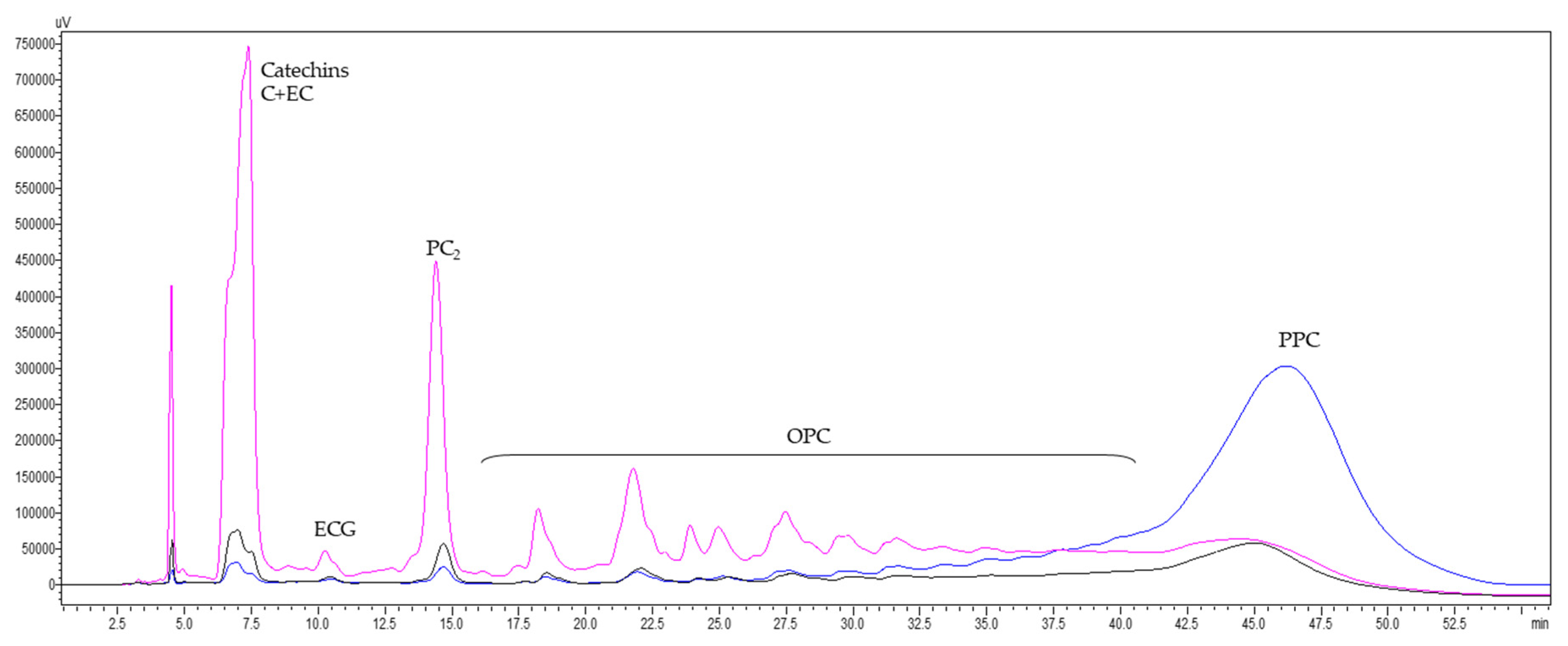
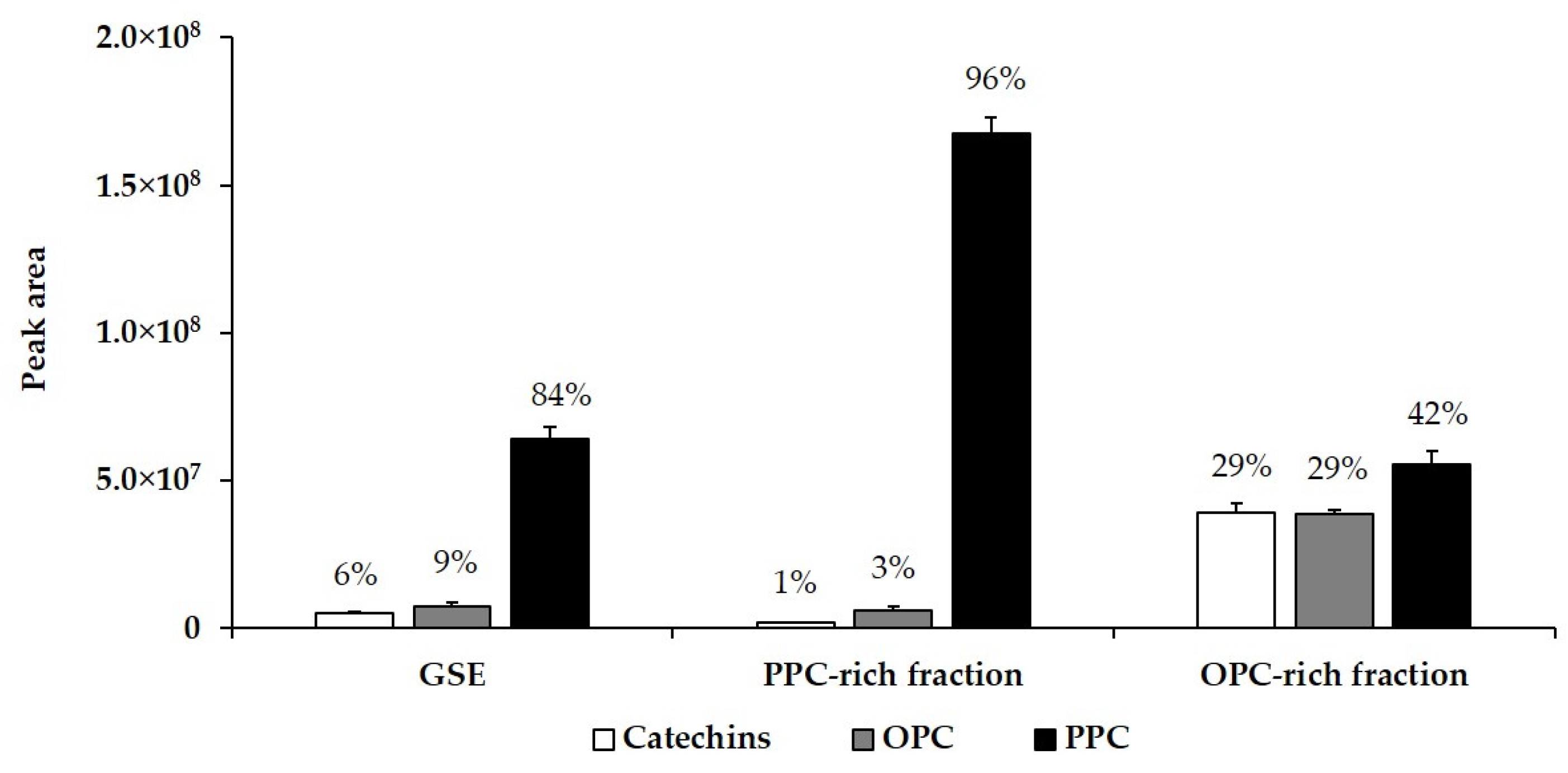
3.6. Characterization of GSE and Its Fractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Percival, S.L.; Williams, D.W. Helicobacter pylori. In Microbiology of Waterborne Diseases: Microbiological Aspects and Risks, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 119–154. [Google Scholar] [CrossRef]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Konturek, P.C.; Konturek, S.J.; Brzozowski, T. Helicobacter pylori infection in gastric cancerogenesis. J. Physiol. Pharmacol. 2009, 60, 3–21. [Google Scholar] [PubMed]
- Santos, M.L.C.; de Brito, B.B.; da Silva, F.A.F.; Sampaio, M.M.; Marques, H.S.; Oliveira, E.; Silva, N.; de Magalhães Queiroz, D.M.; de Melo, F.F. Helicobacter pylori infection: Beyond gastric manifestations. World J. Gastroenterol. 2020, 26, 4076–4093. [Google Scholar] [CrossRef] [PubMed]
- Arima, N.; Tsudo, M. Extragastric mucosa-associated lymphoid tissue lymphoma showing the regression by Helicobacter pylori eradication therapy. Br. J. Haematol. 2003, 120, 790–792. [Google Scholar] [CrossRef]
- Guraya, S.Y.; Ahmad, A.A.; El-Ageery, S.M.; Hemeg, H.A.; Ozbak, H.A.; Yousef, K.; Abdel-Aziz, N.A. The correlation of Helicobacter pylori with the development of cholelithiasis and cholecystitis: The results of a prospective clinical study in Saudi Arabia. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3873–3880. [Google Scholar]
- Chen, C.X.; Mao, Y.S.; Foster, P.; Zhu, Z.W.; Du, J.; Guo, C.Y. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl. Physiol. Nutr. Metab. 2017, 42, 295–301. [Google Scholar] [CrossRef]
- Pellicano, R.; Mazzaferro, V.; Grigioni, W.F.; Cutufia, M.A.; Fagoonee, S.; Silengo, L.; Rizzetto, M.; Ponzetto, A. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J. Gastroenterol. 2004, 10, 598–601. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Sendur, R.; Pawlik, W.W.; Konturek, S.J. Deleterious effect of Helicobacter pylori infection on the course of acute pancreatitis in rats. Pancreatology 2002, 2, 386–395. [Google Scholar] [CrossRef]
- IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC Working group on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 177–240. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, 180–190. [Google Scholar] [CrossRef]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Cheng, T.; Xia, W.; Lai, Y.-T.; Sun, H. Nickel translocation between metallochaperones HypA and UreE in Helicobacter pylori. Metallomics 2014, 6, 1731–1736. [Google Scholar] [CrossRef]
- Eaton, K.A.; Suerbaum, S.; Josenhans, C.; Krakowka, S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 1996, 64, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Roesler, B.M.; Rabelo Gonçalves, E.M.A.; Zeitune, J.M.R. Virulence factors of Helicobacter pylori: A review. Clin. Med. Insights Gastroenterol. 2014, 7, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kwon, D.H.; Graham, D.Y. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 2000, 97, 7533–7538. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kodama, T.; Graham, D.Y.; Kashima, K. Search for putative virulence factors of Helicobacter pylori: The low-molecular-weight (33–35 K) antigen. Dig. Dis. Sci. 1998, 43, 1482–1487. [Google Scholar] [CrossRef]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell. Mol. Gastroenter. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef]
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24, e12638. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Denic, M.; Touati, E.; De Reuse, H. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Parreira, P.; Duarte, M.F.; Reis, C.A.; Martins, C.L. Helicobacter pylori infection: A brief overview on alternative natural treatments to conventional therapy. Crit. Rev. Microbiol. 2016, 42, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Badet, C. Antibacterial activity of grape (Vitis vinifera, Vitis rotundifolia) seeds. In Nuts & Seeds in Health and DISEASE prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 545–552. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Huang, G.H.; Haley-Zitlin, V.; Jiang, X.P. Antibacterial effects of grape extracts on Helicobacter pylori. Appl. Environ. Microbiol. 2009, 75, 848–852. [Google Scholar] [CrossRef]
- Chua, C.S.; Yang, K.C.; Chen, J.H.; Liu, Y.H.; Hsu, Y.H.; Lee, H.C.; Huang, S.Y. The efficacy of blueberry and grape seed extract combination on triple therapy for Helicobacter pylori eradication: A randomised controlled trial. Int. J. Food Sci. Nutr. 2016, 67, 177–183. [Google Scholar] [CrossRef]
- Gonzalez-Quilen, C.; Rodríguez-Gallego, E.; Beltran-Debon, R.; Pinent, M.; Ardevol, A.; Blay, M.T. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeon, E.J.; Cheung, D.Y.; Kim, C.W.; Kim, S.S.; Park, S.H.; Han, S.W.; Kim, M.J.; Lee, Y.S.; Cho, M.L.; et al. Gastroprotective effects of grape seed proanthocyanidin extracts against nonsteroid anti-inflammatory drug-induced gastric injury in rats. Gut Liver 2013, 7, 282–289. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Sangiovanni, E.; Fumagalli, M.; Colombo, E.; Frigerio, G.; Colombo, F.; Peres de Sousa, L.; Altindişli, A.; Restani, P.; Dell’Agli, M. Evaluation of the anti-Inflammatory activity of raisins (Vitis vinifera L.) in human gastric epithelial cells: A comparative study. Int. J. Mol. Sci. 2016, 17, 1156. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Gutiérrez-Docio, A.; Moreno-Fernandez, S.; Alarcón-Cavero, T.; Prodanov, M.; Martinez-Rodriguez, A.J. Procyanidin-rich extract from grape seeds as a putative tool against Helicobacter pylori. Foods 2020, 9, 1370. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Sallas, M.L.; dos Santos, M.P.; Orcini, W.A.; David, É.B.; Peruquetti, R.L.; Payão, S.L.M.; Rasmussen, L.T. Status (on/off) of oipA gene: Their associations with gastritis and gastric cancer and geographic origins. Arch. Microbiol. 2019, 201, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Dossumbekova, A.; Prinz, C.; Mages, J.; Lang, R.; Kusters, J.G.; Van Vliet, A.H.; Reindl, W.; Backert, S.; Saur, D.; Schmid, R.M.; et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: Genetic and functional genomic analysis of hopH gene polymorphisms. J. Infect. Dis. 2006, 194, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Docio, A.; Almodóvar, P.; Moreno-Fernandez, S.; Silvan, J.M.; Martinez-Rodriguez, A.J.; Alonso, G.L.; Prodanov, M. Evaluation of an integrated ultrafiltration/solid phase extraction process for purification of oligomeric grape seed procyanidins. Membranes 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Mingo, E.; Martinez-Rodriguez, A.J. Grape seed extract (GSE) modulates Campylobacter pro-inflammatory response in human intestinal epithelial cell lines. Food Agric. Immunol. 2017, 28, 739–753. [Google Scholar] [CrossRef]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef]
- Martín, M.A.; Cordero-Herrera, I.; Bravo, L.; Ramos, S.; Goya, L. Cocoa flavanols show beneficial effects in cultured pancreatic beta cells and liver cells to prevent the onset of type 2 diabetes. Food Res. Int. 2014, 63, 400–408. [Google Scholar] [CrossRef][Green Version]
- Silvan, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Montilla, A.; van de Lagemaat, J.; Olano, A.; del Castillo, M. Determination of oligosaccharides by conventional high-resolution gas chromatography. Chroma 2006, 63, 453–458. [Google Scholar] [CrossRef]
- Jones, K.R.; Whitmire, J.M.; Merrell, D.S. A tale of two toxins: Helicobacter pylori CagA and VacA modulate host pathways that impact disease. Front. Microbiol. 2010, 1, 115. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Prodanov, M.; Villamiel, M. Effects of high intensity ultrasound on disaggregation of a macromolecular procyanidin-rich fraction from Vitis vinifera L. seed extract and evaluation of its antioxidant activity. Ultrason. Sonochem. 2019, 50, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Wallace, A.J. Dietary amelioration of Helicobacter infection. Nutr. Res. 2015, 35, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Cryer, B.; Sammer, D.; Lee, E.; Spechler, S.J. Influence of H. pylori infection on meal-stimulated gastric acid secretion and gastroesophageal acid reflux. Am. J. Physiol. 1999, 277, G1159–G1164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Hu, J.; Wang, X.; Ren, M.; Lu, G.; Lu, X.; Zhang, D.; He, S. The effect of Helicobacter pylori eradication in patients with gastroesophageal reflux disease: A meta-analysis of randomized controlled studies. Dig. Dis. 2020, 38, 261–268. [Google Scholar] [CrossRef]
- Sugimoto, M.; Murata, M.; Mizuno, H.; Iwata, E.; Nagata, N.; Itoi, T.; Kawai, T. Endoscopic reflux esophagitis and reflux-related symptoms after Helicobacter pylori eradication therapy: Meta-analysis. J. Clin. Med. 2020, 9, 3007. [Google Scholar] [CrossRef] [PubMed]
- Alba, C.; Blanco, A.; Alarcón, T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017, 30, 489–497. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 23 March 2021).
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Forman, D.; Waskito, L.A.; Yamaoka, Y.; Crabtree, J.E. Epidemiology of Helicobacter pylori and CagA-positive infections and global variations in gastric cancer. Toxins 2018, 10, 163. [Google Scholar] [CrossRef]
- Šterbenc, A.; Poljak, M.; Zidar, N.; Luzar, B.; Homan, M. Prevalence of the Helicobacter pylori homA and homB genes and their correlation with histological parameters in children. Microb. Pathog. 2018, 125, 26–32. [Google Scholar] [CrossRef]
- Backert, S.; Mimuro, H.; Israel, D.A.; Peek, R.M. Virulence factors of Helicobacter pylori. In Helicobacter pylori in the 21st Century; Sutton, P., Michell, H., Eds.; CABI: Oxfordshire, UK, 2010; pp. 212–247. [Google Scholar] [CrossRef]
- Kauser, F.; Khan, A.A.; Hussain, M.A.; Carroll, I.M.; Ahmad, N.; Tiwari, S.; Shouche, Y.; Das, B.; Alam, M.; Ali, S.M.; et al. The cag pathogenicity island of Helicobacter pylori is disrupted in the majority of patient isolates from different human populations. J. Clin. Microbiol. 2004, 42, 5302–5308. [Google Scholar] [CrossRef]
- Agudo, S.; Pérez-Pérez, G.; Alarcón, T.; López-Brea, M. High prevalence of clarithromycin-resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J. Clin. Microbiol. 2010, 48, 3703–3707. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.E.; Dowd, C.; O’Morain, C.; McNamara, D.; Smith, S.M. Can bacterial virulence factors predict antibiotic resistant Helicobacter pylori infection? World J. Gastroenterol. 2018, 24, 971–981. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Winter, J.A.; Robinson, K. Differential inflammatory response to Helicobacter pylori infection: Etiology and clinical outcomes. J. Inflamm. Res. 2015, 8, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.B.; Kim, D.Y.; Lee, S.J.; Sun, M.J.; Lee, M.S.; Li, H.; Cho, J.J.; Park, C.S. Inhibition of IL-8 production by green tea polyphenols in human nasal fibroblasts and A549 epithelial cells. Biol. Pharm. Bull. 2006, 29, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lin, J.K. (-)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef]
- Shih, C.M.; Lin, H.; Liang, Y.C.; Lee, W.S.; Bi, W.F.; Juan, S.H. Concentration-dependent differential effects of quercetin on rat aortic smooth muscle cells. Eur. J. Pharmacol. 2004, 496, 41–48. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.I.; Kim, Y.; Choi, M.; Min, S.; Joo, Y.H.; Yim, S.; Chung, N. Grape seed proanthocyanidin inhibits inflammatory responses in hepatic stellate cells by modulating the MAPK, Akt and NF-κB signaling pathways. Int. J. Mol. Med. 2017, 40, 226–234. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. ROS in gastrointestinal inflammation: Rescue or sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Brzozowski, T.; Dembiński, M.; Konturek, S.J.; Pawlik, W.W. Role of capsaicin-sensitive nerves and histamine H1, H2, and H3 receptors in the gastroprotective effect of histamine against stress ulcers in rats. Eur. J. Pharmacol. 2005, 508, 211–221. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Konturek, S.J.; Ceranowicz, P.; Dembinski, M.; Pawlik, W.W.; Kusnierz-Cabala, B.; Naskalski, J.W. Extract of grapefruit-seed reduces acute pancreatitis induced by ischemia/reperfusion in rats: Possible implication of tissue antioxidants. J. Physiol. Pharmacol. 2004, 55, 811–821. [Google Scholar] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Drozdowicz, D.; Konturek, S.J.; Zayachivska, O.; Pajdo, R.; Kwiecien, S.; Pawlik, W.W.; Hahn, E.G. Grapefruit-seed extract attenuates ethanol-and stress-induced gastric lesions via activation of prostaglandin, nitric oxide and sensory nerve pathways. World J. Gastroenterol. 2005, 11, 6450–6458. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Advances in Experimental Medicine and Biology; Lyte, M., Cryan, J., Eds.; Springer: New York, NY, USA, 2014; pp. 39–71. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut--their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.S.; Lee, J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013, 137, 136–141. [Google Scholar] [CrossRef]

| Strains | Antibiotic Resistance (MIC) (mg/L) | Total Resistance | |||||
|---|---|---|---|---|---|---|---|
| AMX | CLR | RIF | LVX | TET | MTZ | ||
| Hp44 | S (0.023) | S (0.125) | S (0.38) | S (0.125) | S (0.125) | S (0.19) | 0/6 |
| Hp48 | S (0.094) | S (<0.016) | S (0.5) | S (0.25) | S (0.25) | R (192) | 1/6 |
| Hp53 | R (0.19) | R (4) | R (4) | S (0.125) | S (0.023) | S (0.75) | 3/6 |
| Hp58 | R (1.5) | R (6) | S (0.75) | R (>32) | S (0.064) | R (96) | 4/6 |
| Hp59 | S (0.023) | S (0.023) | S (1) | S (0.19) | S (0.125) | R (64) | 1/6 |
| Hp61 | S (0.023) | R (12) | R (4) | S (0.38) | S (0.125) | R (>235) | 3/6 |
| Resistant strains | 2/6 | 3/6 | 2/6 | 1/6 | 0/6 | 4/6 | |
| Strains | cagA | cagPAI Essential Genes | vacA Alleles | babA (Alleles) | babB | babC | sabB | oipA (on) |
|---|---|---|---|---|---|---|---|---|
| Hp44 | yes | No cagX, cagV | s1-m2-i1-d1-c2 | yes (A1) | yes | no | yes | yes |
| Hp48 | yes | yes | s1-m2-i2-d2-c2 | yes (A2) | no | no | no | yes |
| Hp53 | no | no | s2-m2-i2-d2-c2 | no | yes | no | no | no |
| Hp58 | no | no | s2-m2-i2-d2-c2 | no | yes | no | no | no |
| Hp59 | yes | yes | s1-m1-i1-d1-c1 | yes (A1) | no | yes | yes | yes |
| Hp61 | yes | yes | s2-m2-i2-d2-c2 | yes (A2) | yes | no | no | yes |
| Strains | Control | GSE | PPC Fraction | OPC Fraction |
|---|---|---|---|---|
| AGS cells | 148.3 ± 11.9 A-a | 156.6 ± 5.3 a | 152.5 ± 10.1 a | 166.6 ± 8.4 a |
| Hp44 | 841.6 ± 52.3 D-d | 295.8 ± 0.9 c (64.9%) | 144.7 ± 11.8 a (82.8%) | 239.2 ± 8.7 b (71.6%) |
| Hp48 | 1525.8 ± 174.4 E-c | 352.5 ± 37.0 b (76.9%) | 255.3 ± 1.6 a (83.3%) | 431.1 ± 10.7 b (71.7%) |
| Hp53 | 445.5 ± 40.5 C-c | 140.6 ± 1.2 a (68.4%) | 149.4 ± 4.4 a (66.5%) | 349.2 ± 0.9 b (21.6%) |
| Hp58 | 196.6 ± 11.7 A-b | 90.0 ± 6.7 a (54.2%) | 94.7 ± 24.4 a (51.8%) | 95.3 ± 11.1 a (51.5%) |
| Hp59 | 1478.1 ± 44.8 E-d | 300.8 ± 62.9 b (79.6%) | 180.8 ± 26.0 a (87.8%) | 510.8 ± 46.0 c (65.4%) |
| Hp61 | 345.8 ± 17.4 B,C-b | 127.5 ± 15.5 a (63.1%) | 164.4 ± 22.5 a (52.5%) | 149.2 ± 11.9 a (56.9%) |
| Strains | Control Growth | GSE | PPC Fraction | OPC Fraction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 mg/mL | log cfu/mL Reduction | MIC (mg/mL) | 2 mg/mL | log CFU/mL Reduction | MIC (mg/mL) | 2 mg/mL | log CFU/mL Reduction | MIC (mg/mL) | ||
| Hp44 | 7.85 ± 0.10 d | 4.98 ± 0.04 a | 2.87 | 0.075 | 5.66 ± 0.03 b | 2.19 | 0.075 | 5.97 ± 0.08 c | 1.88 | 0.25 |
| Hp48 | 7.69 ± 0.06 d | 1.90 ± 0.35 a | 5.79 | 0.5 | 4.62 ± 0.07 b | 3.07 | 0.05 | 6.05 ± 0.01 c | 1.64 | 0.25 |
| Hp53 | 7.44 ± 0.10 d | 2.16 ± 0.04 a | 5.28 | 0.075 | 3.88 ± 0.03 b | 3.56 | 0.05 | 4.84 ± 0.04 c | 2.60 | 0.05 |
| Hp58 | 8.94 ± 0.11 d | 4.90 ± 0.07 b | 4.04 | 1.5 | 4.05 ± 0.02 a | 4.89 | 0.05 | 5.45 ± 0.07 c | 3.49 | 0.1 |
| Hp59 | 7.73 ± 0.09 d | 3.94 ± 0.03 b | 3.79 | 0.5 | 3.38 ± 0.09 a | 4.35 | 0.1 | 6.49 ± 0.04 c | 1.24 | 0.5 |
| Hp61 | 7.56 ± 0.04 c | 4.32 ± 0.09 b | 3.24 | 0.075 | 3.27 ± 0.20 a | 4.29 | 0.1 | 4.36 ± 0.03 b | 3.20 | 1.5 |
| Analytical Parameters | GSE | PPC Fraction | OPC Fraction |
| Total phenolic (TPh) | 25.1 ± 0.5 | 34.9 ± 0.5 | 49.5 ± 1.3 |
| Total procyanidin (TPC) | 8.5 ± 0.3 | 14.6 ± 0.5 | 12.9 ± 0.4 |
| Total carbohydrate (TCH) | 10.5 ± 0.2 | 3.6 ± 0.2 | 0.36 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvan, J.M.; Gutierrez-Docio, A.; Guerrero-Hurtado, E.; Domingo-Serrano, L.; Blanco-Suarez, A.; Prodanov, M.; Alarcon-Cavero, T.; Martinez-Rodriguez, A.J. Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter pylori Infection in Human Gastric Epithelial Cells. Antioxidants 2021, 10, 943. https://doi.org/10.3390/antiox10060943
Silvan JM, Gutierrez-Docio A, Guerrero-Hurtado E, Domingo-Serrano L, Blanco-Suarez A, Prodanov M, Alarcon-Cavero T, Martinez-Rodriguez AJ. Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter pylori Infection in Human Gastric Epithelial Cells. Antioxidants. 2021; 10(6):943. https://doi.org/10.3390/antiox10060943
Chicago/Turabian StyleSilvan, Jose Manuel, Alba Gutierrez-Docio, Esperanza Guerrero-Hurtado, Lucia Domingo-Serrano, Ana Blanco-Suarez, Marin Prodanov, Teresa Alarcon-Cavero, and Adolfo J. Martinez-Rodriguez. 2021. "Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter pylori Infection in Human Gastric Epithelial Cells" Antioxidants 10, no. 6: 943. https://doi.org/10.3390/antiox10060943
APA StyleSilvan, J. M., Gutierrez-Docio, A., Guerrero-Hurtado, E., Domingo-Serrano, L., Blanco-Suarez, A., Prodanov, M., Alarcon-Cavero, T., & Martinez-Rodriguez, A. J. (2021). Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter pylori Infection in Human Gastric Epithelial Cells. Antioxidants, 10(6), 943. https://doi.org/10.3390/antiox10060943








