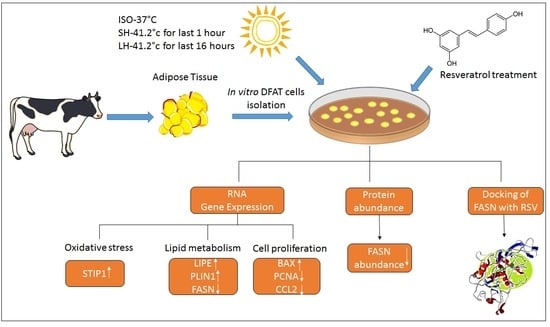
Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Tissue Samples
2.2. Isolation of Mature Adipocyte and Induction of Dedifferentiated Adipocyte-Derived Progeny (DFAT) Cells
2.3. Treatment of DFAT Cells with Resveratrol (RSV)
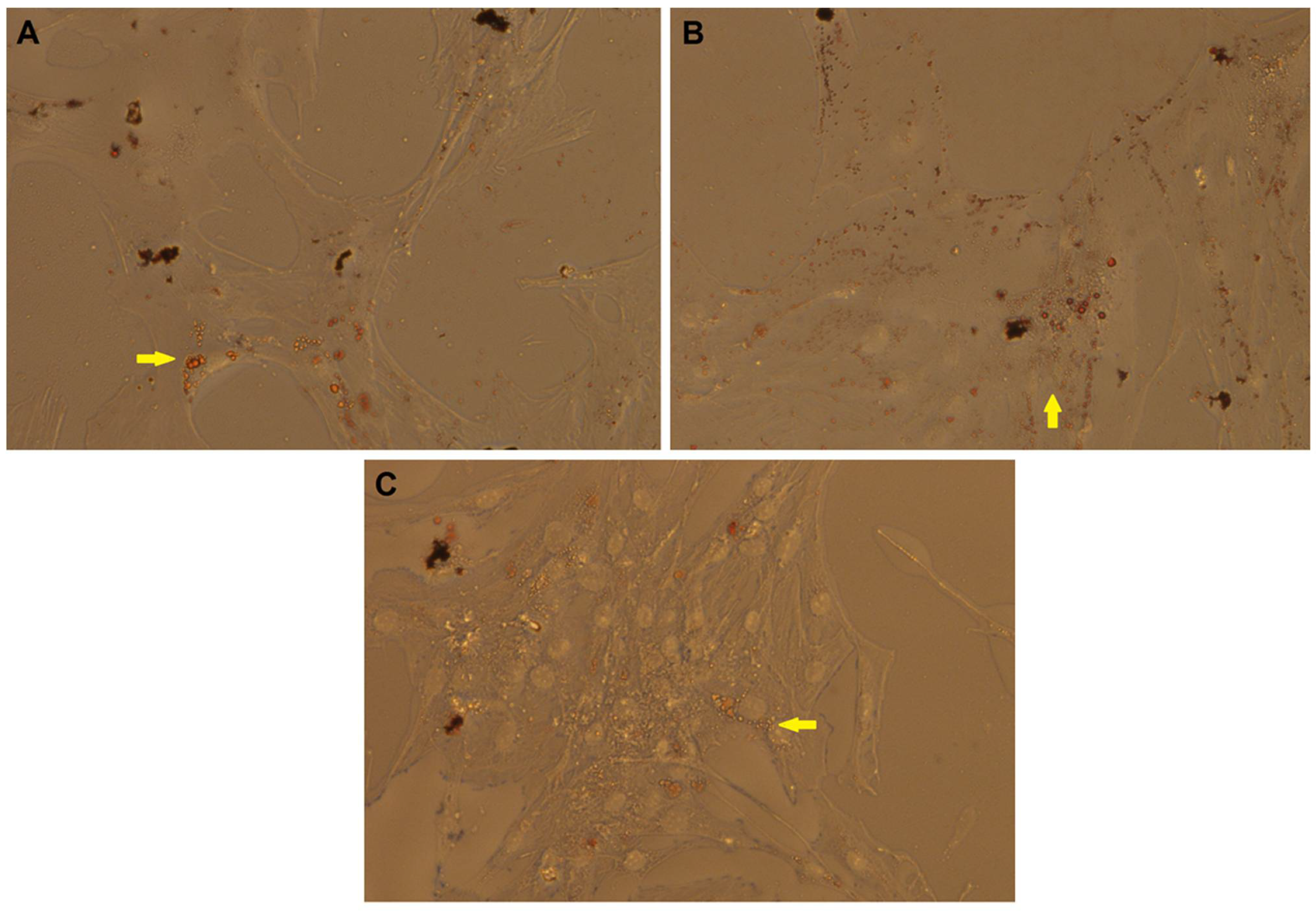
2.4. Morphological Observation and Oil Red O (ORO) Staining of DFAT Cells
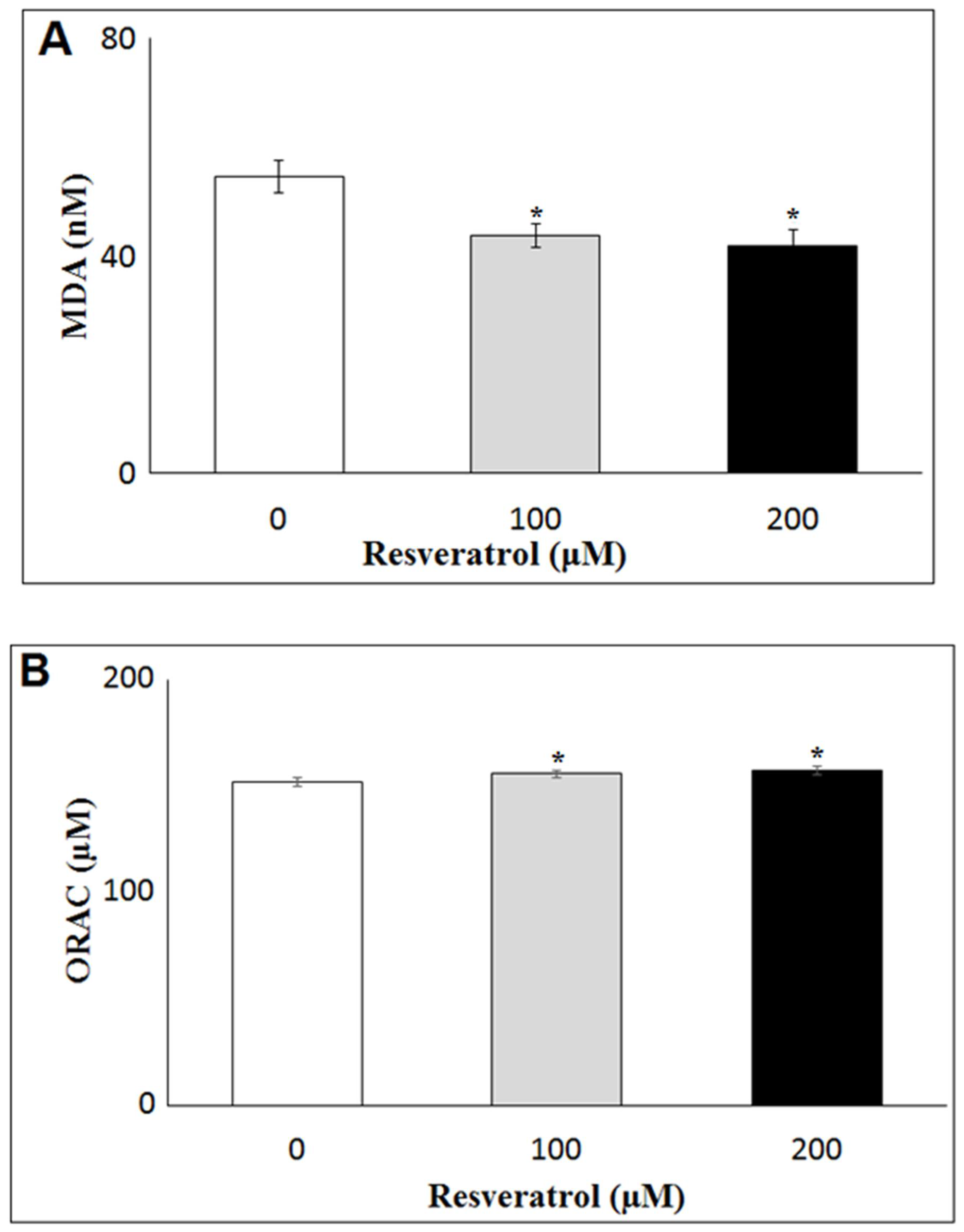
2.5. Antioxidant Activity of RSV
2.5.1. Lipid Peroxidation Assay (TBARS)
2.5.2. Oxygen Radical Absorbance Capacity (ORAC)
2.6. Resveratrol Effects on the DFAT Cells under Different Thermal Treatments
2.7. Extraction and Quantification of RNA by RT- PCR from Treated DFAT Cells
2.8. Immunoblot Analysis of FASN
2.9. In Silico Inhibitory Studies of RSV
2.9.1. Homology Modeling and Active Site Prediction of FASN
2.9.2. Docking Studies with Resveratrol
2.10. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Resveratrol (RSV) in DFAT Cells
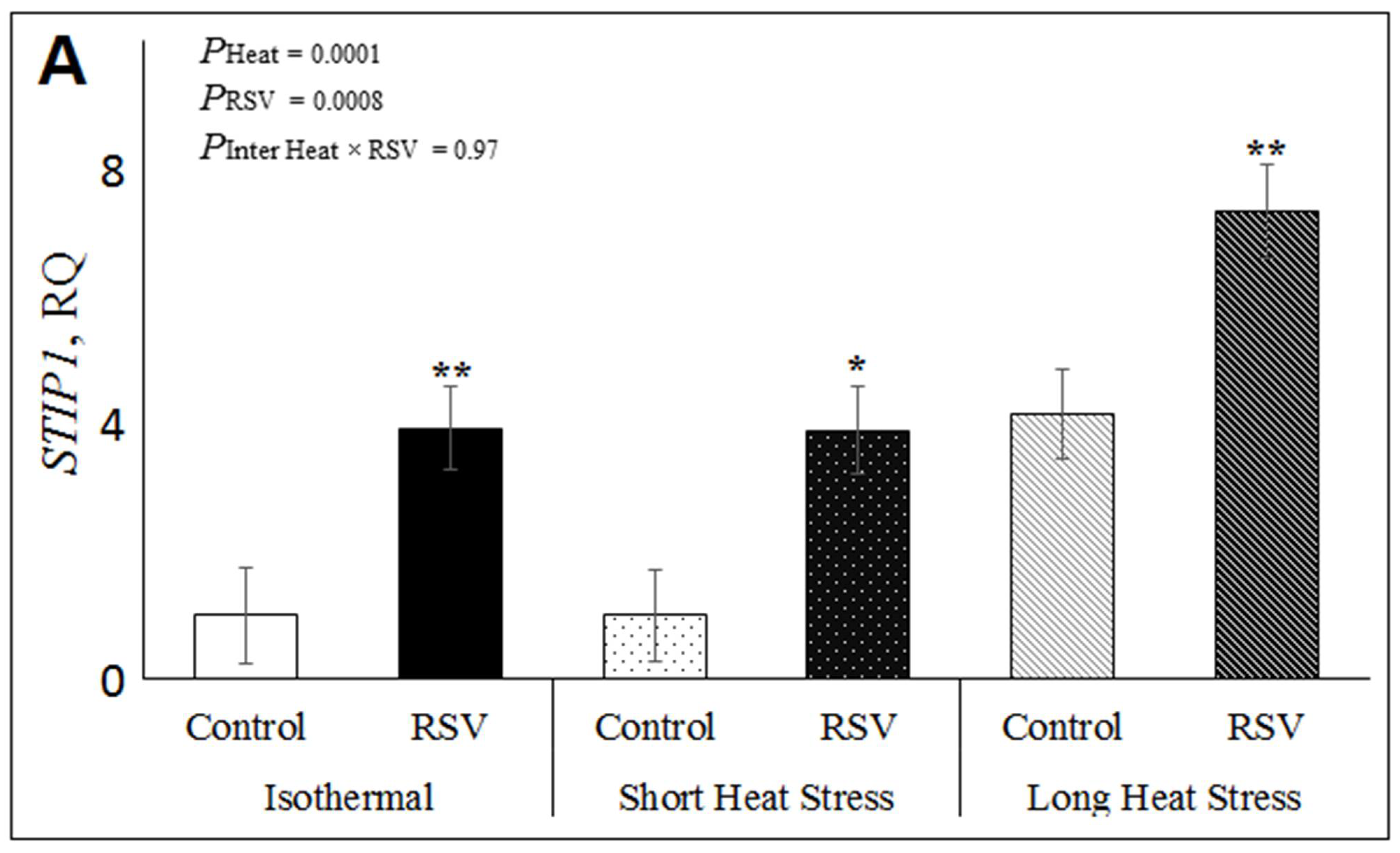
3.2. Effect of Resveratrol, Heat Treatments and Their Interaction on mRNA Expression Related to Oxidative Stress in Bovine DFAT Cells
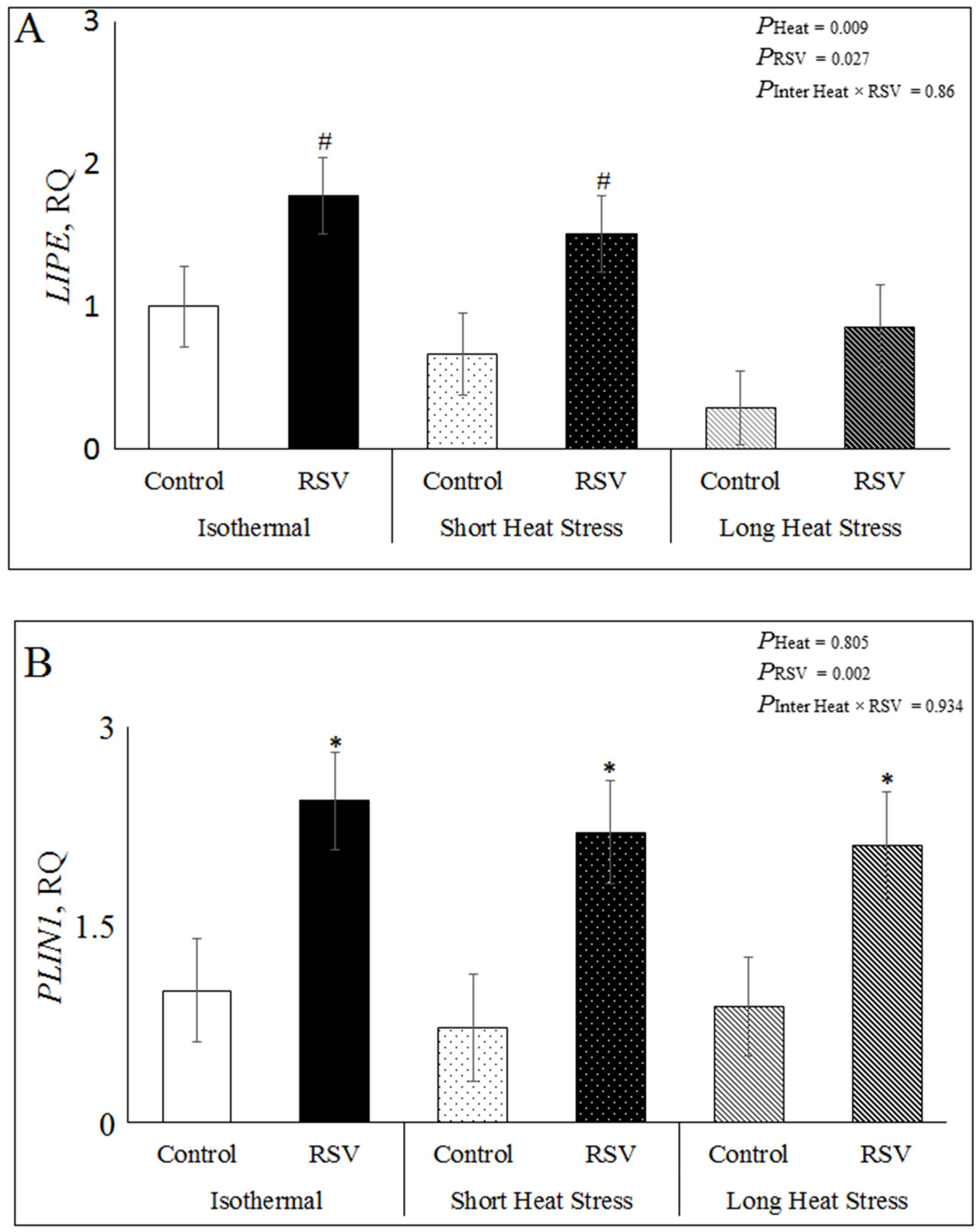
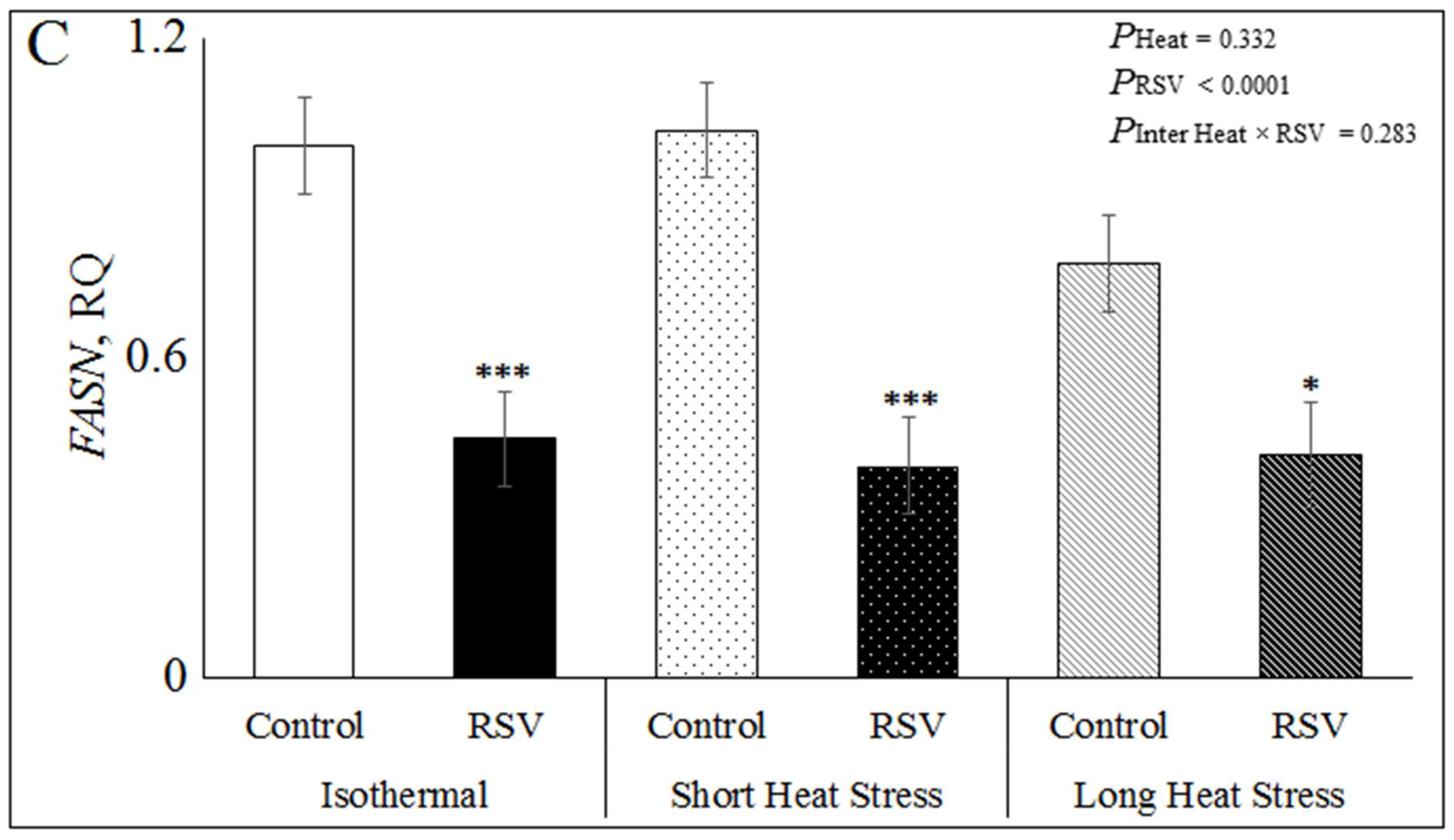
3.3. Effect of Resveratrol, Heat Treatments and Their Interaction on mRNA Expression Related to Lipid Metabolism in Bovine DFAT Cells
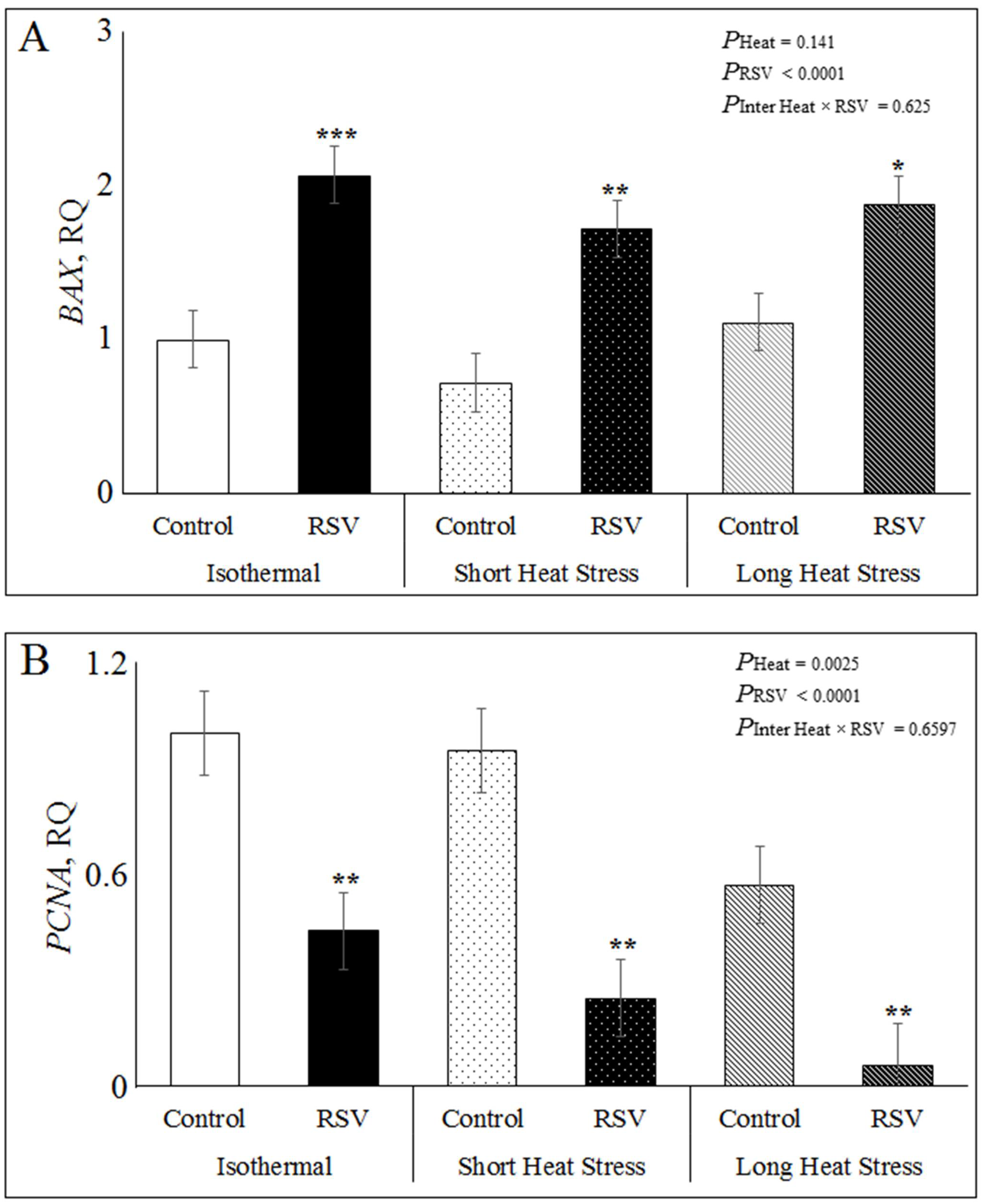
3.4. Effect of Resveratrol, Heat Treatments and Their Interaction on mRNA Expression Related to Apoptosis, Inflammation and SIRT1 Signaling in Bovine DFAT Cells#
3.5. Protein Abundance of FASN in DFAT Cells Treated with RSV under In Vitro HS Conditions
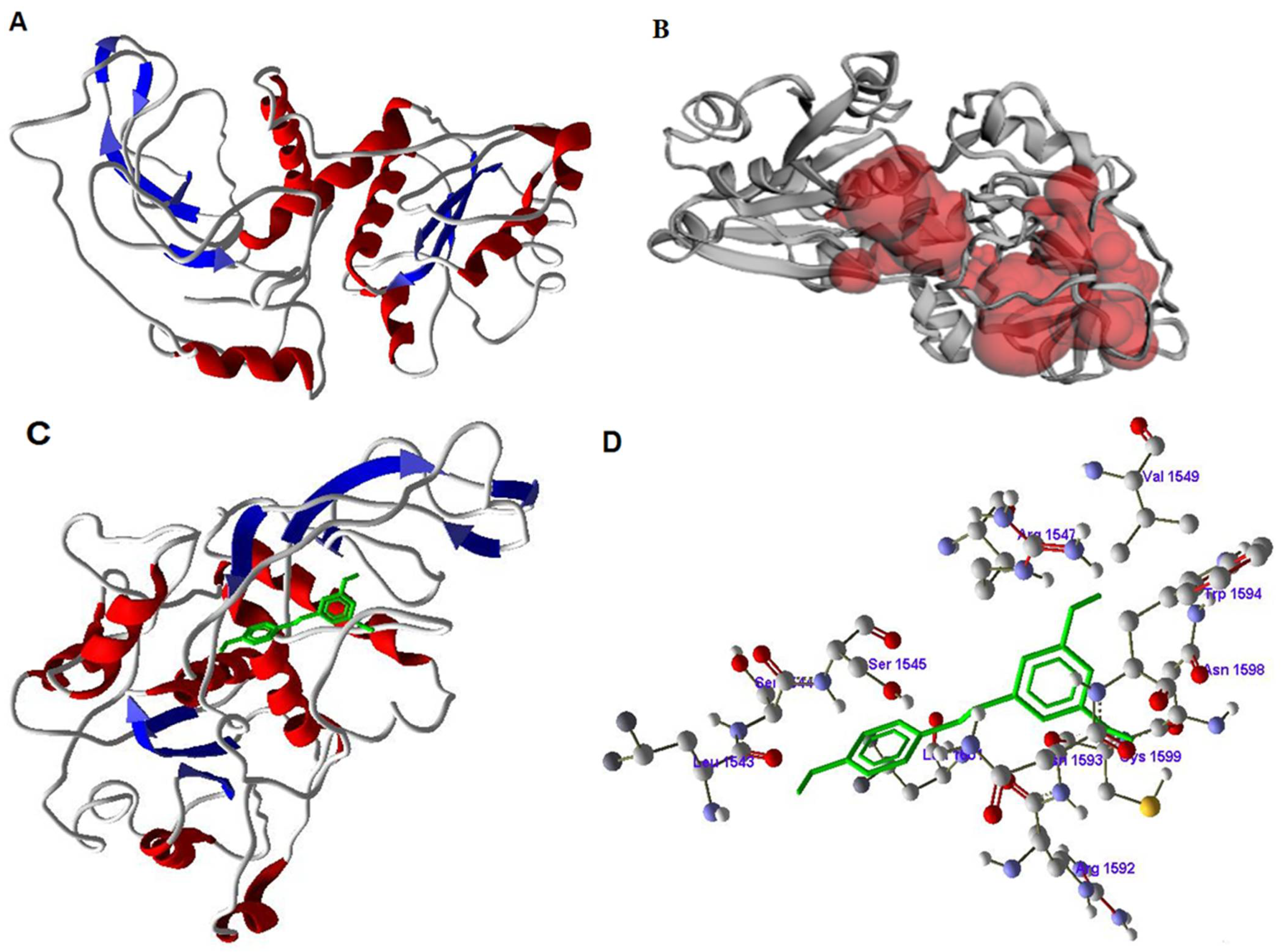
3.6. Inhibitory Activity of Resveratrol on FASN by Docking Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Roth, Z. Effect of heat stress on reproduction in dairy cows: Insights into the cellular and molecular responses of the oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Kra, G.; Livshitz, L.; Portnick, Y.; Yakoby, S.; Friedlander, G.; Levin, Y. Seasonal heat stress affects adipose tissue proteome toward enrichment of the Nrf2-mediated oxidative stress response in late-pregnant dairy cows. J. Proteom. 2017, 158, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Yamashita, R.; Okita, M.; Yoshitoshi, R.; Sugino, T.; Obitsu, T.; Kawamura, K. A comparison of plasma glucose and oxidative status in lactating dairy cows in summer and autumn. Anim. Sci. J. 2016, 87, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function1. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef]
- Zachut, M.; Kra, G.; Nemes-Navon, N.; Ben-Aharon, N.; Moallem, U.; Lavon, Y.; Jacoby, S. Seasonal heat load is more potent than the degree of body weight loss in dysregulating immune function by reducing white blood cell populations and increasing inflammation in Holstein dairy cows. J. Dairy Sci. 2020, 103, 10809–10822. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Alkadi, H. A review on free radicals and antioxidants. Infect. Disord. Drug Targets 2018, 20, 16–26. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Anbudhasan, P.; Surendraraj, A.; Karkuzhali, S.; Sathishkumaran, P. Natural antioxidants and its benefits. Int. J. Food Nutr. Sci. 2014, 3, 225–232. [Google Scholar]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Raal, A.; Pokk, P.; Arend, A.; Aunapuu, M.; Jõgi, J.; Okva, K.; Püssa, T. Trans-resveratrol alone and hydroxystilbenes of rhubarb (Rheum rhaponticum L.) root reduce liver damage induced by chronic ethanol administration: A comparative study in mice. Phytother. Res. 2009, 23, 525–532. [Google Scholar] [CrossRef]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; Van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhao, X.; Fu, J.; Wang, X. Resveratrol increase myocardial Nrf2 expression in type 2 diabetic rats and alleviate myocardial ischemia/reperfusion injury (MIRI). Ann. Palliat. Med. 2019, 8, 565–575. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fu, Q.; Song, X.; Muhammad, A.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Ye, G.; et al. Preparation of resveratrol dry suspension and its immunomodulatory and anti-inflammatory activity in mice. Pharm. Biol. 2020, 58, 8–15. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2019, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B.M. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef] [PubMed]
- Cieślik-Boczula, K. Influence of resveratrol on interactions between negatively charged DPPC/DPPG membranes and positively charged poly-l-lysine. Chem. Phys. Lipids 2018, 214, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Chuah, L.H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef]
- Park, S.H.; Gammon, S.R.; Knippers, J.D.; Paulsen, S.R.; Rubink, D.S.; Winder, W.W.; Rubink, D.S.; Phosphoryla, W.W. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J. Appl. Physiol. 2021, 84602, 2475–2482. [Google Scholar] [CrossRef]
- Faylon, M.P.; Baumgard, L.H.; Rhoads, R.P.; Spurlock, D.M. Effects of acute heat stress on lipid metabolism of bovine primary adipocytes. J. Dairy Sci. 2015, 98, 8732–8740. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Mann, S.; Nydam, D.V.; Abuelo, A.; Yepes, F.A.L.; Overton, T.R.; Wakshlag, J.J. Insulin signaling, inflammation, and lipolysis in subcutaneous adipose tissue of transition dairy cows either overfed energy during the prepartum period or fed a controlled-energy diet. J. Dairy Sci. 2016, 99, 6737–6752. [Google Scholar] [CrossRef]
- Wei, S.; Du, M.; Jiang, Z.; Duarte, M.S.; Fernyhough-Culver, M.; Albrecht, E.; Will, K.; Zan, L.; Hausman, G.J.; Elabd, E.M.Y.; et al. Bovine dedifferentiated adipose tissue (DFAT) cells. Adipocyte 2013, 2, 148–159. [Google Scholar] [CrossRef]
- Kinkel, A.D.; Fernyhough, M.E.; Helterline, D.L.; Vierck, J.L.; Oberg, K.S.; Vance, T.J.; Hausman, G.J.; Hill, R.A.; Dodson, M.V. Oil red-O stains non-adipogenic cells: A precautionary note. Cytotechnology 2004, 46, 49–56. [Google Scholar] [CrossRef]
- Fernyhough, M.E.; Hausman, G.J.; Dodson, M.V. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs 2008, 188, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Daddam, J.R.; Hammon, H.M.; Tröscher, A.; Vogel, L.; Gnott, M.; Kra, G.; Levin, Y.; Sauerwein, H.; Zachut, M. Phosphoproteomic analysis of subcutaneous and omental adipose tissue reveals increased lipid turnover in dairy cows supplemented with conjugated linoleic acid. Int. J. Mol. Sci. 2021, 22, 3227. [Google Scholar] [CrossRef]
- Harathi, N.; Reddy, S.; Sura, M.; Daddam, J.R. Structure prediction, molecular simulations of RmlD from Mycobacterium tuberculosis, and interaction studies of Rhodanine derivatives for anti-tuberculosis activity. J. Mol. Model. 2021, 27, 75. [Google Scholar] [CrossRef]
- Daddam, J.R.; Sreenivasulu, B.; Peddanna, K.; Umamahesh, K. Designing, docking and molecular dynamics simulation studies of novel cloperastine analogues as anti-allergic agents: Homology modeling and active site prediction for the human histamine H1 receptor. RSC Adv. 2020, 10, 4745–4754. [Google Scholar] [CrossRef]
- Daddam, J.R.; Sreenivasulu, B.; Umamahesh, K.; Peddanna, K.; Rao, D.M. In silico studies on anti-stress compounds of ethanolic root extract of Hemidesmus indicus L. Curr. Pharm. Biotechnol. 2019, 21, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Daddam, J.R.; Dowlathabad, M.R.; Panthangi, S.; Jasti, P. Molecular docking and P-glycoprotein inhibitory activity of flavonoids. Interdiscip. Sci. Comput. Life Sci. 2014, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Pakkkianathan, B.C.; Kumar, M.; Daddam, J.R.; Jayavel, S.; Kannan, M.; Pillai, G.G.; Krishnan, M. Computational studies on molecular interactions of 6-thioguanosine analogs with anthrax toxin receptor 1. Interdiscip. Sci. Comput. Life Sci. 2012, 4, 183–189. [Google Scholar] [CrossRef]
- Kurjogi, M.; Satapute, P.; Jogaiah, S.; Abdelrahman, M.; Daddam, J.R.; Ramu, V.; Tran, L.-S.P. Computational modeling of the Staphylococcal enterotoxins and their interaction with natural antitoxin compounds. Int. J. Mol. Sci. 2018, 19, 133. [Google Scholar] [CrossRef]
- Papathoti, N.K.; Saengchan, C.; Daddam, J.R.; Thongprom, N.; Tonpho, K.; Thanh, T.L.; Buensanteai, N. Plant systemic acquired resistance compound salicylic acid as a potent inhibitor against SCF (SKP1-CUL1-F-box protein) mediated complex in Fusarium oxysporum by homology modeling and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2020, 1–8. [Google Scholar] [CrossRef]
- Sahin, K.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Hayirli, A.; Sahin, N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 2010, 89, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P. Ruminant nutrition symposium: Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 2012, 90, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Akiba, Y.; Toyomizu, M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007, 86, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.T.; Marshall, S.; Cutajar, J.; Annois, B.; Pipingas, A.; Tierney, A.; Itsiopoulos, C. Effect of resveratrol supplementation on cognitive performance and mood in adults: A systematic literature review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Dybkowska, E.; Sadowska, A.; Świderski, F.; Rakowska, R.; Wysocka, K. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. A review. Rocz. Panstw. Zakl. Hig. 2018, 69, 5–14. [Google Scholar] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Kwiecien, S.; Jasnos, K.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Brzozowski, B.; Mach, T.; Wojcik, D.; Brzozowski, T. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress-induced gastric injury. J. Physiol. Pharmacol. 2014, 65, 613–622. [Google Scholar] [PubMed]
- Renzo, L.D.; Carraro, A.; Valente, R.; Iacopino, L.; Colica, C.; Lorenzo, A.D. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: A randomized crossover trial. Oxid. Med. Cell. Longev. 2014, 2014, 681318. [Google Scholar] [CrossRef]
- Odunuga, O.O.; Longshaw, V.M.; Blatch, G.L. Hop: More than an Hsp70/Hsp90 adaptor protein. Bioessays 2004, 26, 1058–1068. [Google Scholar] [CrossRef]
- Nicolet, C.M.; Craig, E.A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol. Cell. Biol. 1989, 9, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Sea, K.; Sohn, S.H.; Durazo, A.; Sheng, Y.; Shaw, B.F.; Cao, X.; Taylor, A.B.; Whitson, L.J.; Holloway, S.P.; Hart, P.J.; et al. Insights into the role of the unusual disulfide bond in copper-zinc superoxide dismutase. J. Biol. Chem. 2015, 290, 2405–2418. [Google Scholar] [CrossRef]
- Koltes, D.A.; Spurlock, D.M. Coordination of lipid droplet-associated proteins during the transition period of Holstein dairy cows. J. Dairy Sci. 2011, 94, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Baile, C.A.; Yang, J.Y.; Rayalam, S.; Hartzell, D.L.; Lai, C.Y.; Andersen, C.; Della-Fera, M.A. Effect of resveratrol on fat mobilization. Ann. N. Y. Acad. Sci. 2011, 1215, 40–47. [Google Scholar] [CrossRef]
- Alberdi, G.; Rodríguez, V.M.; Miranda, J.; Macarulla, M.T.; Arias, N.; Andrés-Lacueva, C.; Portillo, M.P. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr. Metab. 2011, 8, 29. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Allemailem, K.S.; Alsahli, M.A.; Khan, S.; Alruwetei, A.M.; Khan, M.A. Fatty acid synthase (FASN) sirna-encapsulatedher-2 targeted fab’-immunoliposomes for gene silencing in breast cancer cells. Int. J. Nanomed. 2020, 15, 5575–5589. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; Koster, J.D. Symposium review: Modulating adipose tissue lipolysis and remodeling to improve immune function during the transition period and early lactation of dairy cows. J. Dairy Sci. 2018, 101, 2737–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Chen, J.; Wu, J. Apoptosis induced by NO via phosphorylation of p38 MAPK that stimulates NF-kappaB, p53 and caspase-3 activation in rabbit articular chondrocytes. Cell Biol. Int. 2007, 31, 1027–1035. [Google Scholar] [CrossRef]
- Tudor, G.; Aguilera, A.; Halverson, D.O.; Laing, N.D.; Sausville, E.A. Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000, 7, 574–586. [Google Scholar] [CrossRef]
- Nguyen, N.U.; Stamper, B.D. Polyphenols reported to shift APAP-induced changes in MAPK signaling and toxicity outcomes. Chem. Biol. Interact. 2017, 277, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.; Mejhert, N.; Kulyté, A.; Balwierz, P.J.; Pachkov, M.; Cormont, M.; Lorente-Cebrián, S.; Ehrlund, A.; Laurencikiene, J.; Hedén, P.; et al. Adipose tissue MicroRNAs as regulators of CCL2 production in human obesity. Diabetes 2012, 61, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H. Invited review: Genes involved in the bovine heat stress response1. J. Dairy Sci. 2008, 91, 445–454. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Zhou, Y.; Wei, X.; Sun, Y.; Lan, X.; Lei, C.; Chen, H. Nicotinamide and resveratrol regulate bovine adipogenesis through a SIRT1-dependent mechanism. J. Funct. Foods 2015, 18, 492–500. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yu, W.; Fu, Y.-C.; Wang, X.; Li, J.-L.; Wang, W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1–FoxO1 pathway. Biochem. Biophys. Res. Commun. 2009, 378, 389–393. [Google Scholar] [CrossRef]
- Costa, C.D.S.; Rohden, F.; Hammes, T.O.; Margis, R.; Bortolotto, J.W.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1–3 mRNA expression in human visceral adipocytes. Obes. Surg. 2011, 21, 356–361. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Jin, Q.; You, W.; Cheng, H.; Liu, Y.; Song, E.; Liu, G.; Tan, X.; Zhang, X.; et al. Resveratrol induces apoptosis and inhibits adipogenesis by stimulating the SIRT1-AMPKα-FOXO1 signalling pathway in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2018, 439, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Stein, J. Biological activities of resveratrol and its analogs. Drugs Future 2002, 27, 949–959. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Zhang, H.; Lia, F.; Zhanga, S. Theoretical study of complexation of resveratrol with cyclodextrins and cucurbiturils: Structure and antioxidative activity. RSC Adv. 2015, 5, 14114–14122. [Google Scholar] [CrossRef]
- Yadav, R.; Srivastava, P. Establishment of resveratrol and its derivatives as neuroprotectant against monocrotophos-induced alteration in NIPBL and POU4F1 protein through molecular docking studies. Environ. Sci. Pollut. Res. 2020, 27, 291–304. [Google Scholar] [CrossRef]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sun, H.; Guo, J.; Fan, J.; Li, G.; Xu, S. Molecular mechanism of the interaction between resveratrol and trypsin: Via spectroscopy and molecular docking. Food Funct. 2019, 10, 3291–3302. [Google Scholar] [CrossRef]



| Gene | GenBank Accession No. | Sequence 5′ > 3′2 |
|---|---|---|
| BAD | NM_001035459.2 | F: GAGGATGAGCGACGAGTTTC |
| R: TCAACCAGGACTGGAGGAAG | ||
| BAX | NM_173894.1 | F: AACATGGAGCTGCAGAGGAT |
| R: CAGTTGAAGTTGCCGTCAGA | ||
| BRPS2 | NM_001033613.2 | F: GGAGCATCCCTGAAGGATGA |
| R: TCCCCGATAGCAACAAACG | ||
| CCL2 | NM_174006.2 | F: ATCTCCATGCAGAGGCTGAT |
| R: GCTTGGGGTCTGCACATAAC | ||
| FASN | NM_001012669.1 | F: ACCTCGTGAAGGCTGTGACTCA |
| R: TGAGTCGAGGCCAAGGTCTGAA | ||
| FOXO1 | XM_025000053.1 | F: TCACGCTGTCGCAGATTTAC |
| R: TGCAGGGACAGATTATGACT | ||
| FOXO3 | NM_001206083.1 | F: CAGACAAACGGCTCACTCTG |
| R: GGTTGTGCCGGATAGAGTTC | ||
| HSF1 | NM_001076809.1 | F: CCAGCAACAGAAAGTCGTCA |
| R: GCATCAGGGGGATCTTTCTC | ||
| LIPE | NM_001080220.1 | F: GAGTTTGAGCGGATCATTCA |
| R: TGAGGCCATGTTTGCTAGAG | ||
| IL1β | NM_174093.1 | F: TCCACCTCCTCTCACAGGAAA |
| R: TACCCAAGGCCACAGGAATCT | ||
| MGLL | NM_001206681.1 | F: GCAACCAGCTGCTCAACAC |
| R: AGCGTCTTGTCCTGGCTCTT | ||
| PCNA | NM_001034494.1 | F: AGGAGGAAGCTGTTGCCATA |
| R: GGAGACAGTGGAGTGGCTTT | ||
| PLIN1 | NM_001083699.1 | F: AGACACTGCCGAGTATGCTG |
| R: TGGAGGGAGGAGGAACTCTA | ||
| PPARG | NM_181024.2 | F: TGCTGTGGGGATGTCTCATA |
| R: GGTCAGCAGACTCTGGGTTC | ||
| SIRT1 | NM_001192980.3 | F: TGGCCAGCTAGACTTGCAAA |
| R: AACTTGGACTCTGGCACGTT | ||
| SOD1 | NM_174615.2 | F: CGAGGCAAAGGGAGATACAG |
| R: TCTCCAAACTGATGGACGTG | ||
| STIP1 | NM_001035492.2 | F: CTGGGGAATGAAGCCTACAA |
| R: GGCTGCTTGGTTGGTTATGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kra, G.; Daddam, J.R.; Gabay, H.; Yosefi, S.; Zachut, M. Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows. Antioxidants 2021, 10, 905. https://doi.org/10.3390/antiox10060905
Kra G, Daddam JR, Gabay H, Yosefi S, Zachut M. Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows. Antioxidants. 2021; 10(6):905. https://doi.org/10.3390/antiox10060905
Chicago/Turabian StyleKra, Gitit, Jayasimha Rayalu Daddam, Hadar Gabay, Sara Yosefi, and Maya Zachut. 2021. "Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows" Antioxidants 10, no. 6: 905. https://doi.org/10.3390/antiox10060905
APA StyleKra, G., Daddam, J. R., Gabay, H., Yosefi, S., & Zachut, M. (2021). Antioxidant Resveratrol Increases Lipolytic and Reduces Lipogenic Gene Expression under In Vitro Heat Stress Conditions in Dedifferentiated Adipocyte-Derived Progeny Cells from Dairy Cows. Antioxidants, 10(6), 905. https://doi.org/10.3390/antiox10060905