Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents
Abstract
1. Introduction
2. Material and Methods
2.1. Chemistry
2.1.1. General Information
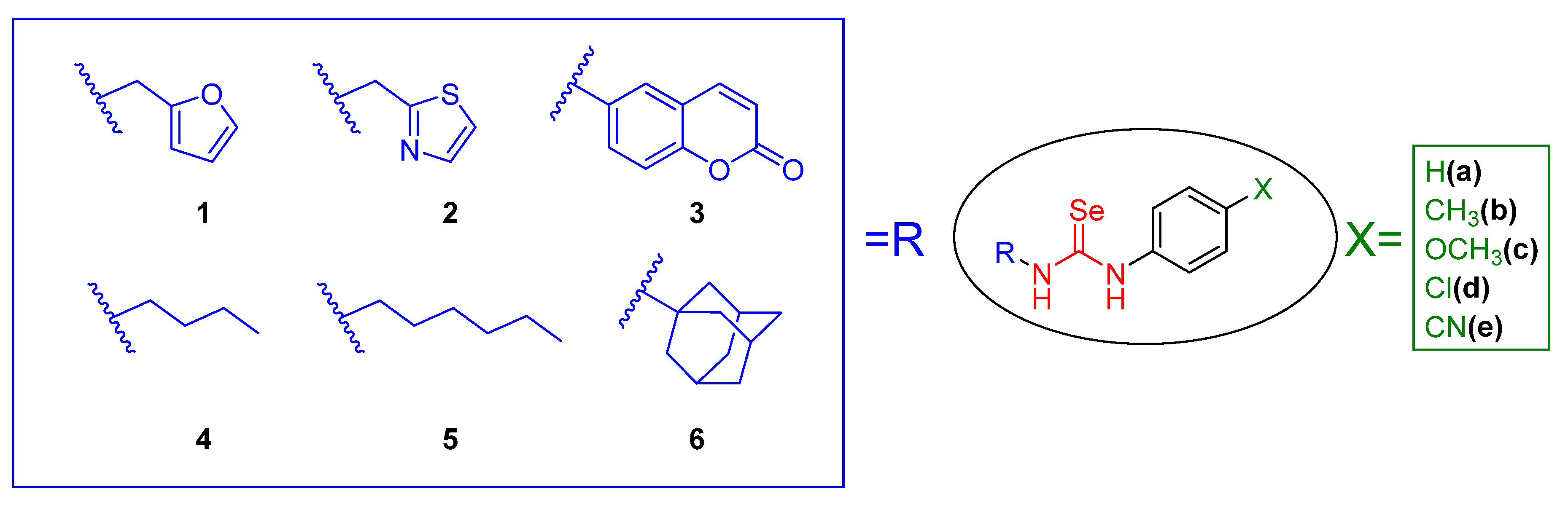
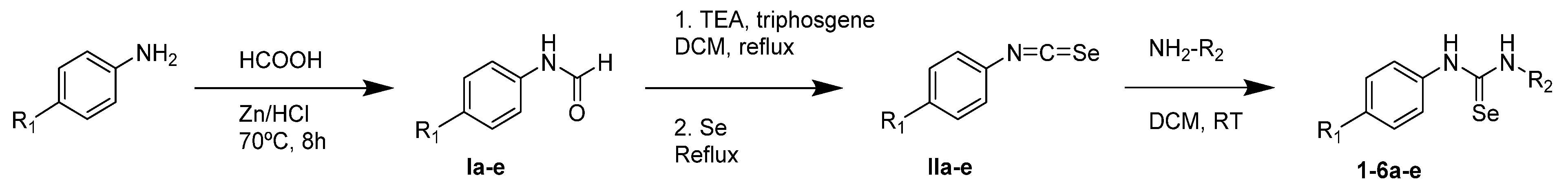
2.1.2. General Procedure for the Preparation of Selenourea Derivatives
2.2. Radical Scavenging Activity
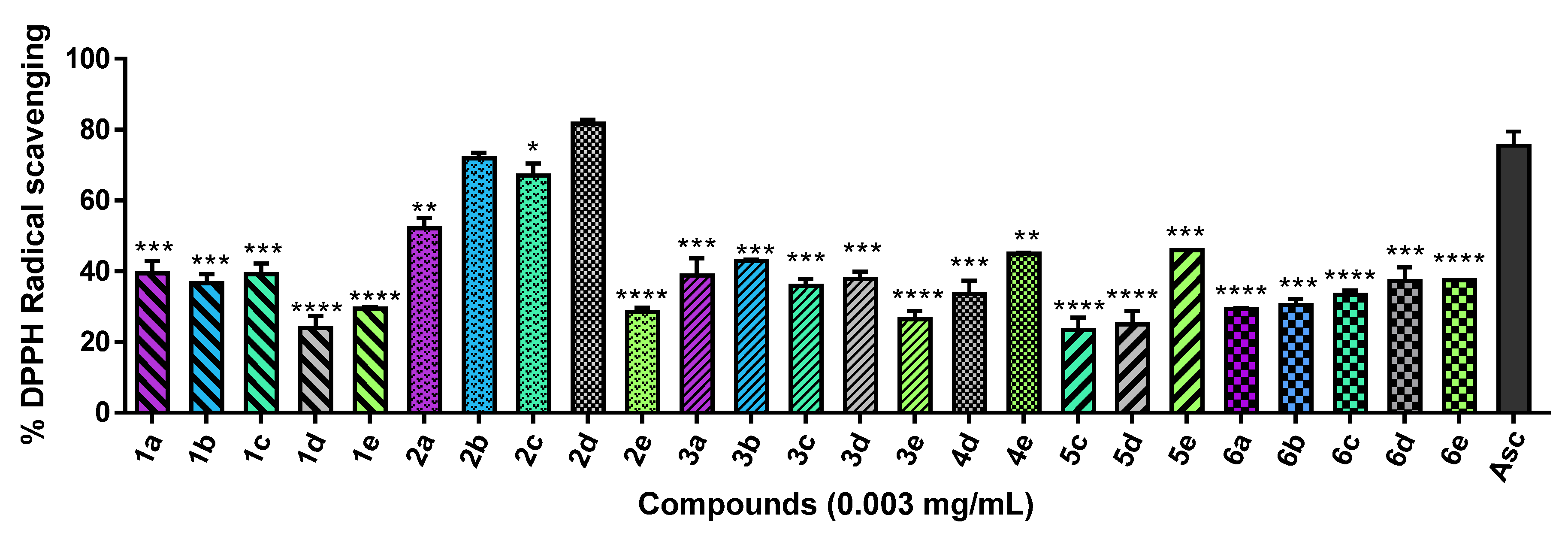
2.2.1. DPPH Radical Scavenging Assay
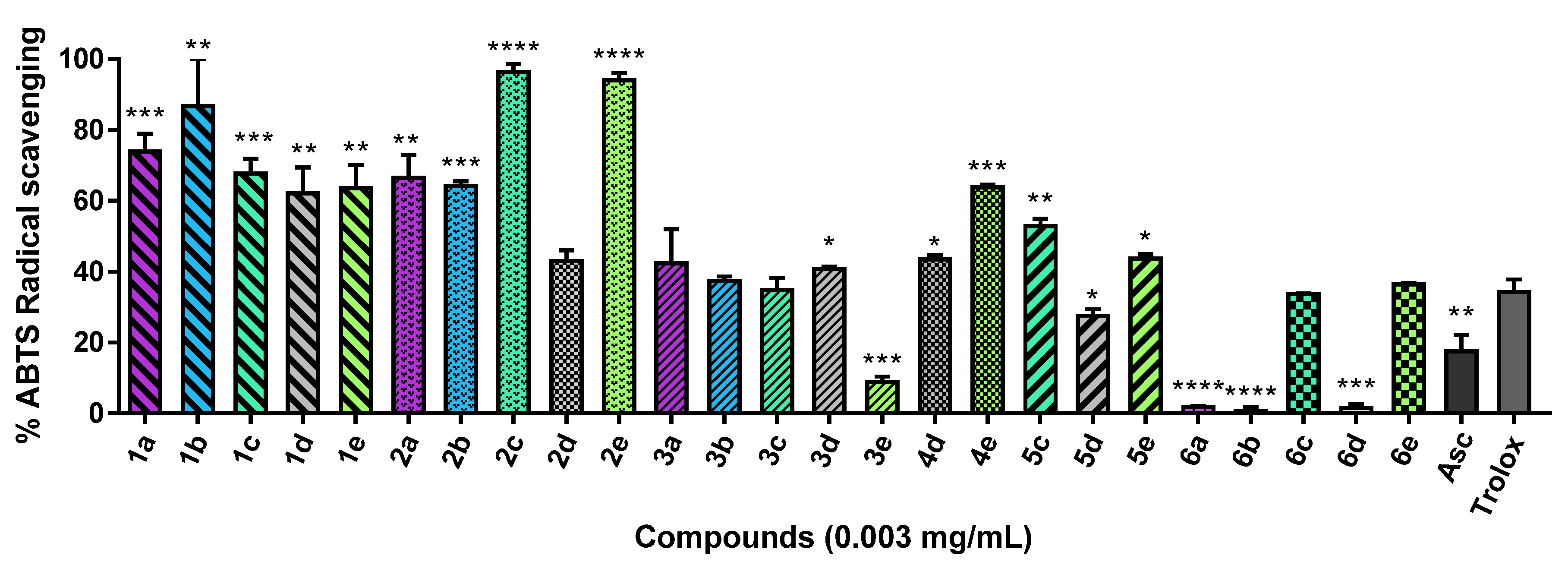
2.2.2. ABTS Radical Scavenging Assay
2.3. Biological Evaluation
2.3.1. Cell Culture Conditions
2.3.2. Cell Viability Assay
2.3.3. Evaluation of Cell Cycle Progression and Apoptosis in HT-29 Cells
3. Results and Discussion
3.1. Chemistry
3.2. Antioxidant Activity
3.2.1. DPPH Radical Scavenging Assay
3.2.2. ABTS Radical Scavenging Assay
3.3. Biological Evaluation
3.3.1. Cytotoxic Activity
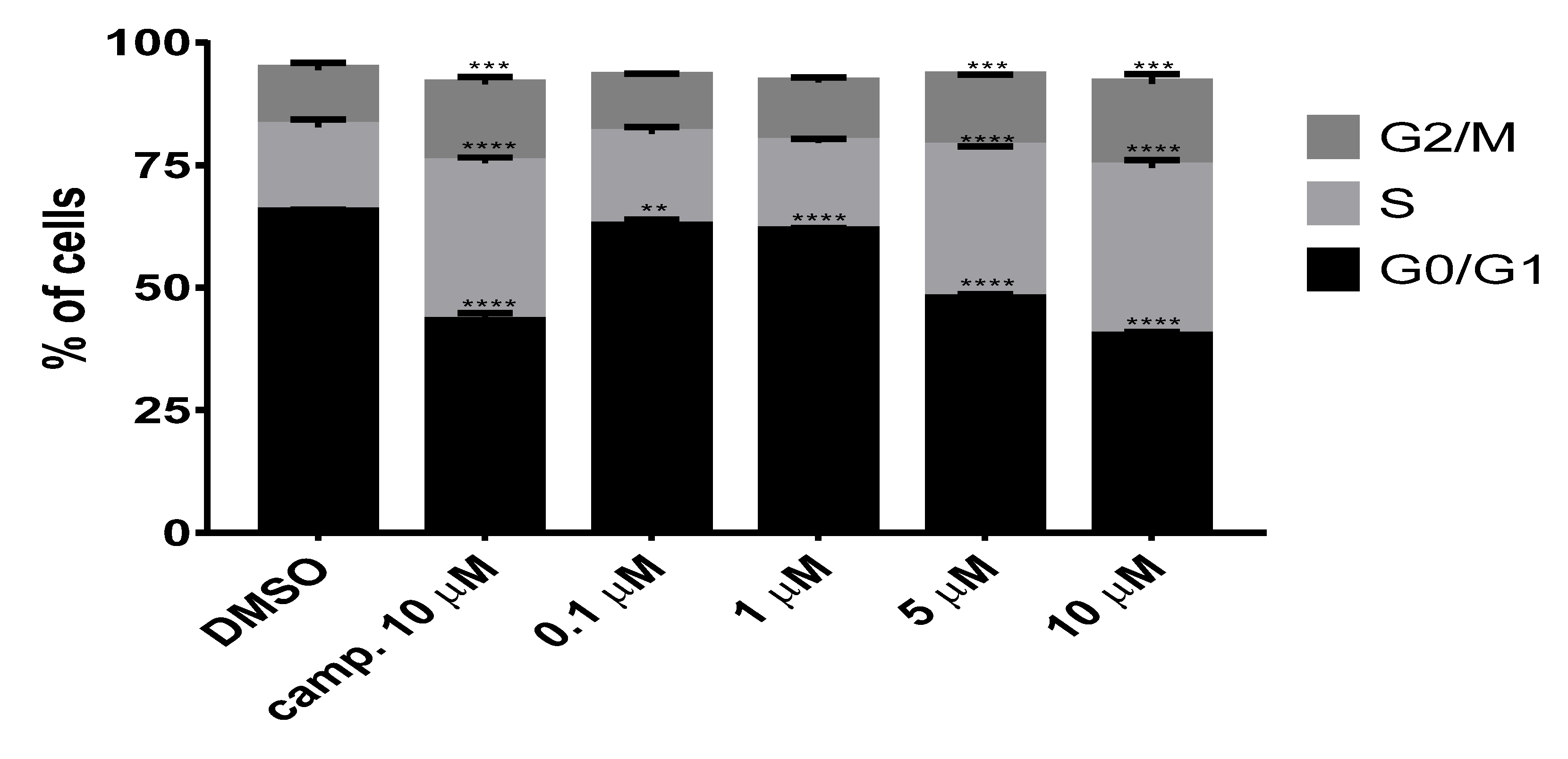
3.3.2. Cell Cycle Modulation by Selenourea 6c
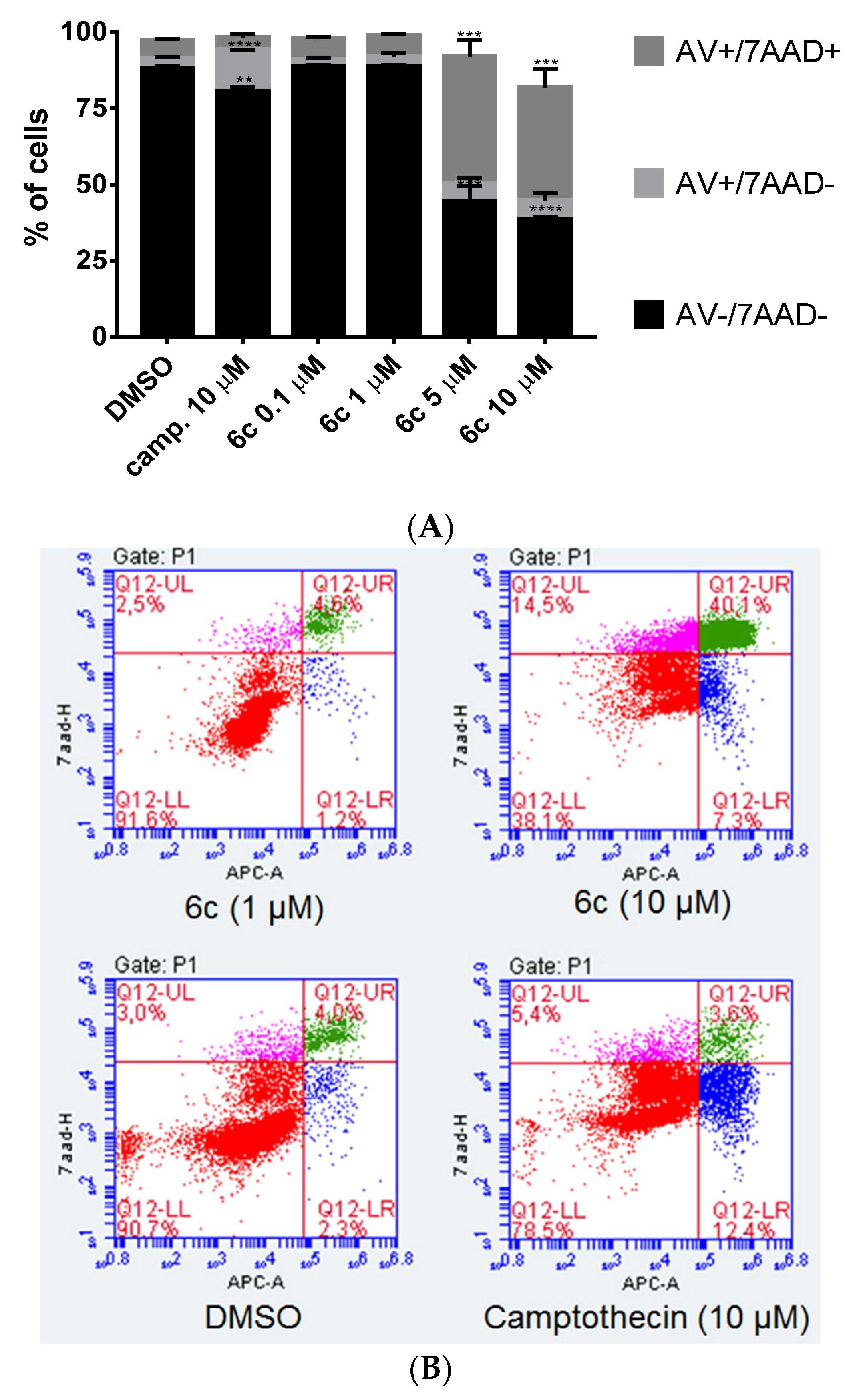
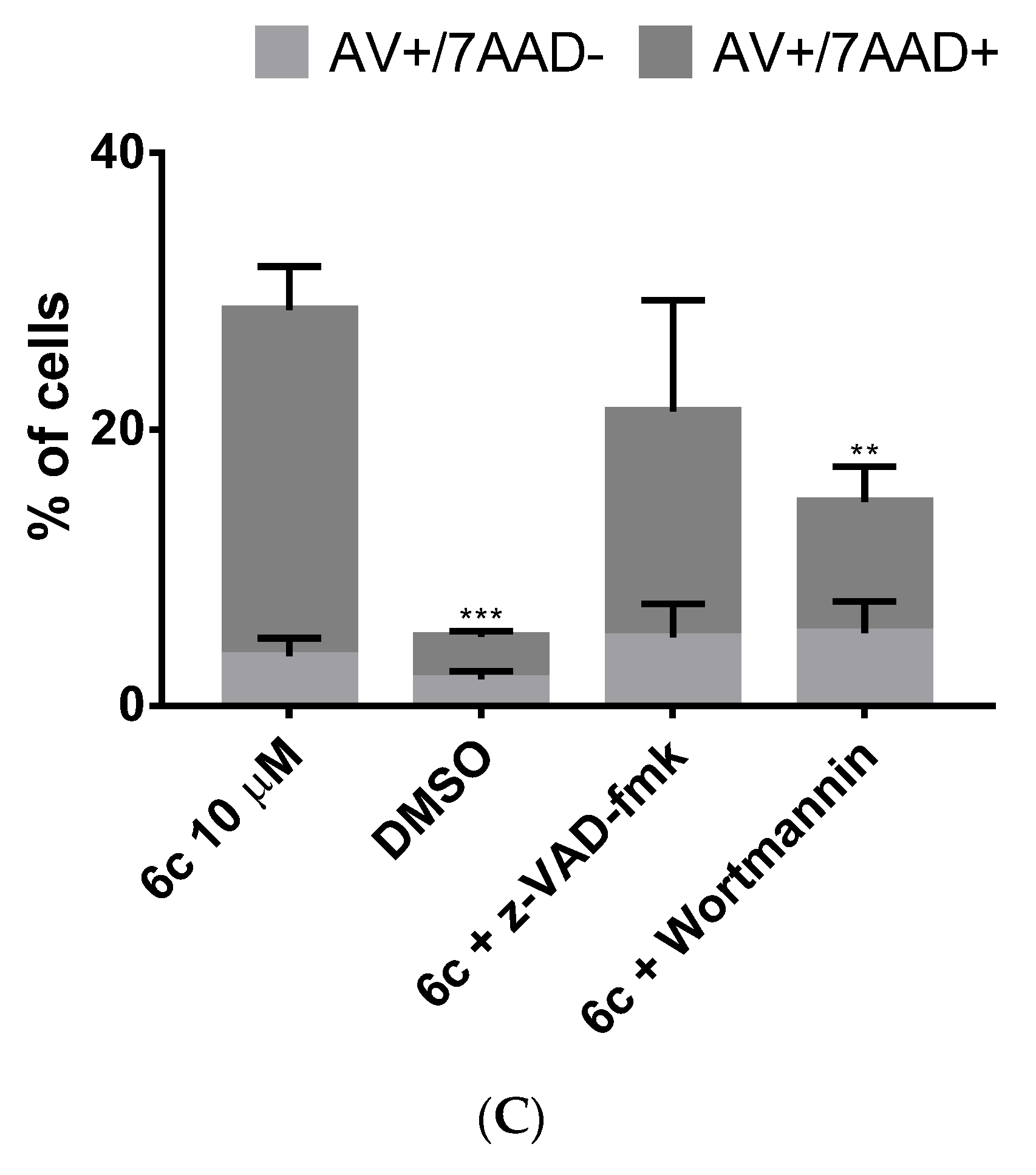
3.3.3. Cell Death Induced by 6c
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 April 2019).
- World Cancer Research Fund International Worldwide Data. Available online: https://www.wcrf.org (accessed on 30 April 2019).
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Kadkol, S.; Diamond, A.M. The Interaction between Dietary Selenium Intake and Genetics in Determining Cancer Risk and Outcome. Nutrients 2020, 12, 2424. [Google Scholar] [CrossRef]
- Diamond, A.M. Selenoproteins of the Human Prostate: Unusual Properties and Role in Cancer Etiology. Biol. Trace Elem. Res. 2019, 192, 51–59. [Google Scholar] [CrossRef]
- Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.M.; Garje, R.; Sieren, J.C.; Buettner, G.R.; Zakharia, Y. Understanding the Redox Biology of Selenium in the Search of Targeted Cancer Therapies. Antioxidants 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263. [Google Scholar] [CrossRef]
- Ekumah, J.-N.; Ma, Y.; Akpabli-Tsigbe, N.D.K.; Kwaw, E.; Ma, S.; Hu, J. Global soil distribution, dietary access routes, bioconversion mechanisms and the human health significance of selenium: A review. Food Biosci. 2021, 41, 100960. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Romero-Hernández, L.L.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Abasolo, I.; Schwartz, S., Jr.; López, Ó.; Fernández-Bolaños, J.G. Diosgenin-based thio(seleno)ureas and triazolyl glycoconjugates as hybrid drugs. Antioxidant and antiproliferative profile. Eur. J. Med. Chem. 2015, 99, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Seliman, A.A.A.; Altaf, M.; Odewunmi, N.A.; Kawde, A.-N.; Zierkiewicz, W.; Ahmad, S.; Altuwaijri, S.; Isab, A.A. Synthesis, X-ray structure, DFT calculations and anticancer activity of a selenourea coordinated gold(I)-carbene complex. Polyhedron 2017, 137, 197–206. [Google Scholar] [CrossRef]
- Barbosa, F.A.R.; Siminski, T.; Canto, R.F.S.; Almeida, G.M.; Mota, N.; Ourique, F.; Pedrosa, R.C.; Braga, A.L. Novel pyrimidinic selenourea induces DNA damage, cell cycle arrest, and apoptosis in human breast carcinoma. Eur. J. Med. Chem. 2018, 155, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, M.; Aneesrahman, K.N.; Perumalsamy, B.; Ramasamy, T.; Ganguly, R.; Sreekanth, A. Synthesis, crystal structure, DFT study, in vitro and in silico molecular docking of novel bis (aroyl selenourea) derivatives. J. Mol. Struct. 2019, 1180, 585–594. [Google Scholar] [CrossRef]
- Hussain, R.A.; Badshah, A.; Ahmed, N.; Pezzuto, J.M.; Kondratyuk, T.P.; Park, E.-J.; Hussain, I. Synthesis, characterization and biological applications of selenoureas having ferrocene and substituted benzoyl functionalities. Polyhedron 2019, 170, 12–24. [Google Scholar] [CrossRef]
- Lagunes, I.; Begines, P.; Silva, A.; Galán, A.R.; Puerta, A.; Fernandes, M.X.; Maya, I.; Fernández-Bolaños, J.G.; López, Ó.; Padrón, J.M. Selenocoumarins as new multitarget antiproliferative agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2019, 179, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, V.; Plano, D.; Karelia, D.N.; Palop, J.A.; Amin, S.; Sanmartín, C.; Sharma, A.K. Novel seleno- and thio-urea derivatives with potent in vitro activities against several cancer cell lines. Eur. J. Med. Chem. 2016, 113, 134–144. [Google Scholar] [CrossRef]
- Garnica, P.; Encío, I.; Plano, D.; Palop, J.A.; Sanmartín, C. Combined Acylselenourea-Diselenide Structures: New Potent and Selective Antitumoral Agents as Autophagy Activators. ACS Med. Chem. Lett. 2018, 9, 306–311. [Google Scholar] [CrossRef]
- Díaz, M.; de Lucio, H.; Moreno, E.; Espuelas, S.; Aydillo, C.; Jiménez-Ruiz, A.; Toro, M.; Gutiérrez, K.J.; Martínez-Merino, V.; Cornejo, A.; et al. Synthesis and Leishmanicidal Activity of Novel Urea, Thiourea, and Selenourea Derivatives of Diselenides. Antimicrob Agents Chemother 2019, 63. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N’-Disubstituted Acylselenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Perin, N.; Cindrić, M.; Vervaeke, P.; Liekens, S.; Mašek, T.; Starčević, K.; Hranjec, M. Benzazole Substituted Iminocoumarins as Potential Antioxidants with Antiproliferative Activity. Med. Chem. 2021, 17, 13–20. [Google Scholar] [CrossRef]
- Kwiecień, H.; Perużyńska, M.; Stachowicz, K.; Piotrowska, K.; Bujak, J.; Kopytko, P.; Droździk, M. Synthesis and biological evaluation of 3-functionalized 2-phenyl- and 2-alkylbenzo[b]furans as antiproliferative agents against human melanoma cell line. Bioorg. Chem. 2019, 88, 102930. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef]
- Peroković, V.P.; Car, Ž.; Usenik, A.; Opačak-Bernardi, T.; Jurić, A.; Tomić, S. Adamantyl pyran-4-one derivatives and their in vitro antiproliferative activity. Mol. Divers. 2020, 24, 253–263. [Google Scholar] [CrossRef]
- Chandra Shekhar, A.; Ravi Kumar, A.; Sathaiah, G.; Luke Paul, V.; Sridhar, M.; Shanthan Rao, P. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, A.; Desai, D.; Madhunapantula, S.V.; Huh, S.J.; Robertson, G.P.; Amin, S. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J. Med. Chem. 2008, 51, 7820–7826. [Google Scholar] [CrossRef] [PubMed]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Plano, D.; Encío, I.; Palop, J.A.; Sanmartín, C. In Vitro radical scavenging and cytotoxic activities of novel hybrid selenocarbamates. Bioorg. Med. Chem. 2015, 23, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Short, S.P.; Williams, C.S. Selenoproteins in Tumorigenesis and Cancer Progression. Adv. Cancer Res. 2017, 136, 49–83. [Google Scholar] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.A.; Badshah, A.; Pezzuto, J.M.; Ahmed, N.; Kondratyuk, T.P.; Park, E.J. Ferrocene incorporated selenoureas as anticancer agents. J. Photochem. Photobiol. B 2015, 148, 197–208. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; Maza, S.; Martos, S.; López, Ó.; Maya, I.; Fernández-Bolaños, J.G. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Plano, D.; Encío, I.; Aydillo, C.; Sharma, A.K.; Sanmartín, C. Novel selenadiazole derivatives as selective antitumor and radical scavenging agents. Eur. J. Med. Chem. 2018, 157, 14–27. [Google Scholar] [CrossRef]
- Lamberto, I.; Plano, D.; Moreno, E.; Font, M.; Palop, J.A.; Sanmartín, C.; Encío, I. Bisacylimidoselenocarbamates cause G2/M arrest associated with the modulation of CDK1 and Chk2 in human breast cancer MCF-7 cells. Curr. Med. Chem. 2013, 20, 1609–1619. [Google Scholar] [CrossRef]
- Arsenyan, P.; Paegle, E.; Domracheva, I.; Gulbe, A.; Kanepe-Lapsa, I.; Shestakova, I. Selenium analogues of raloxifene as promising antiproliferative agents in treatment of breast cancer. Eur. J. Med. Chem. 2014, 87, 471–483. [Google Scholar] [CrossRef]
- Shaldam, M.; Eldehna, W.M.; Nocentini, A.; Elsayed, Z.M.; Ibrahim, T.M.; Salem, R.; El-Domany, R.A.; Capasso, C.; Abdel-Aziz, H.A.; Supuran, C.T. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur. J. Med. Chem. 2021, 216, 113283. [Google Scholar] [CrossRef]
- Abbas, S.Y.; Al-Harbi, R.A.K.; Sh El-Sharief, M.A.M. Synthesis and anticancer activity of thiourea derivatives bearing a benzodioxole moiety with EGFR inhibitory activity, apoptosis assay and molecular docking study. Eur. J. Med. Chem. 2020, 198, 112363. [Google Scholar] [CrossRef] [PubMed]
- Alesawy, M.S.; Al-Karmalawy, A.A.; Elkaeed, E.B.; Alswah, M.; Belal, A.; Taghour, M.S.; Eissa, I.H. Design and discovery of new 1,2,4-triazolo[4,3-c]quinazolines as potential DNA intercalators and topoisomerase II inhibitors. Arch. Pharm 2021, 354, e2000237. [Google Scholar] [CrossRef]
- He, Z.X.; Huo, J.L.; Gong, Y.P.; An, Q.; Zhang, X.; Qiao, H.; Yang, F.F.; Zhang, X.H.; Jiao, L.M.; Liu, H.M.; et al. Design, synthesis and biological evaluation of novel thiosemicarbazone-indole derivatives targeting prostate cancer cells. Eur. J. Med. Chem. 2021, 210, 112970. [Google Scholar] [CrossRef]
- Pantelić, N.; Zmejkovski, B.B.; Božić, B.; Dojčinović, B.; Banjac, N.R.; Wessjohann, L.A.; Kaluđerović, G.N. Synthesis, characterization and in vitro biological evaluation of novel organotin(IV) compounds with derivatives of 2-(5-arylidene-2,4-dioxothiazolidin-3-yl)propanoic acid. J. Inorg. Biochem. 2020, 211, 111207. [Google Scholar] [CrossRef] [PubMed]
- Cetintas, V.B.; Kucukaslan, A.S.; Kosova, B.; Tetik, A.; Selvi, N.; Cok, G.; Gunduz, C.; Eroglu, Z. Cisplatin resistance induced by decreased apoptotic activity in non-small-cell lung cancer cell lines. Cell Biol. Int. 2012, 36, 261–265. [Google Scholar] [CrossRef]
- Weinstein, I.B. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Rapamycin suppresses ROS-dependent apoptosis caused by selenomethionine in A549 lung carcinoma cells. Cancer Chemother. Pharmacol. 2011, 67, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.R.; Jee Jo, M.; Jung, M.J.; Sik Kim, H.; Yoon, S. Selenate specifically sensitizes drug-resistant cancer cells by increasing apoptosis via G2 phase cell cycle arrest without P-GP inhibition. Eur. J. Pharmacol. 2015, 764, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar] [PubMed]

| Compound | IC50 (µM) * | |||||||
|---|---|---|---|---|---|---|---|---|
| Breast | Lung | Colon | ||||||
| MCF-7 | 184B5 | SI ** | HTB-54 | BEAS-2B | SI | HT-29 | HCT-116 | |
| 1d | >10 | N.D. | N.D. | >10 | N.D. | N.D. | 8.07 ± 3.77 | 7.80 ± 0.56 |
| 1e | 9.96 ± 1.45 | 8.09 ± 1.21 | 0.81 | 9.79 ± 0.07 | 6.01 ± 0.94 | 0.61 | 5.89 ± 1.32 | 9.14 ± 1.14 |
| 2a | 9.16 ± 3.49 | 11.00 ± 0.73 | 1.20 | >10 | N.D. | N.D. | >10 | N.D. |
| 2d | >10 | N.D. | N.D. | 8.65 ± 0.29 | 5.22 ± 0.07 | 0.60 | >10 | N.D. |
| 3a | >10 | N.D. | N.D. | >10 | N.D. | N.D. | 6.49 ± 1.58 | 5.86 ± 0.52 |
| 6a | 9.88 ± 1.46 | 22.24 ± 4.07 | 2.25 | >10 | N.D. | N.D. | 5.03 ± 3.06 | 5.69 ± 0.71 |
| 6b | 5.52 ± 1.61 | 12.24 ± 2.42 | 2.21 | 9.01 ± 2.60 | 7.56 ± 0.19 | 0.83 | 5.67 ± 4.67 | 6.29 ± 0.76 |
| 6c | 4.94 ± 1.99 | 7.23 ± 2.28 | 1.46 | 5.09 ± 0.28 | 4.73 ± 0.79 | 0.93 | 5.60 ± 1.24 | 3.60 ± 0.74 |
| 6d | 7.19 ± 1.12 | 15.96 ± 2.74 | 2.22 | 6.56 ± 0.99 | 7.59 ± 0.53 | 1.16 | 8.04 ± 2.17 | 6.95 ± 0.65 |
| 6e | 5.47 ± 3.91 | 7.69 ± 0.31 | 1.41 | 6.09 ± 0.75 | 2.36 ± 0.39 | 0.39 | 4.18 ± 0.46 | 5.43 ± 0.86 |
| Cisplatin | 15.16 ± 1.04 [47] | N.D. | N.D. | 13.68 ± 0.83 [48] | N.D. | N.D. | 14.18 ± 0.73 [47] | 2.18 ± 1.58 |
| Cance Type | Cell Line | GI50 (µM) * | TGI (µM) ** | LC50 (µM) *** |
|---|---|---|---|---|
| Colon cancer | COLO-205 | 1.66 | 3.98 | 9.33 |
| Colon cancer | HCT-116 | 1.29 | 2.75 | 6.03 |
| CNS cancer | SF-593 | 1.91 | 4.17 | 9.33 |
| Melanoma | M14 | 1.38 | 3.31 | 7.94 |
| Melanoma | SK-MEL-5 | 0.56 | 1.78 | 4.27 |
| Ovarian cancer | OVACAR-3 | 1.38 | 3.24 | 7.41 |
| Renal cancer | UO-31 | 1.74 | 4.07 | 9.77 |
| Breast cancer | MDA-MB-231/ATCC | 1.78 | 3.89 | 8.51 |
| Breast cancer | BT-549 | 0.87 | 2.29 | 5.62 |
| Breast cancer | MDA-MB-468 | 1.35 | 3.39 | 8.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Martín, G.; Plano, D.; Encío, I.; Sanmartín, C. Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2021, 10, 777. https://doi.org/10.3390/antiox10050777
Calvo-Martín G, Plano D, Encío I, Sanmartín C. Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants. 2021; 10(5):777. https://doi.org/10.3390/antiox10050777
Chicago/Turabian StyleCalvo-Martín, Gorka, Daniel Plano, Ignacio Encío, and Carmen Sanmartín. 2021. "Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents" Antioxidants 10, no. 5: 777. https://doi.org/10.3390/antiox10050777
APA StyleCalvo-Martín, G., Plano, D., Encío, I., & Sanmartín, C. (2021). Novel N,N′-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants, 10(5), 777. https://doi.org/10.3390/antiox10050777








