The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Conditions, Plant Material and Treatments
2.2. Chemicals and Reagents
2.3. Growth and Shoot and Leaf Weights
2.4. Determination of the Total Phenolic Compounds and Antioxidant Activity (ABTS●+)
2.5. Extraction and Quantification of Total Soluble Sugars
2.6. Extraction of Polyamines and Their Analysis by UHPLC
2.7. Statistical Analysis
3. Results and Discussion
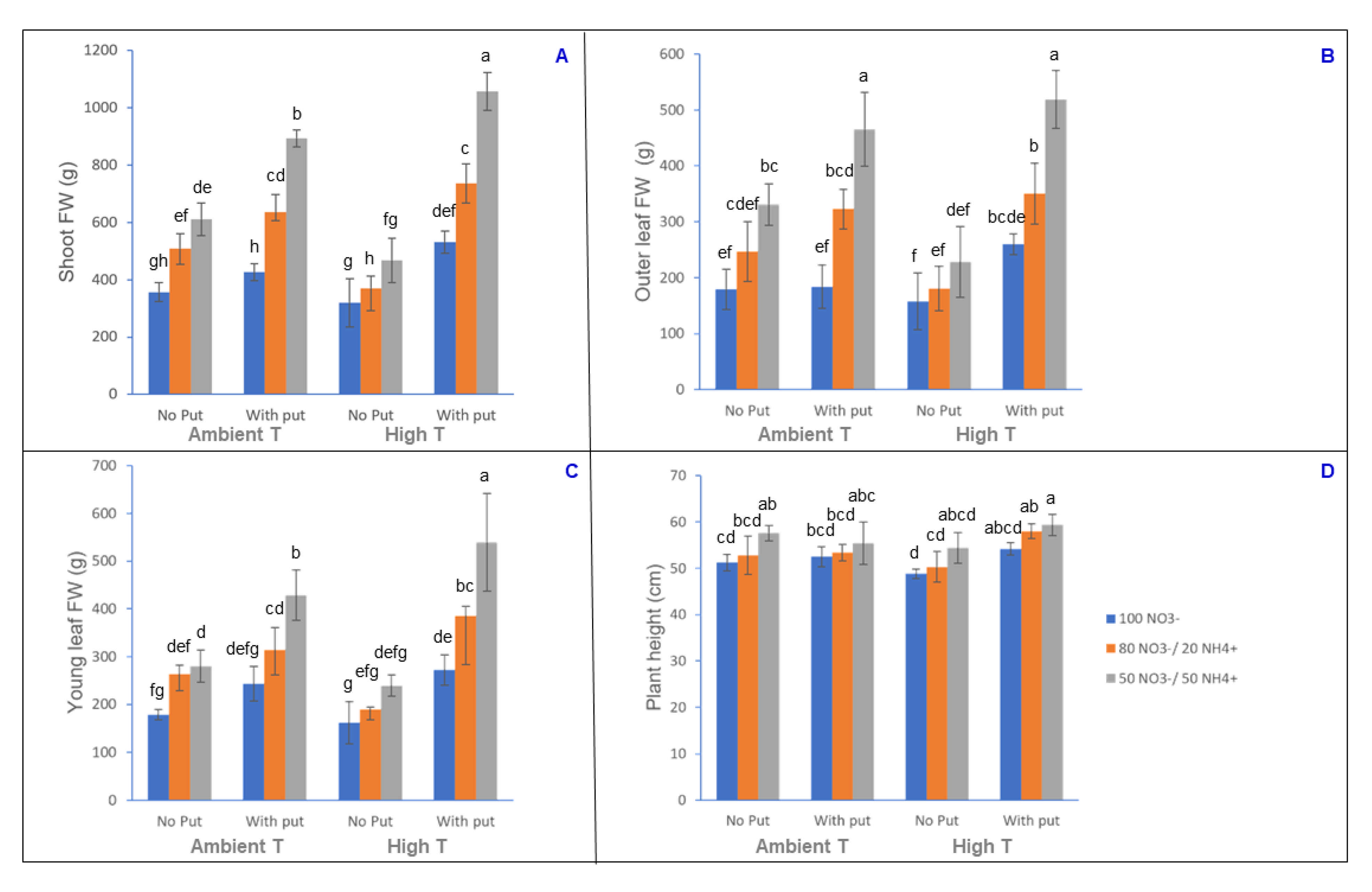
3.1. Analysis of Biomass
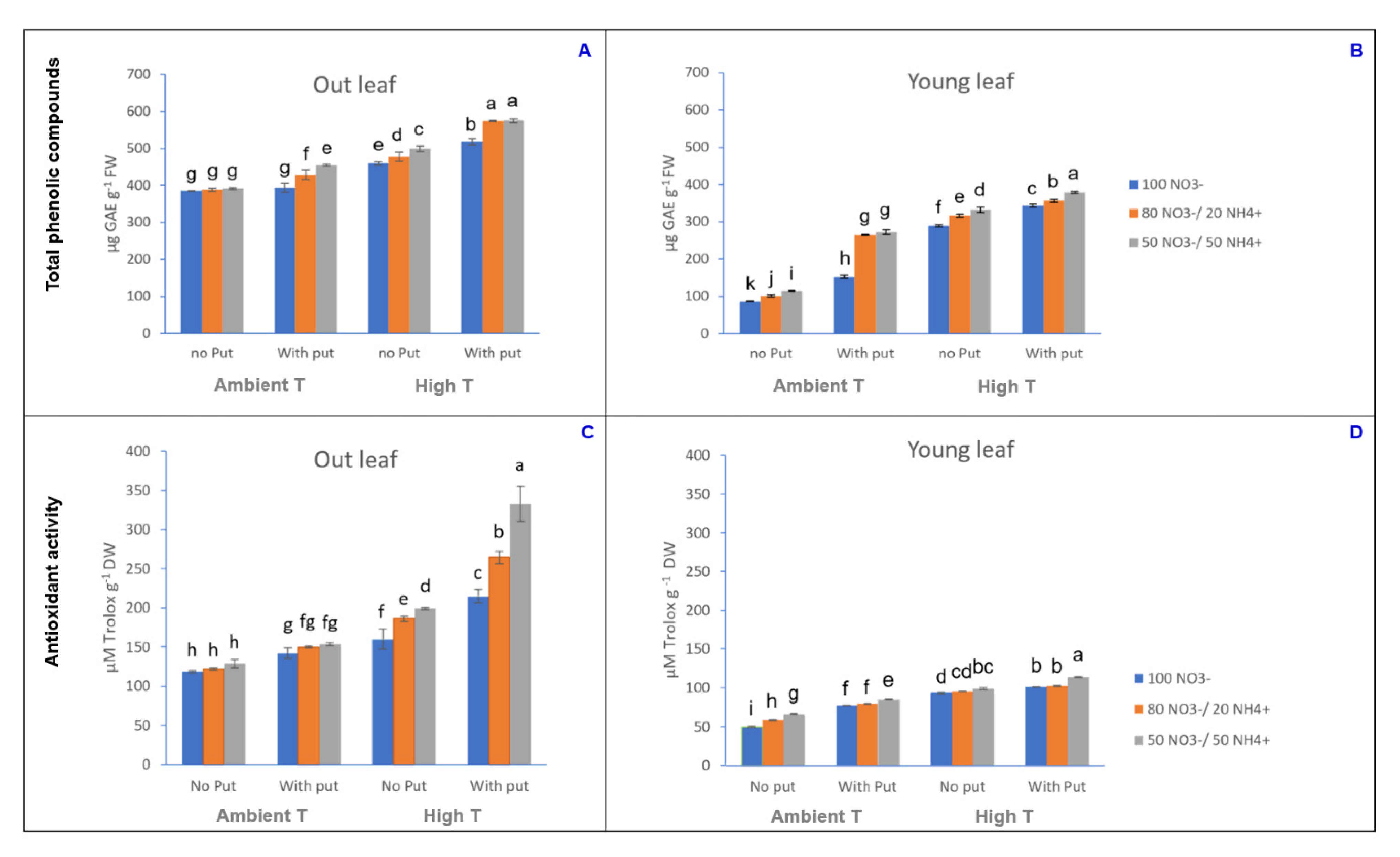
3.2. Evaluation of the Antioxidant Activity and Total Phenolic Compounds
3.3. Determination of Sugars
3.4. Identification and Quantification of Polyamines by UHPLC-DAD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Special Report on Global Warming of 1.5 C (SR15); IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Khan, K.A.; Zaman, K.; Shoukry, A.M.; Sharkawy, A.; Gani, S.; Ahmad, J.; Khan, A.; Hishan, S.S. Natural disasters and economic losses: Controlling external migration, energy and environmental resources, water demand, and financial development for global prosperity. Environ. Sci. Pollut. Res. 2019, 26, 14287–14299. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Rodríguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, L.; Chen, G.-q.; Zhang, J.; Reed, B.M.; Shen, X.-h. ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep. 2015, 34, 1499–1513. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Nortes, P.; Baille, A.; González-Real, M.; Ruiz-Salleres, I.; Verhoef, A.; Martin-Gorriz, B.; Egea, G. Effects of high temperature and vapour pressure deficit on net ecosystem exchange and energy balance of an irrigated orange orchard in a semi-arid climate (Southern Spain). In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium, Lisbon, Portugal, 22 August 2010; pp. 149–156. [Google Scholar]
- Pérez, F.L. Viticultural practices in Jumilla (Murcia, Spain): A case study of agriculture and adaptation to natural landscape processes in a variable and changing climate. AIMS Agric. Food 2016, 1, 265–293. [Google Scholar] [CrossRef]
- Faostat. Agriculture Organization of the United Nations Statistics Division 2014. Production. 2016. Available online: http://faostat3.fao.org/browse/Q/QC/S (accessed on 17 December 2020).
- Ministry of Agriculture, Fisheries and Food. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2019/default.aspx?parte=3&capitulo=07&grupo=6&seccion=32 (accessed on 18 October 2020).
- Kapusta-Duch, J.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kuboń, M.; Leszczyńska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 00108. [Google Scholar] [CrossRef]
- Picchi, V.; Fibiani, M.; Scalzo, R.L. Cauliflower. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambrige, MA, USA, 2020; Volume 1, pp. 19–32. [Google Scholar]
- Huynh, T.N. Biological Treatments of Cauliflower (Brassica oleracea L. var. botrytis) Outer Leaves: Improved Extraction and Conversion of Phenolic Compounds. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Khedkar, M.A.; Nimbalkar, P.R.; Chavan, P.V.; Chendake, Y.J.; Bankar, S.B. Cauliflower waste utilization for sustainable biobutanol production: Revelation of drying kinetics and bioprocess development. Bioprocess Biosyst. Eng. 2017, 40, 1493–1506. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar]
- Orlando, M.; Trivellini, A.; Bartolini, S.; Carmassi, G.; Maggini, R.; Lucchesini, M.; Ferrante, A.; Incrocci, L.; Mensuali, A. Evaluation of by-products of plant food (potato and apple) as potential biostimulants for green leafy vegetables. In Proceedings of the III International Symposium on Growing Media, Composting and Substrate Analysis, Milan, Italy, 24–28 June 2019; Volume 1305, pp. 529–536. [Google Scholar]
- Pirintsos, S.; Munzi, S.; Loppi, S.; Kotzabasis, K. Do polyamines alter the sensitivity of lichens to nitrogen stress? Ecotoxicol. Environ. Saf. 2009, 72, 1331–1336. [Google Scholar] [CrossRef]
- Mostafaei, E.; Zehtab-Salmasi, S.; Salehi-Lisar, Y.; Ghassemi-Golezani, K. Changes in photosynthetic pigments, osmolytes and antioxidants of Indian Mustard by drought and exogenous polyamines. Acta Biol. Hung. 2018, 69, 313–324. [Google Scholar] [CrossRef]
- Pang, X.-M.; Zhang, Z.-Y.; Wen, X.-P.; Ban, Y.; Moriguchi, T. Polyamines, all-purpose players in response to environment stresses in plants. Plant Stress 2007, 1, 173–188. [Google Scholar]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Effects of Different Nitrogen Forms and Exogenous Application of Putrescine on Heat Stress of Cauliflower: Photosynthetic Gas Exchange, Mineral Concentration and Lipid Peroxidation. Plants 2021, 10, 152. [Google Scholar] [CrossRef]
- Luna-Esquivel, E.N.; Ojeda-Barrios, D.L.; Guerrero-Prieto, V.M.; Ruiz-Anchondo, T.; Martínez-Téllez, J.J. Poliaminas como indicadores de estrés en plantas. Rev. Chapingo Ser. Hortic. 2014, 20, 283–295. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Kotakis, C.; Theodoropoulou, E.; Tassis, K.; Oustamanolakis, C.; Ioannidis, N.E.; Kotzabasis, K. Putrescine, a fast-acting switch for tolerance against osmotic stress. J. Plant Physiol. 2014, 171, 48–51. [Google Scholar] [CrossRef]
- Mirdehghan, S.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C. Chemical composition and bioactive properties of Cichorium spinosum L. in relation to nitrate/ammonium nitrogen ratio. J. Sci. Food Agric. 2019, 99, 6741–6750. [Google Scholar] [CrossRef]
- Munene, R.; Changamu, E.; Korir, N.; Joseph, G.-O. Effects of different nitrogen forms on growth, phenolics, flavonoids and antioxidant activity in amaranth species. Trop. Plant Res. 2017, 4, 81–89. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Cuadra-Crespo, P.; Walker, D.J.; Cámara, J.M.; Madrid, R. Effect of foliar application of antitranspirant on photosynthesis and water relations of pepper plants under different levels of CO2 and water stress. J. Plant Physiol. 2010, 167, 1232–1238. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Hernández, F.; Corell, M.; Burló, F.; Legua, P.; Moriana, A.; Carbonell-Barrachina, Á.A. Antioxidant capacity, fatty acids profile, and descriptive sensory analysis of table olives as affected by deficit irrigation. J. Sci. Food Agric. 2017, 97, 444–451. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Cuartero, J.; Bolarín, M.C.; Pérez-Alfocea, F. Sucrolytic activities during fruit development of Lycopersicon genotypes differing in tolerance to salinity. Physiol. Plant. 2003, 118, 38–46. [Google Scholar] [CrossRef]
- Rodriguez, S.; López, B.; Chaves, A.R. Effect of different treatments on the evolution of polyamines during refrigerated storage of eggplants. J. Agric. Food Chem. 2001, 49, 4700–4705. [Google Scholar] [CrossRef]
- Coleto, I.; Vega-Mas, I.; Glauser, G.; González-Moro, M.B.; Marino, D.; Ariz, I. New insights on Arabidopsis thaliana root adaption to ammonium nutrition by the use of a quantitative proteomic approach. Int. J. Mol. Sci. 2019, 20, 814. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium: Nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Juan, L.; Zhou, J.-M.; Duan, Z.-Q. Effects of elevated CO2 concentration on growth and water usage of tomato seedlings under different ammonium/nitrate ratios. J. Environ. Sci. 2007, 19, 1100–1107. [Google Scholar]
- Röth, S.; Paul, P.; Fragkostefanakis, S. Plant heat stress response and thermotolerance. In Genetic Manipulation in Plants for Mitigation of Climate Change; Springer: New Delhi, India, 2015; pp. 15–41. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, J.; Gao, F.; Wang, J.; Su, G. Polyamine-induced nitric oxide generation and its potential requirement for peroxide in suspension cells of soybean cotyledon node callus. Plant Physiol. Biochem. 2014, 79, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hortic. 2021, 277, 109818. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kwak, J.-H. Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Mostafa, H.A.M.; Hassanein, R.A.; Khalil, S.I.; El-Khawas, S.A.; El-Bassiouny, H.M.S.; El-Monem, A.A.A. Effect of arginine or putrescine on growth, yield and yield components of late sowing wheat. Res. J. Appl. Sci. 2010, 177–183. [Google Scholar]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Xia, J.L.; Wu, C.G.; Ren, A.; Hu, Y.R.; Wang, S.L.; Han, X.F.; Shi, L.; Zhu, J.; Zhao, M.W. Putrescine regulates nitric oxide accumulation in Ganoderma lucidum partly by influencing cellular glutamine levels under heat stress. Microbiol. Res. 2020, 239, 126521. [Google Scholar] [CrossRef]
| Main Effects | Total Phenolic Compounds | Antioxidant Activity | ||
|---|---|---|---|---|
| Outer leaves | Young leaves | Outer leaves | Young leaves | |
| T | *** | *** | *** | *** |
| Put | *** | *** | *** | *** |
| NO3−/NH4+ | *** | *** | *** | *** |
| T X Put | ns | ns | *** | * |
| T X NO3−/NH4+ | *** | ** | *** | ns |
| Put X NO3−/NH4+ | *** | *** | *** | *** |
| T X Put X NO3−/NH4+ | *** | *** | *** | *** |
| Temperature | NO3−/NH4+ | Inositol | Glucose | Fructose | Sucrose | Total Free Sugars | |
|---|---|---|---|---|---|---|---|
| Ambient temperature | 100/0 | Without Put | 5.50 h | 39.71 g | 12.91 g | 101.43 f | 159.57 c |
| With Put | 11.04 f | 71.12 e | 18.34 e | 108.03 e | 208.54 b | ||
| 80/20 | Without Put | 8.08 g | 75.80 e | 11.31 h | 114.33 d | 209.53 ab | |
| With Put | 12.38 f | 85.41 cd | 15.84 f | 123.22 c | 236.86 a | ||
| 50/50 | Without Put | 8.75 g | 60.47 f | 9.25 i | 142.90 b | 221.38 ab | |
| With Put | 15.02 e | 83.08 de | 13.84 g | 149.75 a | 261.70 a | ||
| High temperature | 100/0 | Without Put | 18.69 d | 97.25 bc | 25.49 b | 72.78 j | 214.22 ab |
| With Put | 21.80 c | 101.32 ab | 28.14 a | 82.37 i | 233.64 a | ||
| 80/20 | Without Put | 20.34 cd | 105.92 a | 23.49 c | 76.35 j | 226.12 ab | |
| With Put | 23.37 b | 112.18 a | 27.76 a | 88.39 h | 251.71 a | ||
| 50/50 | Without Put | 21.46 c | 93.97 bc | 21.06 d | 95.29 g | 231.79 ab | |
| With Put | 25.34 a | 99.71 ab | 27.38 a | 98.75 fg | 251.20 a | ||
| Main effects | |||||||
| Temperature (T) | *** | *** | *** | *** | *** | ||
| Putrescine (Put) | *** | *** | *** | *** | * | ||
| Nitrate/ammonium (NO3−/NH4+) | *** | *** | *** | *** | *** | ||
| T X Put | ns | *** | ns | ns | * | ||
| T X NO3−/NH4+ | ns | *** | ns | ** | *** | ||
| Put X NO3−/NH4+ | *** | *** | *** | *** | ns | ||
| T X Put X NO3−/NH4+ | * | *** | ** | *** | ** |
| Temperature | NO3−/NH4+ | Inositol | Glucose | Fructose | Sucrose | Total free sugars | |
|---|---|---|---|---|---|---|---|
| Ambient temperature | 100/0 | Without Put | 26.53 i | 117.08 i | 31.75 fg | 65.68 bc | 241.04 g |
| With Put | 30.39 fg | 137.29 fg | 36.12 cd | 70.81 a | 274.62 e | ||
| 80/20 | Without Put | 28.07 hi | 128.75 gh | 31.67 fg | 67.43 b | 256.00 f | |
| With Put | 31.61 ef | 152.33 e | 34.25 de | 71.49 a | 289.68 bcd | ||
| 50/50 | Without Put | 28.64 gh | 122.76 hi | 30.47 h | 69.95 a | 251.82 fg | |
| With Put | 32.62 de | 143.41 f | 32.67 ef | 72.52 a | 281.22 cde | ||
| High temperature | 100/0 | Without Put | 34.28 cd | 153.88 de | 36.98 bc | 51.04 g | 276.18 de |
| With Put | 37.52 b | 158.78 cde | 40.76 a | 59.36 e | 302.45 b | ||
| 80/20 | Without Put | 35.97 bc | 165.02 bc | 36.82 bc | 56.53 f | 292.29 bc | |
| With Put | 37.30 b | 175.32 a | 38.83 b | 61.51 de | 312.99 a | ||
| 50/50 | Without Put | 37.32 b | 162.96 cd | 36.05 cd | 59.54 e | 291.90 bc | |
| With Put | 39.82 a | 172.64 ab | 38.14 bc | 63.38 cd | 313.99 a | ||
| Main effects | |||||||
| Temperature (T) | *** | *** | *** | *** | *** | ||
| Putrescine (Put) | *** | * | ** | *** | ** | ||
| Nitrate/ammonium (NO3−/NH4+) | *** | *** | *** | *** | *** | ||
| T X Put | ns | ns | ns | ns | ns | ||
| T X NO3−/NH4+ | ns | ns | ns | * | ns | ||
| Put X NO3−/NH4+ | *** | *** | *** | *** | *** | ||
| T X Put X NO3−/NH4+ | ns | * | ns | ns | ns |
| Temperature | NO3−/NH4+ | Putrescine | Cadaverine | Spermidine | Spermine | Total | |
|---|---|---|---|---|---|---|---|
| Ambient temperature | 100/0 | Without Put | 6.30 j | 7.33 j | 4.50 k | 2.37 c | 20.49 k |
| With Put | 13.74 g | 9.10 h | 10.63 h | 3.22 abc | 36.69 h | ||
| 80/20 | Without Put | 8.24 i | 8.49 i | 5.85 j | 2.82 bc | 25.41 j | |
| With Put | 15.62 f | 10.03 g | 12.34 g | 3.07 abc | 41.04 g | ||
| 50/50 | Without Put | 11.36 h | 11.09 f | 8.66 i | 2.93 abc | 34.04 i | |
| With Put | 16.45 e | 12.37 d | 13.59 f | 3.31 abc | 45.72 f | ||
| High temperature | 100/0 | Without Put | 17.21 e | 11.76 e | 14.47 e | 3.37 abc | 46.81 e |
| With Put | 21.65 b | 14.99 b | 16.82 c | 3.45 ab | 56.91 c | ||
| 80/20 | Without Put | 18.14 d | 13.31 c | 15.55 d | 3.53 ab | 50.53 d | |
| With Put | 22.34 a | 15.29 ab | 19.51 b | 3.73 ab | 60.87 b | ||
| 50/50 | Without Put | 20.76 c | 14.87 b | 16.14 cd | 3.60 ab | 55.37 c | |
| With Put | 22.74 a | 15.63 a | 20.84 a | 3.95 a | 63.16 a | ||
| Main effects | |||||||
| Temperature (T) | *** | *** | *** | *** | *** | ||
| Putrescine (Put) | *** | *** | *** | * | *** | ||
| Nitrate/ammonium (NO3−/NH4+) | *** | *** | *** | ns | *** | ||
| T X Put | *** | *** | * | ns | *** | ||
| T X NO3−/NH4+ | *** | ** | ** | ns | *** | ||
| Put X NO3−/NH4+ | *** | *** | *** | ns | *** | ||
| T X Put X NO3−/NH4+ | *** | *** | *** | *** | *** |
| Temperature | NO3−/NH4+ | Putrescine | Cadaverine | Spermidine | Spermine | Total | |
|---|---|---|---|---|---|---|---|
| Ambient temperature | 100/0 | Without Put | 23.12 h | 12.44 d | 17.72 h | 3.20 h | 51.84 i |
| With Put | 24.17 g | 16.14 bc | 27.66 efg | 4.45 fg | 68.63 h | ||
| 80/20 | Without Put | 23.73 g | 15.93 c | 24.80 g | 4.17 g | 71.02 h | |
| With Put | 25.15 f | 16.68 abc | 29.61 def | 4.54 fg | 72.41 gh | ||
| 50/50 | Without Put | 24.016 g | 16.12 bc | 26.60 fg | 4.29 fg | 75.98 fgh | |
| With Put | 26.33 e | 17.03 abc | 30.78 de | 4.77 def | 78.91 efg | ||
| High temperature | 100/0 | Without Put | 27.00 d | 17.86 abc | 31.73 cd | 5.01 cde | 81.59 def |
| With Put | 29.73 b | 18.71 abc | 37.57 ab | 5.42 abcd | 84.60 cde | ||
| 80/20 | Without Put | 27.63 c | 18.17 abc | 33.44 cd | 5.37 bcd | 86.97 bcd | |
| With Put | 29.90 b | 19.05 ab | 39.064 ab | 5.94 ab | 91.62 abc | ||
| 50/50 | Without Put | 28.19 c | 18.19 abc | 35.19 bc | 5.61 abc | 93.95 ab | |
| With Put | 30.53 a | 19.44 a | 40.96 a | 6.11 a | 97.05 a | ||
| Main effects | |||||||
| Temperature (T) | *** | *** | *** | *** | *** | ||
| Putrescine (Put) | *** | ns | *** | * | *** | ||
| Nitrate/ ammonium (NO3−/NH4+) | *** | ns | *** | *** | *** | ||
| T X Put | ns | ns | ns | ns | ns | ||
| T X NO3−/NH4+ | ns | ns | ns | ns | ns | ||
| Put X NO3−/NH4+ | *** | ns | *** | *** | *** | ||
| T X Put X NO3−/NH4+ | *** | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Amor, F.M.d. The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste. Antioxidants 2021, 10, 707. https://doi.org/10.3390/antiox10050707
Collado-González J, Piñero MC, Otálora G, López-Marín J, Amor FMd. The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste. Antioxidants. 2021; 10(5):707. https://doi.org/10.3390/antiox10050707
Chicago/Turabian StyleCollado-González, Jacinta, Maria Carmen Piñero, Ginés Otálora, Josefa López-Marín, and Francisco M. del Amor. 2021. "The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste" Antioxidants 10, no. 5: 707. https://doi.org/10.3390/antiox10050707
APA StyleCollado-González, J., Piñero, M. C., Otálora, G., López-Marín, J., & Amor, F. M. d. (2021). The Effect of Foliar Putrescine Application, Ammonium Exposure, and Heat Stress on Antioxidant Compounds in Cauliflower Waste. Antioxidants, 10(5), 707. https://doi.org/10.3390/antiox10050707