Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review
Abstract
1. Introduction
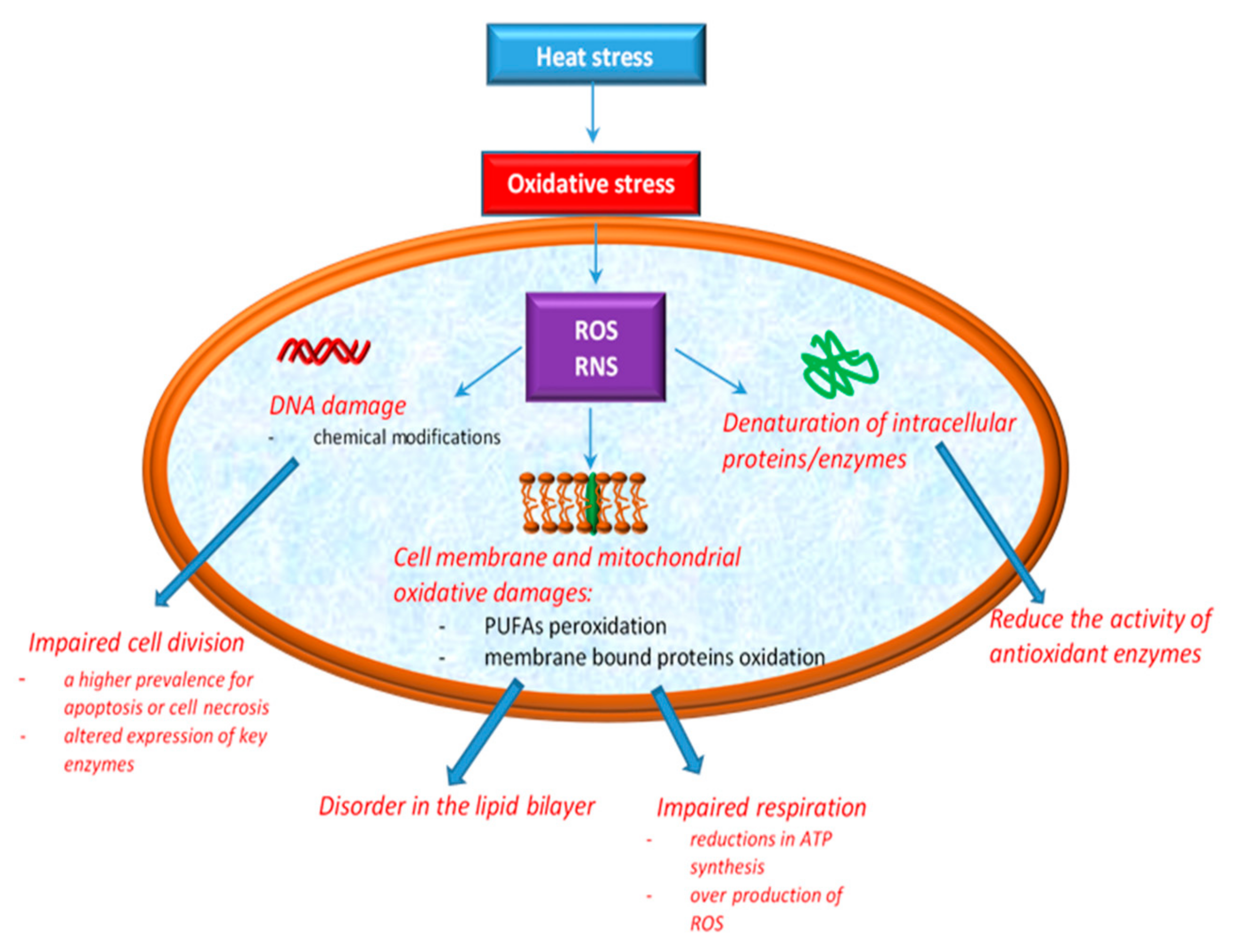
2. Heat Stress and Oxidative Stress in Broiler Chickens
2.1. Reactive Species Involved in Oxidative Stress
2.2. Effect of Heat Stress on Cellular Integrity. Biomarkers of Heat Stress
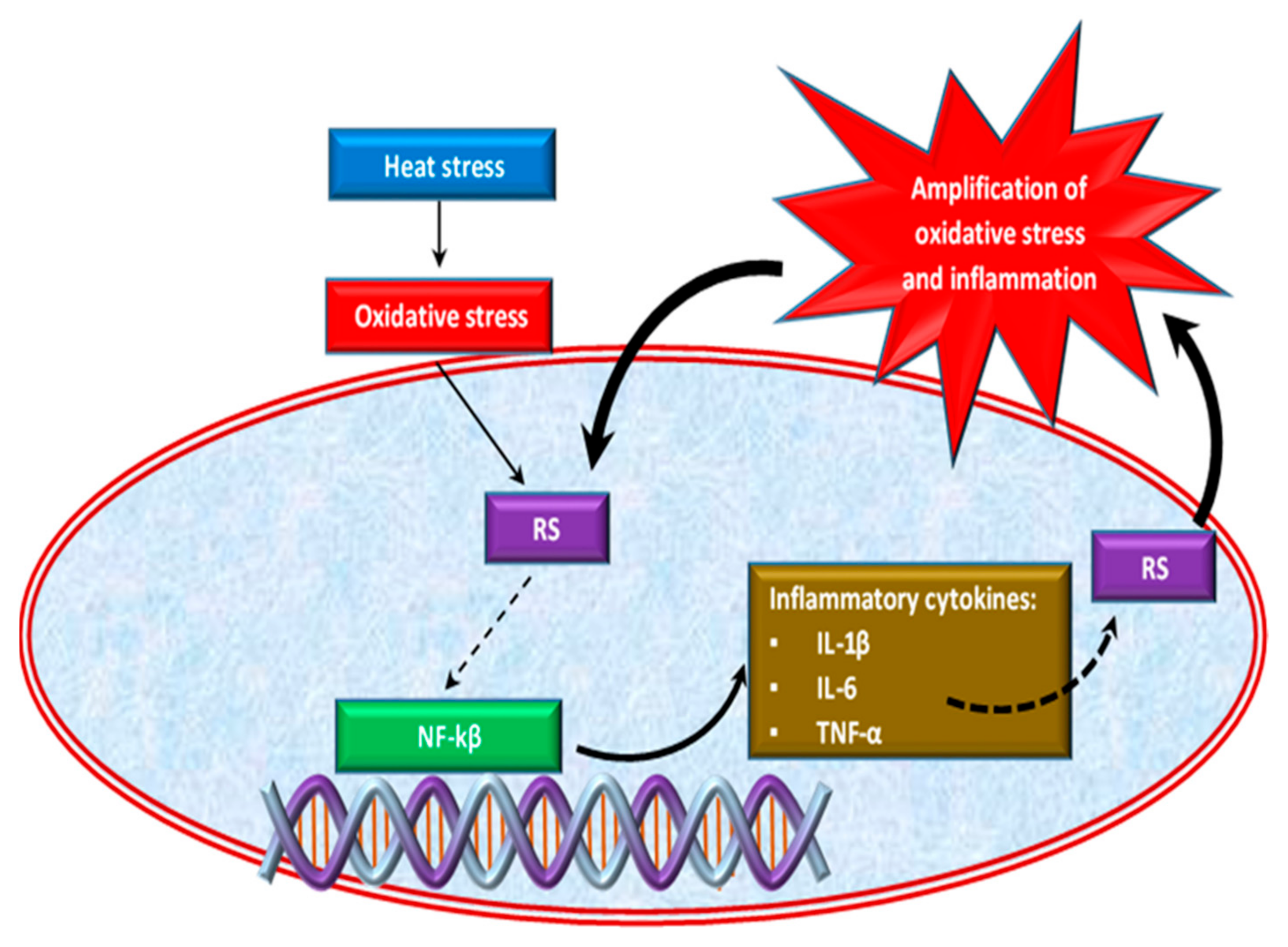
2.3. Effect of Oxidative Stress on Inflammatory Response
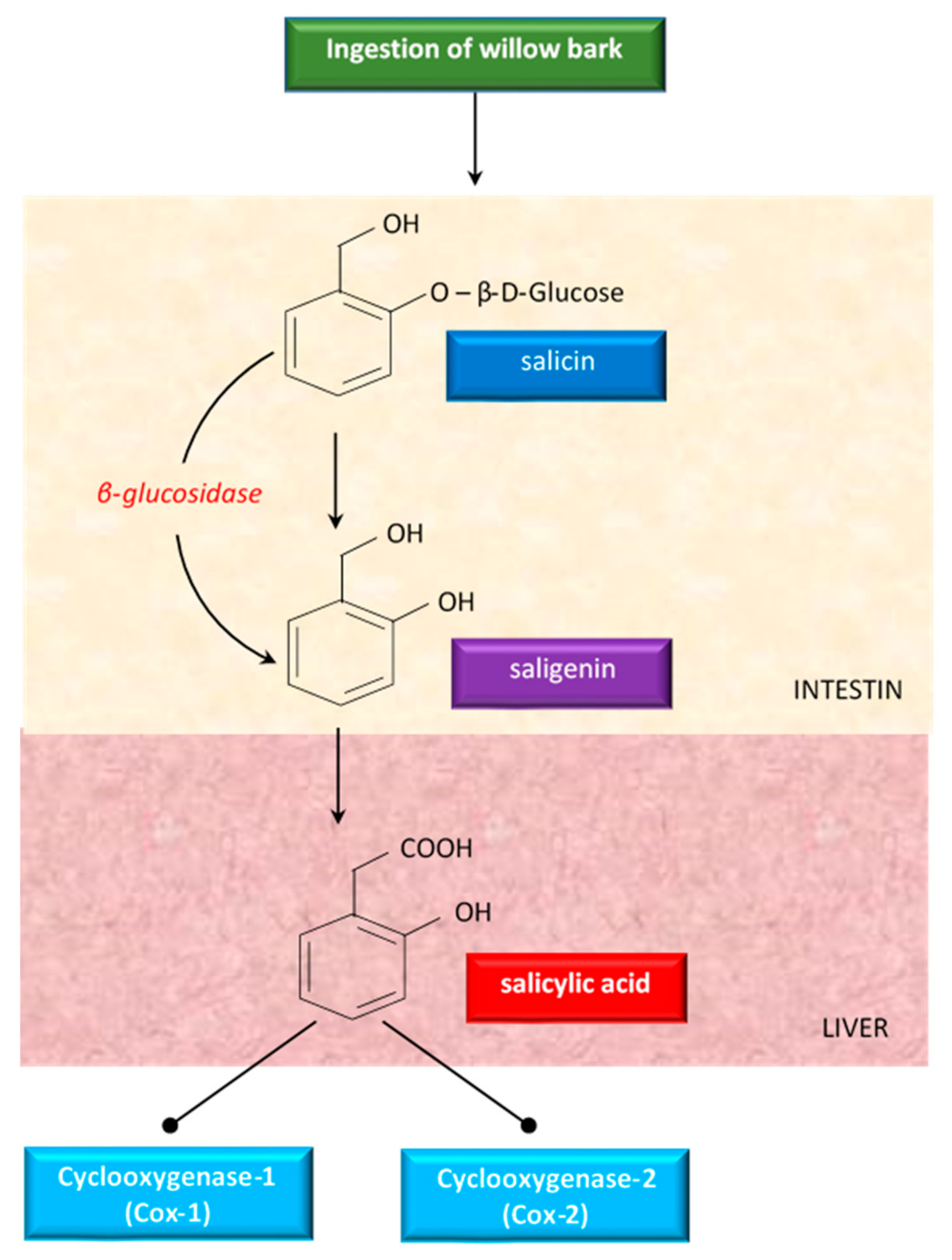
3. Chemical Characterization of Salix spp. Bark
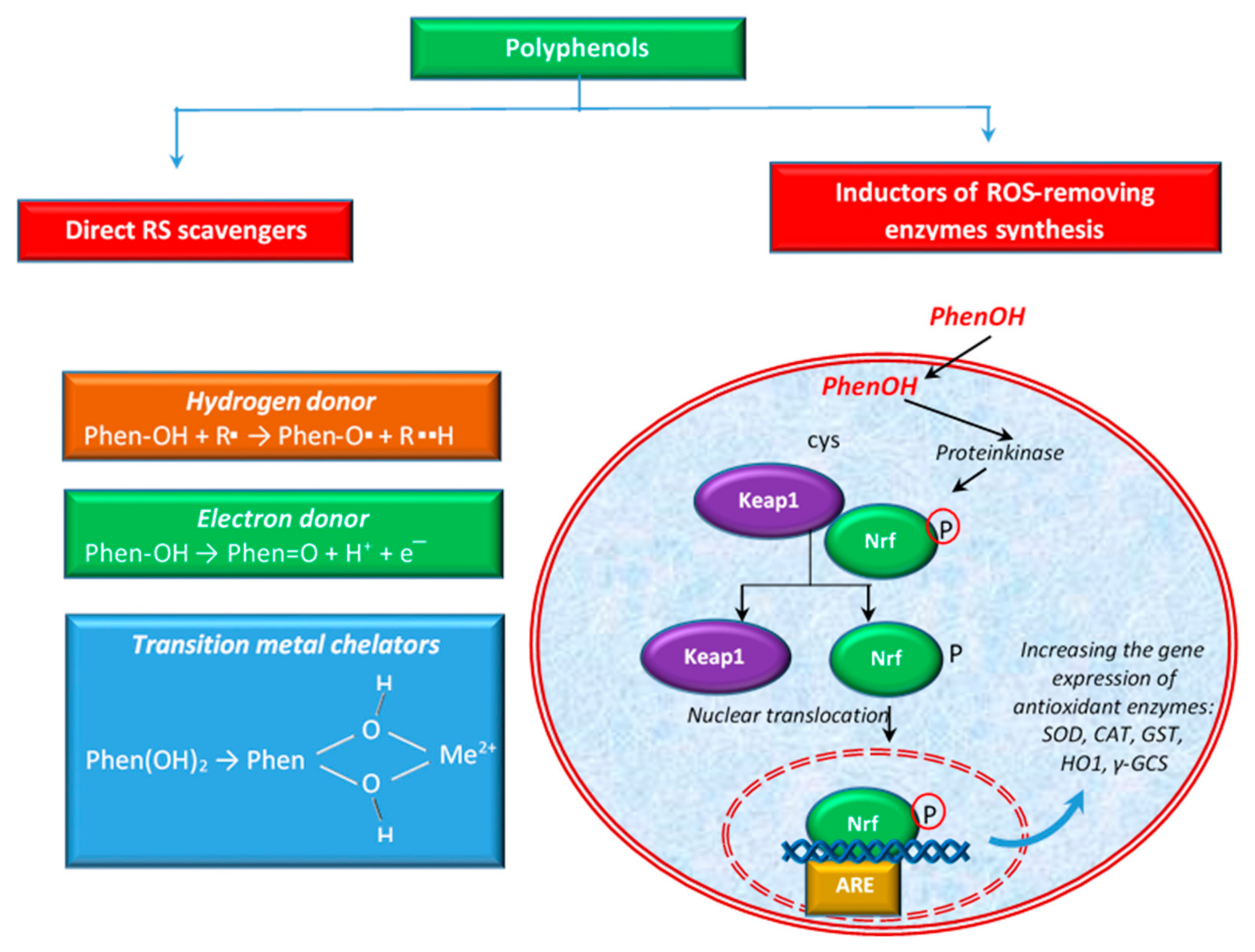
4. Mechanisms of Action of Polyphenols
4.1. Polyphenols as RS Scavengers and ROS-Enzymes Synthesis Modulators
4.2. Polyphenols as Modulator of the Gut Microbial Balance
4.3. Salicin and Salicin Derivatives: Analgesic, Antipyretic, and Anti-Inflammatory Effects
5. Biological Effects of Salix Species Extracts in Different Experimental Models
6. Effects of Different Forms of Inclusion of Salix Species in the Diet of Heat-Stressed Broilers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seifi, S.; Khoshbakht, R.; Sayrafi, R.; Gilani, A. Evaluation of a liquid yeast product on growth performance, anatomical structure, and gut microbiota of broilers challenged with suboptimal diet and heat stress. Rev. Med. Vet. Toulouse 2018, 169, 93–102. [Google Scholar]
- Kamboh, A.A.; Hang, S.Q.; Bakhetgul, M.; Zhu, W.Y. Effects of genistein and hesperidin on biomarkers of heat stress in broilers under persistent summer stress. Poult. Sci. 2013, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Chang, W.H.; Liu, G.H.; Zhang, S.; Zheng, A.J.; Li, Y.; Cai, H.Y. Effects of flavones of sea buckthorn fruits on growth performance, carcass quality, fat deposition and lipometabolism for broilers. Poult. Sci. 2015, 94, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry, mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Smith, P.; Gregory, P. Climate change and sustainable food production. Proc. Nutr. Soc. 2013, 72, 21–28. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef]
- Olfati, A.; Mojtahedin, A.; Sadeghi, T.; Akbari, M.; Martínez-Pastor, F. Comparison of growth performance and immune responses of broiler chicks reared under heat stress, cold stress and thermoneutral conditions. Span. J. Agric. Res. 2018, 16, 15. [Google Scholar] [CrossRef]
- He, S.; Li, S.; Arowolo, M.A.; Yu, Q.; Chen, F.; Hu, R.; He, J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019, 90, 401–411. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; El-Hack, M.E.A.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, G.Y.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Mahmood, S.; Nawaz, H. Effect of dietary inclusion of sodium bicarbonate on blood profile of caged layers during summer. Pak. J. Agric. Sci. 2017, 54, 443–450. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhaoa, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, H.; Ma, T.; Yan, Z.; Zhang, Y.; Geng, Y.; Zhu, Y.; Shi, Y. Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 2020, 91, 102619. [Google Scholar] [CrossRef]
- Tirawattanawanich, C.; Chantakru, S.; Nimitsantiwong, W.; Tongyai, S. The effects of tropical environmental conditions on the stress and immune responses of commercial broilers, Thai indigenous chickens, and crossbred chickens. Poult. Sci. 2011, 20, 409–420. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Cramer, T.; Ogbeifun, O.O.E.; Seo, J.K.; Yan, F.; Cheng, H.W.; Kim, Y.H.B. Meat and muscle biology breast meat quality and protein functionality of broilers with different probiotic levels and cyclic heat challenge exposure. Meat Muscl. Biol. 2017, 1, 81–89. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Ganguly, S.; Trivedi, S.P.; Patil, S.S.; Das, O.; Gohil, B. A Review on the Nutritional Management of Climatic Heat Stress in Poultry. In Recent Research Trends in Veterinary Sciences and Animal Husbandry; Ganguly, S., Trivedi, S.P., Eds.; AkiNik Publications: New Delhi, India, 2018; pp. 71–96. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649. [Google Scholar] [CrossRef]
- Aarestrup, F. Sustainable farming: Get pigs off antibiotics. Nature 2012, 486, 465–466. [Google Scholar] [CrossRef]
- EU. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. 2005. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687 (accessed on 1 April 2021).
- Sharma, S.; Sahu, D.; Das, H.R.; Sharma, D. Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2011, 49, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 6.0; Council of Europe: Strasbourg, France, 2004; p. 3562.
- Fayaz, M.; Sivakumaar, P.K. Phytochemical analysis and antimicrobial activity of Salix alba against dental biofilm forming bacteria. Int. J. Pharm. Biol. Arch. 2010, 5, 137–140. [Google Scholar] [CrossRef]
- Islam, M.S.; Zahan, R.; Nahar, L.; Alam, M.B.; Naznin, M.; Sarkar, G.C.; Mosaddik, M.A.; Haque, M.E. Antibacterial, insecticidal and in vivo cytotoxicity activities of Salix tetrasperma. Int. J. Pharm. Sci. Res. 2011, 2, 2103–2108. [Google Scholar] [CrossRef]
- Popova, T.P.L.; Kaleva, M.D. Antimicrobial effect in vitro of aqueous extracts of leaves and branches of willow (Salix babylonica L.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 146–152. [Google Scholar]
- Ramos, P.A.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.; Freire, C.S.R.; et al. The health-promoting potential of Salix spp. bark polar extracts: Key insights on phenolic composition and in vitro bioactivity and biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef]
- Zaugg, S.E.; Cefalo, D.; Walker, E.B. Capillary electrophoretic analysis of salicin in Salix spp. J. Chromatogr. A 1997, 781, 487–490. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Okpanyi, S.N.; Kelber, O.; Weiser, D. Mechanisms involved in the anti-inflammatory effect of a standardized willow bark extract. Arzneimittelforschung 2005, 55, 677–687. [Google Scholar] [CrossRef]
- Pop, C.; Vodnar, D.; Ranga, F.; Socaciu, C. Comparative antibacterial activity of different plant extracts in relation to their bioactive molecules, as determined by LC-MS analysis. Bull. UASVM Anim. Sci. Biotechnol. 2013, 70, 86–94. [Google Scholar]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- Mujahid, A.; Sato, K.; Akiba, Y.; Toyomizu, M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006, 85, 1259–1265. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef]
- Fellenberg, M.A.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. Worlds Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Crit. Rev. Food Sci. Nutr. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Predescu, C.; Papuc, C.; Petcu, C.; Goran, G.; Rus, A.E. The effect of some polyphenols on minced pork during refrigeration compared with ascorbic acid. Bull. UASVM Food Sci. Technol. 2018, 75, 36–42. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Durand, D.; Damon, M.; Gobert, M. Oxidative stress in farm animals: General aspects. Cah. Nutr. Diet. 2013, 48, 218–224. [Google Scholar] [CrossRef]
- Zhong, R.; Zhou, D. Oxidative stress and role of natural plant derived antioxidants in animal reproduction. J. Integr. Agric. 2013, 12, 1826–1838. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Hayirli, A.; Bilgili, S.; Kucuk, O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016, 95, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J. The role of free radicals and antioxidants. Nutrition 2000, 16, 716–718. [Google Scholar] [CrossRef]
- Ozcan, A.; Ogun, M. Biochemistry of reactive oxygen and nitrogen species. Basic principles and clinical significance of oxidative stress. IntechOpen 2015, 3, 37–58. [Google Scholar] [CrossRef]
- Mishra, B.; Rajesh, J. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Van der Paal, J.; Neyts, E.C.; Verlackt, C.C.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. The mitochondrial permeability transition pore: A molecular target for amyotrophic lateral sclerosis therapy. Biochim. Biophys. Acta 2010, 1802, 186–197. [Google Scholar] [CrossRef]
- Wei, Y.H.; Lu, C.Y.; Wei, C.Y.; Ma, Y.S.; Lee, H.C. Oxidative stress in human aging and mitochondrial disease? Consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001, 44, 1–11. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Ștefan, G. Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells. Crit. Rev. Food Sci. Nutr. 2020, 9, 1–28. [Google Scholar] [CrossRef]
- Tan, G.Y.; Yang, L.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010, 89, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wazir, H.; Chay, S.Y.; Zarei, M.; Hussin, F.S.; Mustapha, N.A.; Wan Ibadullah, W.Z.; Saari, N. Effects of storage time and temperature on lipid oxidation and protein co-oxidation of low-moisture shredded meat products. Antioxidants 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015, 94, 1635–1644. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef]
- Ruff, J.; Barros, T.L.; Tellez, G., Jr.; Blankenship, J.; Lester, H.; Graham, B.D.; Selby, C.A.M.; Vuong, C.N.; Dridi, S.; Greene, E.S.; et al. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poult. Sci. 2019, 99, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidant signaling in skeletal muscle: A brief review. Exp. Gerontol. 2007, 42, 582–593. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Collins, T. Acute and Chronic Inflammation. In Robbins Pathologic Basis of Disease; Cotran, R.S., Kumar, V., Collins, T., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 50–80. [Google Scholar]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Gomes, A.V.; Pinheiro, M.L.; Ribeiro, A.; Ferraz-de-Paula, V.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella enteritidis. Avian Pathol. 2012, 41, 421. [Google Scholar] [CrossRef]
- Abdelqader, A.M.; Abuajamieh, M.; Hammad, H.M.; Al-Fataftah, A.R. Effects of dietary butyrate supplementation on intestinal integrity of heat-stressed cockerels. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1115–1121. [Google Scholar] [CrossRef]
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Li, S.; Sun, X.; Wang, Y.; Shao, Y.; Hou, Z.; Xing, M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor dependent pathways in chicken immune organs. Oncotarget 2017, 8, 40327–40344. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the effectiveness of willow bark for musculoskeletal pain. Phytother. Res. 2009, 23, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Gligoric, E.; Igic, R.; Suvajdžic, L.; Grujic-Letic, N. Species of the Genus Salix L.: Biochemical screening and molecular docking approach to potential acetylcholinesterase inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Altınterim, B. Effects of willow bark (Salix alba) and its salicylates on blood coagulant. Karaelmas Sci. Eng. J. 2013, 3, 37–39. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Appel, K. Anti-inflammatory effects of willow bark extract. Clin. Pharmacol. Ther. 2003, 1, 96. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Chrubasik, S. Effects of an ethanolic Salix extract on the release of selected inflammatory mediators in vitro. Phytomedicine 2004, 11, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Yoshino, T.; Kobashi, K.; Hattori, M. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002, 68, 714–718. [Google Scholar] [CrossRef]
- Khan, A.S. Antipyretic and Analgesic Activities of Some Economically Important Woody Plants. In Medicinally Important Trees; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–309. [Google Scholar] [CrossRef]
- Albrecht, M.; Nahrsted, A.; Luepke, N.P.; Theisen, N.L.; Garon, G. Anti-inflammatory activity of flavonol glycosides and salicin derivatives from the leaves of Populus tremuloides. Planta Med. 1990, 56, 660. [Google Scholar] [CrossRef]
- Schmid, B.; Kotter, I.; Heide, L. Pharmacokinetics of salicin after oral administration of a standardised willow bark extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Badawy, A.; Elshazly, A.; Elsayed, A.; Krohn, K.; Riaz, M.; Schulz, B. Chemical constituents and antimicrobial activity of Salix subserrata. Rec. Nat. Prod. 2011, 5, 133–137. [Google Scholar]
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Łojkowska, E. Chromatographic analysis of simple phenols in some species from the genus Salix. Phytochem. Anal. 2010, 21, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Enayat, S.; Banerjee, S. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 2009, 116, 23–28. [Google Scholar] [CrossRef]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Pohjamo, S.P.; Hemming, J.E.; Willför, S.M.; Reunanen, M.H.T.; Holmbom, B.R. Phenolic extractives in Salix caprea wood and knots. Phytochemistry 2003, 63, 165–169. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U. The study of interactions between active compounds of coffee and willow (Salix sp.) bark water extract. BioMed Res. Int. 2014, 1–11. [Google Scholar] [CrossRef]
- Zabihi, A.N.; Mahmoudabady, M.; Soukhtanloo, M.; Hayatdavoudi, P.; Beheshti, F.; Niazmand, S. Salix alba attenuated oxidative stress in the heart and kidney of hypercholesterolemic rabbits. Avicenna J. Phytomed. 2018, 8, 63–72. [Google Scholar]
- Saracila, M.; Panaite, T.D.; Soica, C.; Tabuc, C.; Olteanu, M.; Predescu, C.; Rotar, M.C.; Criste, R.D. Use of a hydroalcoholic extract of Salix alba L. bark powder in diets of broilers exposed to high heat stress. S. Afr. J. Anim. Sci. 2019, 49, 942–954. [Google Scholar] [CrossRef]
- Shivatare, R.S.; Phopase, M.L.; Nagore, D.H.; Nipanikar, S.U.; Chitlange, S.S. Development and validation of HPLC analytical protocol for quantification of salicin from Salix alba L. Inventi Rapid Pharm. Anal. Qual. Assur. 2015, 2015, 61–66. [Google Scholar] [CrossRef]
- El-Shazly, A.; El-Sayed, A.; Fikrey, E. Bioactive secondary metabolites from Salix tetrasperma Roxb. Zeitschrift fur Naturforschung. C J. Biosci. 2012, 67, 353–359. [Google Scholar]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Saracila, M.; Tabuc, C.; Panaite, T.D.; Papuc, C.P.; Olteanu, M.; Criste, R.D. Effect of the dietary willow bark extract (Salix alba) on the caecal microbial population of broilers (14–28 days) reared at 32 °C. Agric. Life Life Agric. Conf. Proc. 2018, 1, 1155–1161. [Google Scholar] [CrossRef][Green Version]
- Bonaterra, G.A.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. In vitro anti-proliferative effects of the willow bark extract STW 33-I. Arzneimittelforschung 2010, 60, 330–335. [Google Scholar] [CrossRef]
- Jukic, M.; Burcul, F.; Carev, I.; Politeo, O.; Milos, M. Screening for acetylcholinesterase inhibition and antioxidant activity of selected plants from Croatia. Nat. Prod. Res. 2012, 26, 1703–1707. [Google Scholar] [CrossRef]
- Stajner, D.; Popovic, B.M.; Boza, P. Evaluation of willow herb’s (Epilobium angustofolium L.) antioxidant and radical scavenging capacities. Phytother. Res. 2007, 21, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (−)-epigallocatechin-3-gallate and quercetin in particle-stabilized W/O/W emulsion gels: Controlled release and bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.V.; Anil Kumar, N.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, D. Plant-Derived Antioxidants as Food. In Plants as a Source of Natural Antioxidants; Dubey, N.K., Ed.; CAB International: Wallingford, UK, 2014. [Google Scholar]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases. In Phytochemicals: Source of Antioxidants and Role in Disease Prevention; Toshiki, A., Asaduzzaman, M.D., Eds.; IntechOpen: London, UK, 2018; pp. 49–73. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Jifon, J.L.; Patil, B.S. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum. Nutr. 2012, 67, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Surh, Y.J.; Na, H.K. NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008, 2, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Menegon, S.; Columbano, A.; Giordano, S. The dual roles of NRF2 in cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Navarro, B.C.H.; Perez, J.A.M. Endogenous Antioxidants: A Review of Their Role in Oxidative Stress. In A Master Regulator of Oxidative Stress-the Transcription Factor nrf2; Morales-Gonzalez Morales, J.A., Gonzalez, A., Madrigal-Santillan, E.O., Eds.; IntechOpen: London, UK, 2016; pp. 3–19. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Singhal, S.S.; Awasthi, S.; Pandya, U.; Piper, J.T.; Saini, M.K.; Cheng, J.Z.; Awasthi, Y.C. The effect of curcumin on glutathione-linked enzymes in K562 human leukemia cells. Toxicol. Lett. 1999, 109, 87–95. [Google Scholar] [CrossRef]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C. Studies on modulation of gut microbiota by wine polyphenols: From isolated cultures to omic approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Koskinen, K.; Moissl-Eichinger, C.; Bauer, R. A combined LC-MS metabolomics-and 16S rRNA sequencing platform to assess interactions between herbal medicinal products and human gut bacteria in vitro: A pilot study on willow bark extract. Front. Pharmacol. 2017, 8, 893. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Koskinen, K.; Moissl-Eichinger, C.; Bauer, R. In vitro study of the interaction between human gut microbiota and herbal extracts using willow bark extract as an example. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Corêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- González-Alamilla, E.N.; Gonzalez-Cortazar, M.; Valladares-Carranza, B.; Rivas-Jacobo, M.A.; Herrera-Corredor, C.A.; Ojeda-Ramírez, D.; Zaragoza-Bastida, A.; Rivero-Perez, N. Chemical constituents of Salix babylonica L. and their antibacterial activity against gram-positive and gram-negative animal bacteria. Molecules 2019, 24, 2992. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Sugier, D.; Dziki, D.; Sugier, P. Bioaccessibility in vitro of nutraceuticals from bark of selected Salix species. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sun, H.X. New advances in the immunosuppressant effect of traditional Chinese medicines. Tradit. Chin. Med. 2004, 2, 36–40. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Robida-Stubbs, S.; Okunoa, A.; Goto, M.; King, G.L.; Blackwell, T.K.; Makino, T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic. Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Allaway, D.; Nebrich, S.; Mobasheri, A. Botanical extracts from rosehip (Rosa canina), willow bark (Salix alba), and nettle leaf (Urtica dioica) suppress IL-1β-inducedNF-κB activation in canine articular chondrocytes. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Kelber, O.; Bonnaterra, G.; Kinscherf, R.; Weiser, D.; Metz, J. Inhibitorische Effekte von Weidenrindenextrakten auf proinflammatorische Prozesse in LPS aktivierten Humanmonozyten. Z. Rheumatol. 2006, 65, S31. [Google Scholar]
- Roze, L.V.; Koptina, A.V.; Laivenieks, M.; Beaudry, R.M.; Jones, D.A.; Kanarsky, A.V.; Linz, J.E. Willow volatiles influence growth, development, and secondary metabolism in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 92, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P. The antioxidant response element and oxidative stress modifiers in airway diseases. Curr. Mol. Med. 2008, 8, 376–383. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Chen, X.L.; Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Polyphenol compound as a transcription factor inhibitor. Nutrients 2015, 7, 8987–9004. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sullivan, K.E. Gamma interferon and lipopolysaccharide interact at the level of transcription to induce tumor necrosis factor alpha expression. Infect. Immun. 2001, 69, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Budzynska, A.; Rozalska, B.; Sadowska, B. The immunomodulatory role of plant polyphenols (in Polish). Post. Hig. Med. Dosw. 2012, 66, 637–646. [Google Scholar] [CrossRef]
- Al-Fataftah, A.R.; Abdelqader, A. Effect of Salix babylonica, Populus nigra and Eucalyptus camaldulensis extracts in drinking water on performance and heat tolerance of broiler chickens during heat stress. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 1309–1313. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Vlaicu, P.A.; Tabuc, C.; Palade, M.L.; Gavris, T.; Criste, R.D. Dietary willow bark extract for broilers reared under heat stress. Anim. Sci. Biotechnol. 2018, 75, 92–98. [Google Scholar] [CrossRef]
- Sugito, S.; Rahmi, E.; Delima, M.; Nurliana, N.; Rusli, R.; Isa, M. Effect of Salix tetrasperma roxb. extract on the value of feed conversion ratio, carcass weight, and abdominal fat content of broiler chicken with heat stress condition. E3S Web Conf. 2020, 151, 01034. [Google Scholar] [CrossRef]
- Roussan, D.A.; Khwaldeh, G.Y.; Haddad, R.R.; Shaheen, I.A.; Salameh, G.; Al Rifai, R. Effect of ascorbic acid, acetylsalicylic acid, sodium bicarbonate, and potassium chloride supplementation in water on the performance of broiler chickens exposed to heat stress. J. Appl. Poult. Res. 2008, 17, 141–144. [Google Scholar] [CrossRef]
- Wu, D.; Xu, J.; Song, E.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperones 2015, 20, 687–696. [Google Scholar] [CrossRef]
- Salah, A.S.; Mahmoud, M.A.; Ahmed-Farid, O.A.; El-Tarabany, M.S. Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. J. Therm. Biol. 2019, 84, 259–265. [Google Scholar] [CrossRef]
- Panaite, T.D.; Saracila, M.; Papuc, C.P.; Predescu, C.N.; Soica, C. Influence of dietary supplementation of Salix alba bark on performance, oxidative stress parameters in liver and gut microflora of broilers. Animals 2020, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.; Dunshea, F.R. Gut microbiota-polyphenol interactions in chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Shabat, S.K.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 62, 1–15. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Liao, X.; Shao, Y.; Sun, G.; Yang, Y.; Zhang, L.; Guo, Y.L.X.; Lu, L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020, 99, 5883–5895. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Hu, L.; Suo, Y.; Zhang, L.; Jin, F.; Feng, X.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.C.; Williams, B.A.; Kwakkel, R.P.; Li, H.S.; Li, X.P.; Luo, J.Y.; Li, W.K.; Verstegen, M. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004, 83, 175–182. [Google Scholar] [CrossRef]
- Chamorro, S.; Romero, C.; Brenes, A.; Sánchez-Patán, F.; Bartolomé, B.; Viveros, A.; Arija, I. Impact of a sustained consumption of grape extract on digestion, gut microbial metabolism and intestinal barrier in broiler chickens. Food Funct. 2019, 10, 1444–1454. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, J.; Zhang, B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020, 99, 4892–4903. [Google Scholar] [CrossRef]
- Angelakis, E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World's Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Altan, O.; Altan, A.; Oguz, I.; Pabuccuoglu, A.; Konyalioglu, S.İ.B.E.L. Effects of heat stress on growth, some blood variables and lipid oxidation in broilers exposed to high temperature at an early age. Br. Poult. Sci. 2000, 41, 489–493. [Google Scholar] [CrossRef]
- Chhetree, R.R.; Dash, G.K.; Mondal, S.; Parhi, R. Studies on the hypoglycaemic activity of the bark of Salix tetrasperma roxburgh. Int. J. Drug Dev. Res. 2010, 2, 799–805. [Google Scholar]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: A Review. Int. J. Mol. Sci. 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Conseil, G.; Baubichon-Cortay, H.; Dayan, G.; Jault, J.M.; Barron, D.; Di Pietro, A. Flavonoids: A class of modulators with bifunctional interactions at vicinal ATPand steroid-binding sites on mouse P-glycoprotein. Proc. Natl. Acad. Sci. USA 1998, 95, 9831–9836. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Mao, Q.; Oleschuk, C.J.; Deeley, R.G.; Cole, S.P. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and ATPase activities by interaction with dietary flavonoids. Mol. Pharmacol. 2001, 59, 1171–1180. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef]
- Jastrebski, S.F.; Lamont, S.J.; Schmidt, C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS ONE 2017, 12, e0181900. [Google Scholar] [CrossRef]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broiler. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Sulaiman, R.H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Yan, F.F.; Hu, J.Y.; Amen, O.A.; Cheng, H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018, 96, 1654–1666. [Google Scholar] [CrossRef]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
| Species | Origin and Sampling Period | Extraction Method | Compounds Detection Method | Identified/Quantified (mg/g) Compounds | Reference | |
|---|---|---|---|---|---|---|
| Polyphenols | Saligenin Derivatives | |||||
| S. alba | Pecenjevce (June) | 70% Aqueous ethanol by maceration for 48 h at room temperature (25 °C) | HPLC with diode array detection | Gallic acid (0.17) Chlorogenic acid (1.65) p-Hydroxybenzoic acid (0.32) Syringic acid (0.22) Epicatechin (1.17) p-Coumaric acid (0.15) Rutin (1.75) Quercetin (0.38) trans-Cinnamic acid (0.15) Naringenin (0.20) | Salicin (3.99) | [79] |
| S. babylonica | Bosut riverside, Morovic (September) | Gallic acid (0.17) Chlorogenic acid (1.92) p-Hydroxybenzoic acid (1.21) Syringic acid (0.34) Epicatechin (2.68) p-Coumaric acid (0.15) Rutin (1.36) Quercetin (0.52) trans-Cinnamic acid (0.57) Naringenin (0.27) | Salicin (3.11) | |||
| S. purpurea | Mountain Deli Jovan (August) | Chlorogenic acid (1.14) Caffeic acid (1.05) Epicatechin (2.08) p-Coumaric acid (1.53) Rutin (4.30) Quercetin (1.13) | Salicin (7.53) | |||
| S. purpurea | Mountain Deli Jovan (August) | 70% Aqueous ethanol by maceration for 48 h at room temperature (25 °C) | HPLC with diode array detection | trans-Cinnamic acid (0.13) Naringenin (0.26) | [79] | |
| S. triandra | Vlasina Lake (July) | Gallic acid (0.26) Chlorogenic acid (1.63) Epicatechin (1.77) p-Coumaric acid (0.22) Rutin (1.73) Quercetin (0.67) trans-Cinnamic acid (0.53) Naringenin (0.33) | Salicin (2.87) | |||
| S. subserrata | Sharkia, Egypt, (March) | Exhaustively extraction with methanol at room temperature | Silica gel column chromatography | (+) Catechin 1,2-Benzenedicarboxylic acid, Methyl 1-hydroxy-6- oxocyclohex-2-enecarboxylate Catechol propyl acetate | bis (2-ethylhexyl) ester saligenin | [87] |
| S. alba S. daphnoides | Willow cultures of the University of Warmia and Mazury (Olsztyn, Poland) | Exhaustively extraction with methanol (3 × 120 mL, 60 °C) | MGD-HPTL | p-Hydroxybenzoic acid Vanillic acid Cinnamic acid p-Coumaric acid Ferulic acid Caffeic acid | [88] | |
| S. acutifolia S. daphnoides, S. purpurea L. S. triandra | From their natural habitat in west Poland (March) | Exhaustively extraction with methanol (3 × 120 mL, 60 °C) | MGD-HPTL | p-Hydroxybenzoic acid Vanillic acid Cinnamic acid p-Coumaric acid Ferulic acid Caffeic acid | [88] | |
| S. herbacea S. sachalinensis S. viminalis | Garden of Medicinal Plants (Medical University of Gdańsk, Poland) | |||||
| S. purpurea | Labofarm (Starogard Gd., Poland, March) | |||||
| S. aegyptiaca | Ghaene ghom, Iran, (unknown season) | Ethanol extraction (1:10, w/v) by sonication 20 min | HPLC method with a PDA detector | Gallic acid (0.69) Caffeic acid (0.06) Vanillin (1.53) p-Coumaric acid (0.80) Myricetin (5.87) Catechin (0.93) Epigallocatechin gallate (2.39) Rutin (quercetin-3-rhamnosyl glucoside) (4.59) Quercetin (1.47) | - | [89] |
| S. daphnoides | Finzelberg, Andernach, Germany (unknown season) | Methanol extraction, 1:25 (w:v) by stiring for 2 h | RP-HPLC coupled to electrospray triple-quadrupole MS and MS/MS | Naringenin-7-O-glucoside Isosalipurposide Salipurposide | Saligenin, Salicylic acid Salicin Isosalicin Picein Salidroside Triandrin Salicoylsalicin Salicortin Tremulacin | [90] |
| Species | Origin and Sampling Period | Extraction Method | Total Phenolics (mg GAE/g 1) | Total Flavonoids (mg QE 2/g dw 4) | Salicin Content (mg/mL) | Antioxidant Activity | Reference |
|---|---|---|---|---|---|---|---|
| S. alba | Pecenjevce, June | 70% Aqueous ethanol extraction by maceration for 48 h at room temperature (25 °C) | 40.9 | 3.48 | 3.99 mg/g | IC50 DPPH 3 = 1.83 μg/mL | [79] |
| S. babylonica | Bosut riverside, Morovic, September | 20.17 | 3.13 | 3.11 mg/g | IC50 DPPH 3 = 2.59 μg/mL | ||
| S. purpurea | Mountain Deli Jovan, August | 69.1 | 31 | 7.53 mg/g | IC50 DPPH 3 = 4.73 μg/mL | ||
| S. triandra | Vlasina Lake, July | 18.41 | 2.88 | 2.87 mg/g | IC50 DPPH 3 = 7.79 μg/mL | ||
| S. alba | Plant Extract, Radaia, Cluj County, unknown season | Hydroalcoholic extraction in grain alcohol and water by maceration | 4.67 | 98% | 35.13 mM equivalent ascorbic acid 35.97 mM equivalent vitamin E | [95] | |
| S. alba | Phyto concentrate India, unknown season | Methanol extraction by sonication for 30 min | - | - | 1.92% | - | [96] |
| S. purpurea | cultivated on the sandy soil (heavy loamy sand) of experimental fields at the University of Life Sciences in Lublin, November | Water extraction by shaken for 60 min at room temperature | 20.04 | - | - | EC50 LPO 5 = 8.06 mg/mL | [93] |
| S. myrsinifolia | 23.10 | - | - | EC50 LPO 5 = 8.31 mg/mL | |||
| S. alba | - | Soxhlet extraction with ethanol for 7 h | 162 | - | - | DPPH 3 In% = 12.50, 37.50 and 80.00% of 10, 50 and 100 μg/mL | [35] |
| S. tetrasperma Roxb. | Zagazig City, Sharkia Province, Egypt, unknown season | Methanol extraction | - | - | - | IC50 DPPH 3 = 94.5 µg/mL | [97] |
| S. aegyptiaca | Ghaene ghom, Iran, unknown season | Ethanol extraction (1:10, w/v) by sonication for 20 min. | 212 | 479 | 3.1 | 169 mg QE 2/g dried sample | [89] |
| S. aegyptiaca | Water extraction (1:10, w/v) by sonication for 20 min. | 139 | 243 | 0.07 | 78 mg QE 2/g dried sample | ||
| S. caprea | Finnish origin, unknown season | 80% Aqueous methanol extraction, using Ultra Turrax mixer, 1 min. | 75.5 | - | - | 96% Inhibition (500 ppm) MLO 5 | [98] |
| S. alba | Finnish origin, unknown season | 80% Aqueous methanol extraction, using Ultra Turrax mixer, 1 min. | 58.6 | - | - | 96% Inhibition (500 ppm) MLO 5 |
| Species/Origin | Experimental Model | Effect | Reference |
|---|---|---|---|
| Willow bark extract (Shamanshop, Camden, NY, USA) | Human umbilical vein endothelial cells | —willow bark extract failed to activate ARE 1 —luciferase activity, whereas a salicin-free willow bark extract fraction had intensive activity —induced antioxidant enzymes and prevented oxidative stress through activation of Nrf2 2 independent of salicin | [140] |
| S. alba (chloroform extract) | Primary canine articular chondrocytes | —anti-inflammatory and anabolic effects on chondrocytes, reducing cytokine induced activation and up regulation of pro-inflammatory enzymes (MMPs and COX-2 5) and NF-κB 3 | [141] |
| 5 fractions of a willow bark extract from S. daphnoides, purpurea and fragilis | Human monocytes | —↓ nitrite and NO 7 release —inhibited the inflammatory cytokines (IFN-γ 9), and lipopolysaccharide (LPS) | [142] |
| Salix extract 1520 L (ethanol extract) | Primary human monocytes | —inhibits COX-2 5-mediated PGE2 8 release through other compounds than salicin or salicylate | [82] |
| Willow bark extract STW 33-I (water extract) and a polyphenol-rich fraction of STW 33-I | Colon-carcinoma cell line HT-29 | —anti-proliferative and pro- apoptotic effects on HT-29 —inhibited COX-1 6 expression | [100] |
| S. alba (ethanol extract) | Bacterial strains and one yeast HL-60 4 cells | —significant antioxidant activity and antimicrobial activities against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans —highly cytotoxic to HL-60 cells, dependently on the dose and time | [35] |
| S. alba (water extract) | Bacterial strains | —antimicrobial activity against E. coli, Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus and Salmonella enteritis | [34] |
| S. acutifolia (ethanol extract) | A. parasiticus | —↓ aflatoxin production of A. parasiticus | [143] |
| Species/Origin | Part of Plant and form Used | Dose of Inclusion | Animal Model | Temperature | Effect | Reference |
|---|---|---|---|---|---|---|
| S. babylonica (Zai Natural parks, Jordan) | Leaves (extract) | 100 mL/day | Arbor Acres broiler chickens | 35 °C | —↑ final body weight, average daily gain, and average daily feed intake —↓ feed conversion ratio —↓ rectal temperature —↓ panting rate (breath/minute) —↓ mortality (%) | [153] |
| S. alba (Plant Extract, Radaia, Cluj County) | Bark (hydroalcoholic extract) | 0.025% and 0.05% in diet | Cobb 500 broiler chickens | 32 °C | —↓ the serum cholesterol, triglycerides, and ALT —↓ the malondialdehyde concentration in liver —↓ the number of E. coli and staphylococci in the caecum —↑ the number of lactobacilli in the caecum | [95] |
| S. alba (Plant Extract, Radaia, Cluj County) | Bark (hydroglyceroalcoholic extract) | 1% in diet | Cobb 500 broiler chickens | 32 °C | —↓ serum cholesterol and glucose level —↓ the pathogenic bacteria in the caecum | [154] |
| S. tetrasperma Roxb (unknown origin) | Leaves (extract) | 50 and 100 mg/L of drinking water | No data | 34 °C | —no effect on performance —↑ the weight of abdominal fat | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saracila, M.; Panaite, T.D.; Papuc, C.P.; Criste, R.D. Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review. Antioxidants 2021, 10, 686. https://doi.org/10.3390/antiox10050686
Saracila M, Panaite TD, Papuc CP, Criste RD. Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review. Antioxidants. 2021; 10(5):686. https://doi.org/10.3390/antiox10050686
Chicago/Turabian StyleSaracila, Mihaela, Tatiana Dumitra Panaite, Camelia Puia Papuc, and Rodica Diana Criste. 2021. "Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review" Antioxidants 10, no. 5: 686. https://doi.org/10.3390/antiox10050686
APA StyleSaracila, M., Panaite, T. D., Papuc, C. P., & Criste, R. D. (2021). Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review. Antioxidants, 10(5), 686. https://doi.org/10.3390/antiox10050686








