Effects of Drying Methods on Antioxidant, Anti-Inflammatory, and Anticancer Potentials of Phenolic Acids in Lovage Elicited by Jasmonic Acid and Yeast Extract
Abstract
1. Introduction
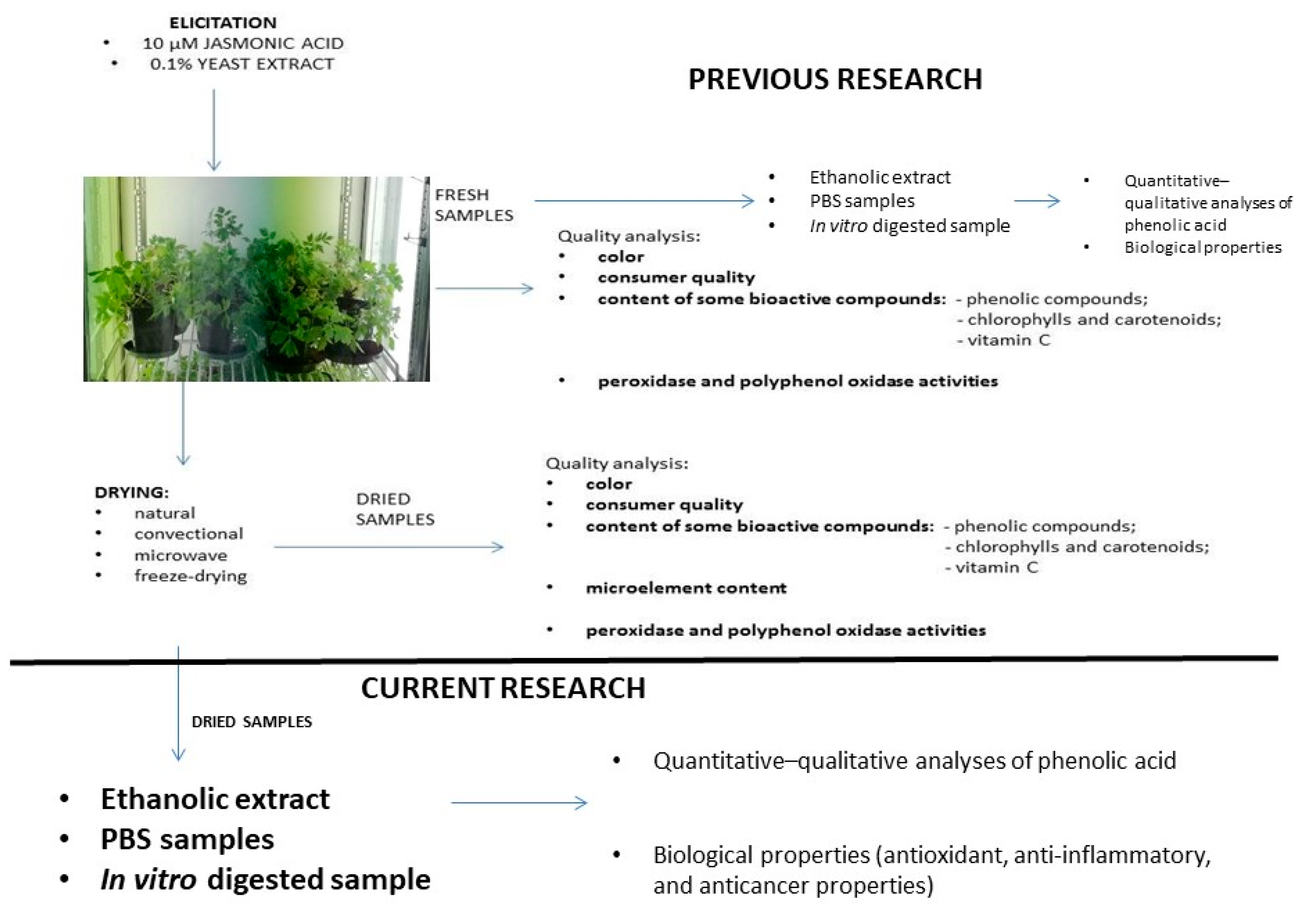
2. Materials and Methods
2.1. Growth Conditions
2.2. Drying Techniques
2.3. Preparation of Extracts
2.3.1. Ethanolic Extracts
2.3.2. PBS Extracts
2.3.3. In Vitro Digestion
2.4. Analysis of Phenolic Acids
2.5. Antioxidant Activities
2.5.1. Free Radical Scavenging Assay
2.5.2. Ferric Reducing Antioxidant Power
2.5.3. Chelating Power
2.6. Determination of Potential Anti-Inflammatory Properties
2.6.1. Lipoxygenase Inhibitory Activity
2.6.2. Cyclooxygenase-2 Inhibitory Activity
2.7. Anticancer Properties
2.7.1. Cell Culture
2.7.2. MTT and NR Tests
2.7.3. Cell Viability and Type of Cell Death
2.7.4. Cell Cycle
2.8. Statistical Analysis
3. Results
3.1. Phenolic Acid Analysis
3.2. Antioxidant Activities
3.3. Lipoxygenase and Cyclooxygenase 2 Inhibition
3.4. Influence of Analyzed Extracts on Healthy and Cancer Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical knowledge of Apiaceae family in Iran: A review. J. Phytomed. 2016, 6, 621–635. [Google Scholar]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Złotek, U.; Szymanowska, U.; Jęderka, K.; Rybczyńska-Tkaczyk, K.; Lewicki, S. In vitro Antioxidant, Anti-inflammatory, Anti-metabolic Syndrome, Antimicrobial, and Anticancer Effect of Phenolic Acids Isolated from Fresh Lovage Leaves [ Levisticum o ffi cinale Koch ] Elicited with Jasmonic Acid and Yeast Extract. Antioxidants 2020, 9, 554. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Pecio, Ł.; Kozachok, S.; Jakubczyk, A. Antioxidative and Potentially Anti-inflammatory Activity of Phenolics from Lovage Leaves Levisticum officinale Koch Elicited with Jasmonic Acid and Yeast Extract. Molecules 2019, 24, 1441. [Google Scholar] [CrossRef]
- Śledź, M.; Nowacka, M.; Wiktor, A.; Witrowa-Rajchert, D. Selected chemical and physico-chemical properties of microwave-convective dried herbs. Food Bioprod. Process. 2013, 91, 421–428. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Rybczyńska-Tkaczyk, K.; Jakubczyk, A. Effect of jasmonic acid, yeast extract elicitation, and drying methods on the main bioactive compounds and consumer quality of lovage (Levisticum officinale Koch). Foods 2020, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B. Nutritional and antioxidant potential of lentil sprouts affected by elicitation with temperature stress. J. Agric. Food Chem. 2014, 62, 3306–3313. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar]
- Szymanowska, U.; Jakubczyk, A.; Baraniak, B.; Kur, A. Characterisation of lipoxygenase from pea seeds (Pisum sativum var. Telephone L.). Food Chem. 2009, 116, 906–910. [Google Scholar] [CrossRef]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Rózewska, E.; Skopiński, P. Inhibition of proliferation, migration and invasiveness of endothelial murine cells culture induced by resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M.; Zdanowski, R.; Suska, M.; Brewczyńska, A.; Stankiewicz, W.; Kloc, M.; Kubiak, J.Z.; Lewicki, S. Effects of Hexachlorophene, a Chemical Accumulating in Adipose Tissue, on Mouse and Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 211–222. [Google Scholar] [CrossRef]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food matrix effects of polyphenol bioaccessibility from almond skin during simulated human digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Ramirez-Gomez, X.S.; Beltran-Campos, V.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Feregrino-Pérez, A.A. Changes in the content of phenolic compounds and biological activity in traditional Mexican herbal infusions with different drying methods. Molecules 2020, 25, 1601. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Demirci, M.; Selen, S.; Toydemir, G.; Boyacioglu, D.; Capanoglu, E. Home processing of tomatoes (Solanum lycopersicum): Effects on in vitro bioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity. J. Sci. Food Agric. 2014, 94, 2225–2233. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E. Investigating the in vitro bioaccessibility of polyphenols in fresh and sun-dried figs (Ficus carica L.). Int. J. Food Sci. Technol. 2013, 48, 2621–2629. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Kuete, V.; McGaw, L.J.; Eloff, J.N. The 15-lipoxygenase inhibitory, antioxidant, antimycobacterial activity and cytotoxicity of fourteen ethnomedicinally used African spices and culinary herbs. J. Ethnopharmacol. 2014, 156, 1–8. [Google Scholar] [CrossRef]
- Dogan, K.; Tornuk, F. Improvement of bioavailability of bioactive compounds of medicinal herbs by drying and fermentation with Lactobacillus plantarum. Funct. Foods Health Dis. 2019, 9, 735–748. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Yi, W.; Wetzstein, H.Y. Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience 2011, 46, 70–73. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sȩczyk, Ł.; Złotek, U.; Rózyło, R.; Kaszuba, K.; Ryszawy, D.; Czyz, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. BioMed Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szychowski, K.A.; Świeca, M. Potential in vitro antioxidant, anti-inflammatory, antidiabetic, and anticancer effect of arachidonic acid-elicited basil leaves. J. Funct. Foods 2017, 36, 290–299. [Google Scholar] [CrossRef]
- Rosa, L.; Silva, N.; Soares, N.; Monteiro, M.; Teodoro, A. Anticancer Properties of Phenolic Acids in Colon Cancer—A Review. J. Nutr. Food Sci. 2016, 6, 1–7. [Google Scholar]
- Bogucka-Kocka, A.; Smolarz, H.D.; Kocki, J. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia 2008, 79, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; Zin El-Abedin, T.K.A.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
| Sample | Compounds [mg/g DW] | |||||||
|---|---|---|---|---|---|---|---|---|
| Protocatechuic Acid | p-hydroxybenzoic Acid | Caffeic Acid | Syringic Acid | Vanillic Acid | Ferulic Acid | Sinapic Acid | Salicylic Acid | |
| Freeze-Dried Samples | ||||||||
| Ethanolic extracts | ||||||||
| CL-E | 159.59 ± 0.42 dC | nd | 177.32 ± 0.98 eE | 15.34 ± 0.02 aA | 7.71 ± 0.02 bAB | 10.40 ± 0.06 aA | 102.14 ± 0.52 aC | 492.75 ± 1.37 aC |
| JAL-E | 413.56 ± 0.78 iE | nd | 440.86 ± 1.43 jG | 38.55 ± 0.23 eC | nd | 15.84 ± 0.01 cC | 260.91 ± 0.93 hF | 1149.10 ± 6.23 hF |
| YEL-E | 264.84 ± 0.41 hD | nd | 312.03 ± 0.62 iF | 22.02 ± 0.12 cB | 6.19 ± 0.03 aA | 13.52 ± 0.05 bB | 232.78 ± 0.49 fgE | 1036.70 ± 8.10 gE |
| PBS extracts | ||||||||
| CL-P | nd | 15.65 ± 0.06 cdC | 10.22 ± 0.01 aA | nd | 12.58 ± 0.49 cD | nd | 84.73 ± 4.20 cB | nd |
| JAL-P | 9.56 ± 0.08 aA | 13.64 ± 1.92 cB | 12.45 ± 0.16 abB | nd | 10.42 ± 0.13 bC | nd | 110.77 ± 7.57 dC | 33.65 ± 0.44 aA |
| YEL-P | nd | 9.45 ± 0.15 aA | nd | nd | 8.42 ± 1.58 aB | nd | 61.44 ± 3.21 bA | nd |
| GD extracts | ||||||||
| CL-GD | 31.27 ± 9.91 abcB | 150.22 ± 12.05 bD | 18.73 ± 4.47 abcC | 14.13 ± 3.67 abcA | 16.88 ± 0.76 bcE | nd | 99.14 ± 7.89 abBC | 185.15 ± 34.42 abB |
| JAL-GD | 126.67 ± 29.42 eC | 273.40 ± 22.48 deE | 138.38 ± 2.19 eD | 54.39 ± 7.79 eD | 26.63 ± 3.12 deF | nd | 303.54 ± 40.57 fG | 693.13 ± 74.54 eD |
| YEL-GD | nd | 290.60 ± 0.21 eF | nd | nd | 18.40 ± 0.02 cE | nd | 188.43 ± 1.05 deD | nd |
| Microwave-Dried Samples | ||||||||
| Ethanolic extracts | ||||||||
| CM-E | 111.43 ± 0.34 bD | nd | 148.59 ± 3.46 dD | 34.12 ± 0.20 dC | 10.68 ± 0.07 cA | 20.82 ± 0.06 dA | 186.76 ± 2.82 bcD | 654.54 ± 16.19 cF |
| JAM-E | 235.26 ± 6.39 gE | nd | 257.94 ± 7.48 hF | 52.38 ± 0.69 hE | 15.91 ± 0.57 fC | 33.94 ± 2.17 hC | 210.74 ± 8.11 dE | 968.39 ± 74.54 fH |
| YEM-E | 130.39 ± 6.43 cD | nd | 173.08 ± 5.51 eE | 44.66 ± 1.46 fD | 12.32 ± 0.12 dB | 25.96 ± 3.38 gB | 223.97 ± 13.16 efE | 781.96 ± 55.85 eG |
| PBS extracts | ||||||||
| CM-P | 46.45 ± 0.21 dB | nd | 58.92 ± 0.33 dC | 15.17 ± 0.11 cB | 15.93 ± 0.14 dC | nd | 108.55 ± 0.56 dB | 146.09 ± 1.19 eB |
| JAM-P | 240.68 ± 0.65 hE | nd | 290.26 ± 0.61 iG | 71.93 ± 0.19 hF | 30.65 ± 0.08 fD | nd | 190.61 ± 0.12 fD | 557.83 ± 1.30 hE |
| YEM-P | 22.88 ± 0.16 cA | 37.42 ± 0.25 gA | 18.80 ± 0.06 cA | 10.01 ± 0.04 bA | 12.04 ± 0.25 cB | nd | 86.61 ± 0.22 cA | 117.31 ± 0.23 cA |
| GD extracts | ||||||||
| CM-GD | 74.52 ± 16.44 dC | 210.29 ± 19.73 cC | 36.61 ± 6.50 dB | 29.62 ± 5.29 dC | 16.72 ± 1.60 bcC | nd | 159.91 ± 24.89 cdC | 409.11 ± 91.18 cD |
| JAM-GD | 253.35 ± 27.51 fE | 272.01 ± 2.78 deD | 153.74 ± 14.10 fD | 102.83 ± 10.04 fG | 30.36 ± 1.09 eD | nd | 278.95 ± 1.77 fF | 1019.41 ± 23.70 fH |
| YEM-GD | 50.05 ± 16.66 abcdC | 109.75 ± 26.71 aB | 27.97 ± 7.26 bcdAB | 20.18 ± 5.99 abcdBC | 14.05 ± 1.92 bC | nd | 93.20 ± 26.45 abAB | 197.93 ± 84.82 abC |
| Traditionally Dried Samples | ||||||||
| Ethanolic extracts | ||||||||
| CT-E | 67.93 ± 0.25 aB | 61.63 ± 0.06 dB | 73.88 ± 0.33 bE | 20.54 ± 0.30 bC | 16.35 ± 0.10 fC | 16.67 ± 0.05 cA | 183.75 ± 0.29 bD | 544.54 ± 0.92 bE |
| JAT-E | 109.24 ± 0.61 bC | 97.05 ± 0.19 fC | 119.42 ± 0.82 cG | 46.42 ± 0.04 gE | 18.73 ± 0.08 iE | 21.49 ± 0.02 deB | 261.88 ± 0.32 hF | 770.86 ± 0.55 eF |
| YET-E | 62.99 ± 0.45 aB | 63.64 ± 0.14 eB | 65.42 ± 0.14 aD | 19.50 ± 0.06 bC | 16.03 ± 0.12 fC | 16.48 ± 0.01 cA | 194.22 ± 0.07 bcD | 536.23 ± 0.98 abE |
| PBS extracts | ||||||||
| CT-P | 20.10 ± 0.22 cA | 25.47 ± 0.12 eA | 20.55 ± 0.13 cB | 9.82 ± 0.07 bB | 9.41 ± 0.13 abB | nd | 48.32 ± 0.03 aA | 74.72 ± 0.60 bA |
| JAT-P | 106.57 ± 0.33 fC | 99.79 ± 0.06 hC | 96.23 ± 0.15 gF | 48.25 ± 0.10 gF | 29.18 ± 0.21 eG | nd | 228.75 ± 0.03 gE | 438.91 ± 1.48 gD |
| YET-P | 15.59 ± 0.46 bA | 27.46 ± 0.09 fA | 15.24 ± 0.11 bA | 6.73 ± 0.04 aA | 8.61 ± 0.04 aA | nd | 63.59 ± 0.20 bB | 74.10 ± 0.08 bA |
| GD extracts | ||||||||
| CT-GD | 21.49 ± 3.90 abA | 273.23 ± 16.57 deD | 16.14 ± 0.40 abA | 10.21 ± 0.02 abB | 18.75 ± 1.50 c | nd | 171.10 ± 33.59 dD | 298.74 ± 49.77 bcC |
| JAT-GD | 62.93 ± 0.46 cdB | 296.99 ± 1.38 eE | 36.94 ± 0.27 dC | 25.10 ± 0.19 cdD | 26.18 ± 0.14 dF | nd | 226.70 ± 0.89 eE | 546.00 ± 4.45 dE |
| YET-GD | 26.81 ± 0.47 abA | 174.08 ± 0.18 bD | 17.81 ± 0.42 bAB | 11.74 ± 0.10 abB | 17.41 ± 0.02 bcD | nd | 115.97 ± 0.22 bcC | 189.18 ± 0.10 abB |
| Convectionally Dried Samples | ||||||||
| Ethanolic extracts | ||||||||
| CC-E | 186.76 ± 0.52 fE | 27.05 ± 0.16 aC | 202.31 ± 0.66 gG | 63.05 ± 0.07 jH | 17.20 ± 0.17 gE | 24.19 ± 0.10 fgC | 197.18 ± 0.63 cG | 812.49 ± 1.04 eH |
| JAC-E | 171.51 ± 0.29 eE | 40.76 ± 0.16 bD | 188.60 ± 0.38 fF | 58.11 ± 0.26 iG | 14.79 ± 0.09 eC | 23.29 ± 0.03 efB | 215.38 ± 0.19 deH | 775.73 ± 0.59 eG |
| YEC-E | 129.45 ± 0.14 cD | 42.06 ± 0.02 cD | 153.35 ± 0.29 dE | 51.98 ± 0.11 hF | 17.71 ± 0.05 hE | 22.52 ± 0.01 defA | 236.07 ± 0.20 gI | 712.59 ± 0.26 dF |
| PBS extracts | ||||||||
| CC-P | 65.00 ± 0.59 eC | 11.36 ± 0.03 bA | 68.90 ± 0.50 fD | 26.33 ± 0.13 eD | 11.90 ± 0.04 cB | nd | 57.19 ± 0.04 bB | 127.35 ± 0.72 dB |
| JAC-P | 63.31 ± 1.37 eBC | 14.55 ± 0.07 cdB | 65.20 ± 1.16 eD | 23.59 ± 0.23 dC | 9.60 ± 0.06 abA | nd | 64.71 ± 1.26 bC | 160.75 ± 1.45 fC |
| YEC-P | 126.44 ± 2.94 gD | nd | 157.84 ± 3.83 hE | 42.82 ± 0.66 fE | 16.89 ± 0.01 dD | nd | 174.35 ± 5.69 eE | 440.20 ± 8.90 gE |
| GD extracts | ||||||||
| CC-GD | 52.58 ± 11.53 bcdB | 162.02 ± 21.63 bF | 32.07 ± 3.97 cdC | 24.36 ± 3.61 cdCD | 17.16 ± 2.83 bcE | nd | 121.51 ± 11.87 bcD | 279.64 ± 55.88 bD |
| JAC-GD | 50.07 ± 0.42 abcdB | 240.28 ± 0.27 cdG | 23.97 ± 0.06 abcdB | 20.58 ± 0.13 bcdB | 24.69 ± 0.05 dF | nd | 187.68 ± 0.13 deF | 287.81 ± 1.49 bcD |
| YEC-GD | 17.09 ± 0.05 aA | 79.95 ± 0.08 aE | 12.92 ± 0.09 aA | 8.65 ± 0.03 aA | 10.17 ± 0.02 aA | nd | 55.19 ± 0.14 aA | 86.44 ± 0.19 aA |
| Extract Type | Sample | Antioxidant Activities | Potential Anti-Inflammatory Activities | |||
|---|---|---|---|---|---|---|
| ABTS [µMTrolox/g DW] | RP [mg Trolox/g DW] | CHP [mg EDTA/g DW] | LOX Inhibition EC50 [mg DW/mL] | COX2 Inhibition EC50 [mg DW/mL] | ||
| Freeze-Dried Samples | ||||||
| Ethanolic extracts | CL-E | 1.87 ± 0.21 bdAB | 19.97 ± 5.98 bcB | 3.18 ± 0.35 gB | 0.115 ± 0.004 dD | 0.113 ± 0.0004 deDE |
| JAL-E | 1.99 ± 0.10 dB | 18.35 ± 5.73 bcB | 3.19 ± 0.40 gB | 0.145 ± 0.004 eE | 0.021 ± 0.000 aA | |
| YEL-E | 1.53 ± 0.17 bcA | 7.90 ± 2.94 aA | 2.28 ± 0.32 cefA | 0.208 ± 0.015 fDE | 0.048 ± 0.0001 bB | |
| PBS extracts | CL-P | 3.98 ± 0.79 cdeC | 22.03 ± 4.52 dB | 6.50 ± 0.34 abC | 0.044 ± 0.001 aA | 0.075 ± 0.004 cC |
| JAL-P | 4.25 ± 0.62 eC | 19.64 ± 0.72 bcdB | 5.95 ± 0.63 aC | 0.068 ± 0.003 cC | 0.122 ± 0.006 eE | |
| YEL-P | 4.17 ± 0.98 deC | 15.73 ± 1.32 abcdAB | 6.68 ± 0.50 abC | 0.063 ± 0.0005 cC | 0.103 ± 0.001 dD | |
| GD extracts | CL-GD | 6.82 ± 0.36 eE | 98.81 ± 4.83 bcdC | 14.25 ± 0.49 bD | 0.059 ± 0.004 abcABC | 0.019 ± 0.0001 aA |
| JAL-GD | 6.40 ± 0.41 deDE | 115.93 ± 6.04 cdeC | 14.48 ± 0.35 bD | 0.061 ± 0.002 bcBC | 0.038 ± 0.003 bB | |
| YEL-GD | 5.71 ± 0.32 dD | 108.29 ± 4.36 cdC | 14.77 ± 0.14 bD | 0.047 ± 0.004 abAB | 0.013 ± 0.0005 aA | |
| Microwave-Dried Samples | ||||||
| Ethanolic extracts | CM-E | 0.92 ± 0.16 aA | 14.17 ± 0.93 abA | 2.41 ± 0.17 efA | 0.296 ± 0.032 eE | 0.209 ± 0.011 fF |
| JAM-E | 1.46 ± 0.16 bB | 17.88 ± 1.25 bcA | 0.98 ± 0.12 aA | 0.164 ± 0.008 cC | 0.147 ± 0.012 dD | |
| YEM-E | 0.95 ± 0.12 aAB | 9.42 ± 0.46 aA | 2.25 ± 0.16 cefA | 0.224 ± 0.013 dD | 0.171 ± 0.0003 eE | |
| PBS extracts | CM-P | 3.05 ± 0.95 bcdC | 20.90 ± 1.13 cdA | 6.92 ± 0.71 abB | 0.072 ± 0.011 abAB | 0.044 ± 0.0004 bB |
| JAM-P | 4.59 ± 0.57 eD | 20.90 ± 8.03 cdA | 7.30 ± 1.15 bB | 0.097 ± 0.002 bB | 0.058 ± 0.002 bcBC | |
| YEM-P | 4.66 ± 0.24 eD | 20.31 ± 6.81 bcdA | 7.03 ± 0.33 abB | 0.072 ± 0.0001 abAB | 0.061 ± 0.003 cC | |
| GD extracts | CM-GD | 4.18 ± 0.22 bcD | 115.46 ± 4.47 cdeC | 13.97 ± 1.29 bD | 0.068 ± 0.004 abAB | 0.012 ± 0.0001 aA |
| JAM-GD | 4.06 ± 0.37 bcD | 131.72 ± 6.39 eD | 12.11 ± 1.86 aC | 0.059 ± 0.002 aA | 0.013 ± 0.0006 aA | |
| YEM-GD | 4.12 ± 0.15 bcD | 101.66 ± 9.20 bcdB | 14.03 ± 0.53 bD | 0.069 ± 0.001 abAB | 0.013 ± 0.0002 aA | |
| Traditionally Dried Samples | ||||||
| Ethanolic extracts | CT-E | 1.40 ± 0.17 bA | 25.98 ± 2.06 cC | 1.62 ± 0.40 abcA | 0.221 ± 0.028 bB | 0.051 ± 0.001 dD |
| JAT-E | 1.82 ± 0.16 cdAB | 20.70 ± 4.23 bcBC | 2.32 ± 0.14 cefAB | 0.198 ± 0.0005 bB | 0.024 ± 0.001 bB | |
| YET-E | 2.05 ± 0.11 cBC | 15.20 ± 2.01 abAB | 2.75 ± 0.49 fgB | 0.205 ± 0.002 bB | 0.031 ± 0.001 cC | |
| PBS extracts | CT-P | 1.89 ± 0.14 aB | 8.83 ± 3.93 aA | 7.29 ± 0.35 bC | 0.051 ± 0.008 aA | 0.055 ± 0.004 eD |
| JAT-P | 4.53 ± 0.34 eF | 11.88 ± 3.56 abcdA | 7.08 ± 0.12 abC | 0.049 ± 0.004 aA | 0.057 ± 0.002 eD | |
| YET-P | 3.00 ± 0.20 abcD | 10.15 ± 2.06 abA | 6.72 ± 0.19 abC | 0.060 ± 0.0001 aA | 0.055 ± 0.001 deD | |
| GD extracts | CT-GD | 4.64 ± 0.61 cF | 37.69 ± 3.43 aD | 14.10 ± 0.91 bD | 0.049 ± 0.002 aA | 0.013 ± 0.0007 aA |
| JAT-GD | 4.03 ± 0.25 bcF | 42.60 ± 2.51 aD | 13.69 ± 0.84 abD | 0.043 ± 0.003 aA | 0.019 ± 0.002 abAB | |
| YET-GD | 3.70 ± 0.24 bE | 41.34 ± 3.84 aD | 13.44 ± 0.69 abD | 0.051 ± 0.002 aA | 0.030 ± 0.001 cC | |
| Convectionally Dried Samples | ||||||
| Ethanolic extracts | CC-E | 1.99 ± 0.20 dAB | 25.98 ± 2.06 cA | 2.50 ± 0.30 efgA | 0.175 ± 0.011 cC | 0.083 ± 0.0003 cC |
| JAC-E | 2.00 ± 0.15 dAB | 20.70 ± 4.23 bcA | 1.86 ± 0.38 bceA | 0.179 ± 0.001 cC | 0.167 ± 0.005 eE | |
| YEC-E | 1.46 ± 0.09 bA | 15.20 ± 2.01 abA | 1.36 ± 0.84 abA | 0.212 ± 0.003 dD | 0.176 ± 0.004 eE | |
| PBS extracts | CC-P | 2.43 ± 0.50 abBC | 11.02 ± 3.60 abcA | 6.49 ± 1.14 abB | 0.146 ± 0.001 bB | 0.099 ± 0.010 dD |
| JAC-P | 2.40 ± 0.44 abBC | 13.14 ± 4.19 abcdA | 6.20 ± 0.73 abB | 0.211 ± 0.012 dD | 0.091 ± 0.001 cdD | |
| YEC-P | 3.97 ± 0.50 cdeD | 13.60 ± 4.43 abcdA | 7.13 ± 0.27 abB | 0.22 ± 0.001 dD | 0.093 ± 0.0003 cdD | |
| GD extracts | CC-GD | 3.93 ± 0.65 bcD | 98.54 ± 24.95 bcB | 13.81 ± 0.32 bC | 0.131 ± 0.010 abAB | 0.03 ± 0.002 bB |
| JAC-GD | 6.23 ± 0.35 deE | 80.82 ± 0.34 bB | 13.51 ± 0.43 abC | 0.122 ± 0.006 aA | 0.018 ± 0.001 abAB | |
| YEC-GD | 2.84 ± 0.50 aC | 121.30 ± 11.08 deC | 14.28 ± 0.56 bC | 0.117 ± 0.005 aA | 0.016 ± 0.001 aA | |
| Protocatechuic Acid | p-hydroxybenzoic Acid | Caffeic Acid | Syringic Acid | Vanillic Acid | Ferulic Acid | Sinapic Acid | Salicylic Acid | |
|---|---|---|---|---|---|---|---|---|
| ABTS | −0.32 | 0.63 | −040 | −0.11 | 0.50 | −0.69 | −0.04 | −0.40 |
| RP | −0.14 | 0.72 | −0.29 | 0.08 | 0.36 | −0.45 | 0.08 | −0.10 |
| CHP | −0.43 | 0.76 | −0.54 | −0.22 | 0.38 | −0.78 | −0.19 | −0.47 |
| LOX inhibition * | 0.37 | −0.48 | 0.47 | 0.29 | −0.26 | 0.66 | 0.28 | 0.50 |
| COX2 inhibition * | 0.14 | −0.60 | 0.27 | 0.18 | −0.27 | 0.57 | 0.01 | 0.19 |
| inhibition of proliferation of NCI-N87 cell line | 0.13 | 0.27 | 0.14 | 0.06 | 0.11 | 0.45 | 0.43 | 0.40 |
| inhibition of viability of NCI-N87 cell line | 0.34 | 0.12 | 0.38 | 0.16 | −0.01 | 0.62 | 0.54 | 0.59 |
| inhibition of proliferation of VCaP cell line | 0.07 | 0.37 | 0.09 | 0.08 | 0.18 | 0.36 | 0.47 | 0.33 |
| inhibition of viability of VCaP cell line | 0.18 | 0.27 | 0.21 | 0.14 | 0.13 | 0.52 | 0.52 | 0.45 |
| inhibition of proliferation of HPrEC cell line | 0.36 | 0.11 | 0.38 | 0.02 | −0.14 | 0.39 | 0.41 | 0.49 |
| inhibition of viability of HPrEC cell line | 0.37 | 0.14 | 0.40 | 0.21 | −0.02 | 0.63 | 0.56 | 0.62 |
| Sample | NCI-N87 (ATCC® CRL5822 ™) | VCaP—(ATCC® CRL-2876 ™) | HPrEC (ATCC® PCS-440-010 ™) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proliferation | Viability | Cell Cycle | Proliferation | Viability | Cell Cycle | Proliferation | Viability | Cell Cycle | |
| Freeze-Dried Samples | |||||||||
| Ethanolic extracts | |||||||||
| CL-E | ↓↓ | ↓↓↓ | ↑G1↓G2 | ↓ | ↓↓ | n.c. | ↓↓↓↓ | ↓↓↓ | ↑S↓G1 |
| JAL-E | ↓↓ | ↓↓↓ | ↑G1↓G2 | ↓ | ↓↓ | ↑G2 | ↓↓↓↓ | ↓↓↓ | ↑S↓G1 |
| YEL-E | ↓ | ↓↓↓ | ↑G1↓S | ↓ | ↓ | ↑G2 | ↓↓↓↓ | ↓↓↓ | ↑S↓G1 |
| PBS extracts | |||||||||
| CL-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| JAL-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| YEL-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| GD extracts | |||||||||
| CL-GD | ↓ | ↓↓ | n.c. | ↓ | ↓ | ↑G1↓S | n.c. | ↓ | ↑S↓G1,G2 |
| JAL-GD | ↓ | ↓↓ | n.c. | ↓↓ | ↓↓ | ↑G1↓S,G2 | ↓ | ↓↓ | ↑S↓G2 |
| YEL-GD | ↓ | ↓ | n.c. | ↓↓ | ↓↓ | ↑G1↓S,G2 | ↓ | ↓↓ | ↑S |
| Microwave-Dried Samples | |||||||||
| Ethanolic extracts | |||||||||
| CM-E | ↓↓ | ↓↓↓ | ↑S↓G2 | ↓↓↓ | ↓↓ | ↑G1↓S | ↓ | ↓↓↓ | n.c. |
| JAM-E | ↓↓ | ↓↓↓ | ↑G1 | ↓ | ↓↓ | ↑G1↓S | ↓ | ↓↓↓ | n.c. |
| YEM-E | ↓↓ | ↓↓↓ | n.c. | n.c. | ↓ | ↑G1↓S | ↓ | ↓↓↓ | ↑G2 |
| PBS extracts | |||||||||
| CM-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| JAM-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| YEM-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| GD extracts | |||||||||
| CM-GD | n.c. | n.c. | ↑S↓G1 | n.c. | n.c. | ↑G1 | n.c. | n.c. | ↑G2 |
| JAM-GD | n.c. | n.c. | ↑S↓G1 | ↑ | n.c. | n.c. | n.c. | ↓ | ↑G2 |
| YEM-GD | ↑ | n.c. | n.c. | n.c. | n.c. | ↑G1 | n.c. | n.c. | ↑G2 |
| Traditionally Dried Samples | |||||||||
| Ethanolic extracts | |||||||||
| CT-E | ↓↓↓ | ↓↓↓ | ↓G2 | ↓↓ | ↓↓↓ | ↓G2 | ↓↓↓ | ↓↓↓ | ↑G2↓G1 |
| JAT-E | ↓↓↓ | ↓↓↓ | ↓G2 | ↓↓ | ↓↓↓ | n.c. | ↓↓↓ | ↓↓↓ | ↑G2↓G1 |
| YET-E | ↓↓↓ | ↓↓↓ | ↑G2 | ↓↓ | ↓↓↓ | ↑G1↓G2 | ↓↓↓ | ↓↓↓ | ↑G2↓G1 |
| PBS extracts | |||||||||
| CT-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| JAT-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| YET-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| GD extracts | |||||||||
| CT-GD | ↓↓↓ | ↓↓↓ | n.c. | ↓↓ | ↓↓↓ | ↑S | ↓↓↓ | ↓↓↓ | ↓S |
| JAT-GD | ↓↓↓ | ↓↓↓ | ↓G1 | ↓↓ | ↓↓↓ | ↑S | ↓↓↓ | ↓↓↓ | ↓S |
| YET-GD | ↓↓↓ | ↓↓↓ | ↓G1 | ↓↓ | ↓↓↓ | ↓G2 | ↓↓↓ | ↓↓↓ | ↓S |
| Convectionally Dried Samples | |||||||||
| Ethanolic extracts | |||||||||
| CC-E | ↓↓ | ↓↓↓ | n.c. | ↓↓ | ↓↓↓ | ↑G1↓S,G2 | ↓↓ | ↓↓↓ | ↑S↓G2 |
| JAC-E | ↓↓ | ↓↓↓ | n.c. | ↓↓ | ↓↓↓ | ↑G1↓S,G2 | ↓↓↓ | ↓↓↓ | ↑S↓G2 |
| YEC-E | ↓↓ | ↓↓↓ | n.c. | ↓↓ | ↓↓↓ | ↑G1↓S | ↓↓ | ↓↓↓ | ↑S↓G2 |
| PBS extracts | |||||||||
| CC-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| JAC-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| YEC-P | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
| GD extracts | |||||||||
| CC-GD | ↓↓↓ | ↓↓ | ↑S | ↓↓ | ↓ | n.c. | ↓↓↓ | ↓↓↓ | ↑G2↓S |
| JAC-GD | ↓↓↓ | ↓↓↓ | ↑S | ↓↓ | ↓↓ | n.c. | ↓↓ | ↓ | ↑G2 |
| YEC-GD | n.c. | n.c. | ↑S | ↑↑ | n.c. | ↑G1 | ↓ | ↓ | ↑G1↓S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Złotek, U.; Lewicki, S.; Markiewicz, A.; Szymanowska, U.; Jakubczyk, A. Effects of Drying Methods on Antioxidant, Anti-Inflammatory, and Anticancer Potentials of Phenolic Acids in Lovage Elicited by Jasmonic Acid and Yeast Extract. Antioxidants 2021, 10, 662. https://doi.org/10.3390/antiox10050662
Złotek U, Lewicki S, Markiewicz A, Szymanowska U, Jakubczyk A. Effects of Drying Methods on Antioxidant, Anti-Inflammatory, and Anticancer Potentials of Phenolic Acids in Lovage Elicited by Jasmonic Acid and Yeast Extract. Antioxidants. 2021; 10(5):662. https://doi.org/10.3390/antiox10050662
Chicago/Turabian StyleZłotek, Urszula, Sławomir Lewicki, Anna Markiewicz, Urszula Szymanowska, and Anna Jakubczyk. 2021. "Effects of Drying Methods on Antioxidant, Anti-Inflammatory, and Anticancer Potentials of Phenolic Acids in Lovage Elicited by Jasmonic Acid and Yeast Extract" Antioxidants 10, no. 5: 662. https://doi.org/10.3390/antiox10050662
APA StyleZłotek, U., Lewicki, S., Markiewicz, A., Szymanowska, U., & Jakubczyk, A. (2021). Effects of Drying Methods on Antioxidant, Anti-Inflammatory, and Anticancer Potentials of Phenolic Acids in Lovage Elicited by Jasmonic Acid and Yeast Extract. Antioxidants, 10(5), 662. https://doi.org/10.3390/antiox10050662








