Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Diet
2.3. Collagen Type II Induction of Arthritis Disease
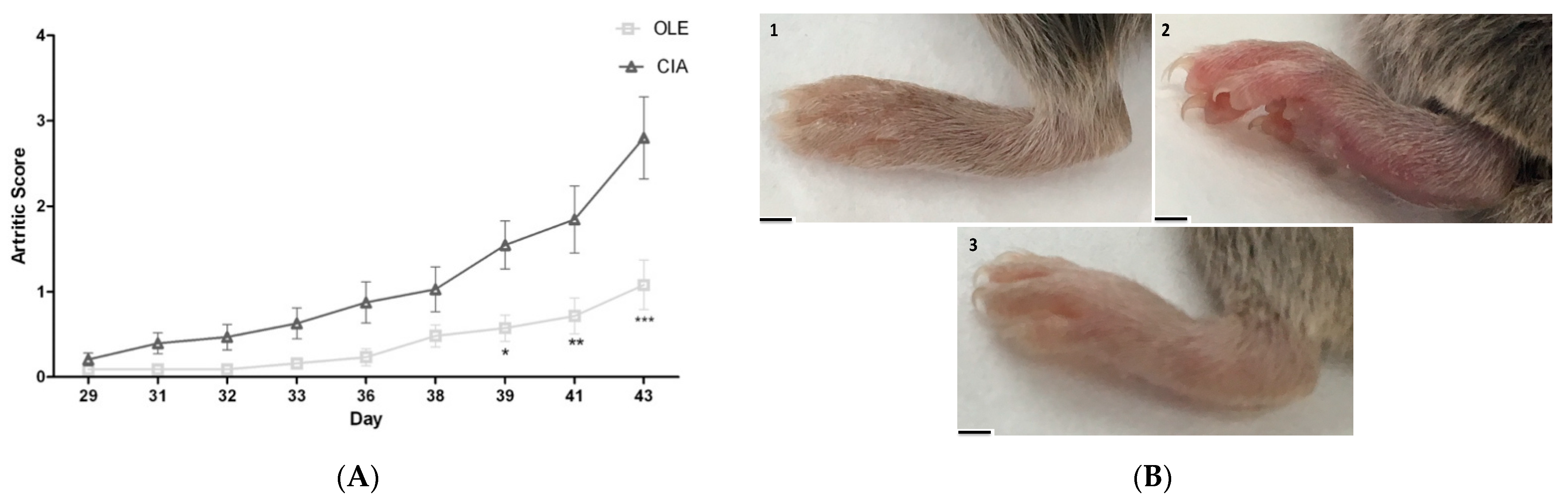
2.4. Clinical Assessment of Joints
2.5. Histological Analysis
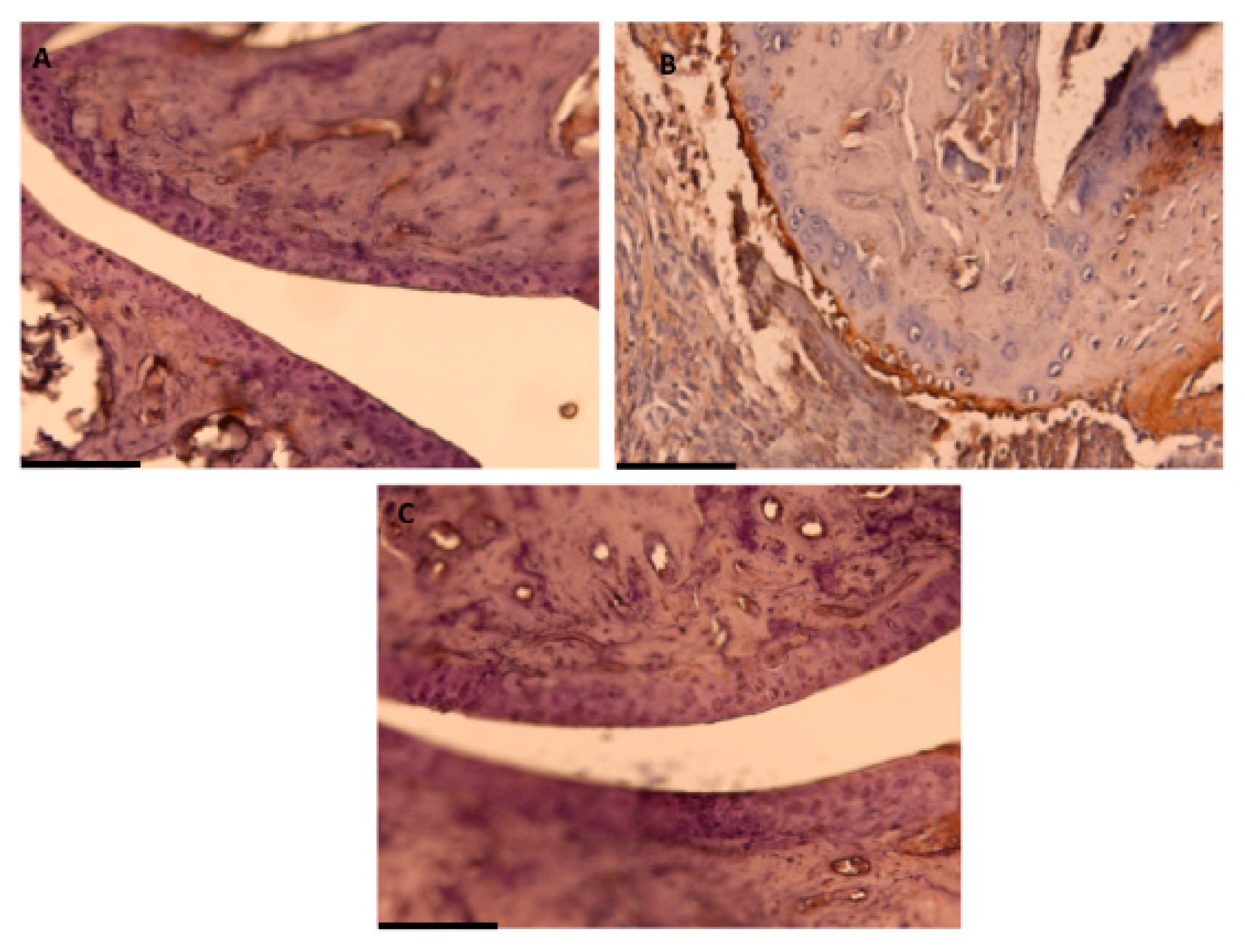
2.6. Immunohistochemical Detection of COX-2 Expression
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Western Blotting
2.9. Data Analysis
3. Results
3.1. Effects of OLE Supplemented Diet on Collagen-Induced Arthritis Model
3.2. Effects of Dietary OLE on MMP-3 and Joint Inflammatory Biomarkers Levels
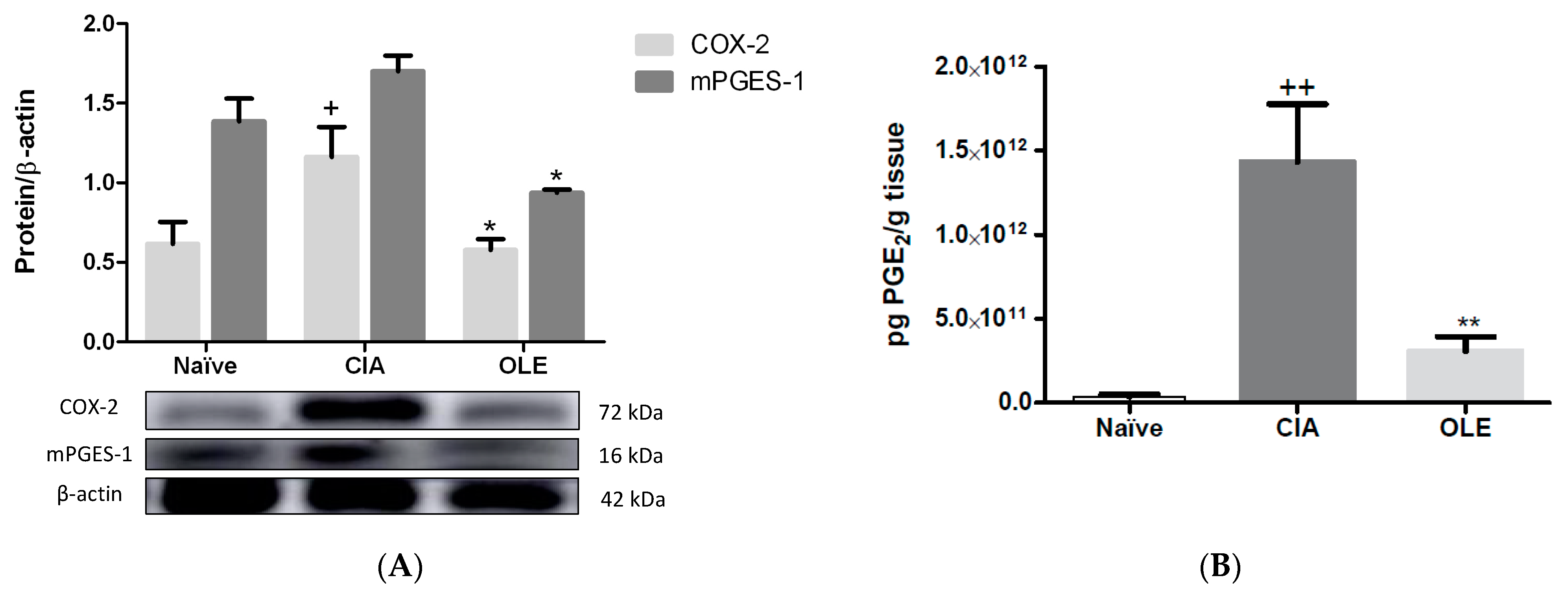
3.3. Effects of Dietary OLE on COX-2 Protein, mPGES-1 Expression and PGE2 Production
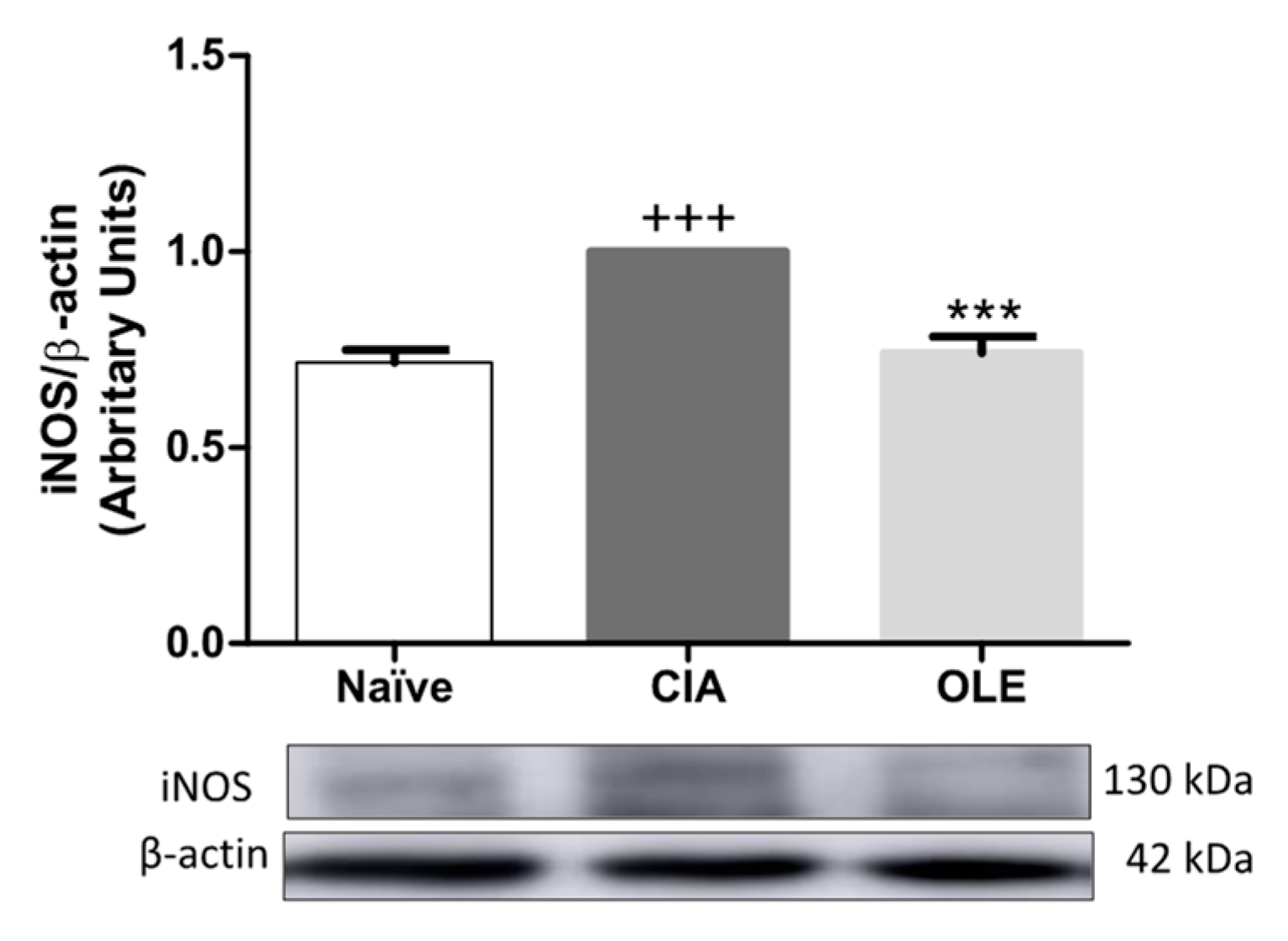
3.4. OLE Experimental Diet Attenuated iNOS Overexpression
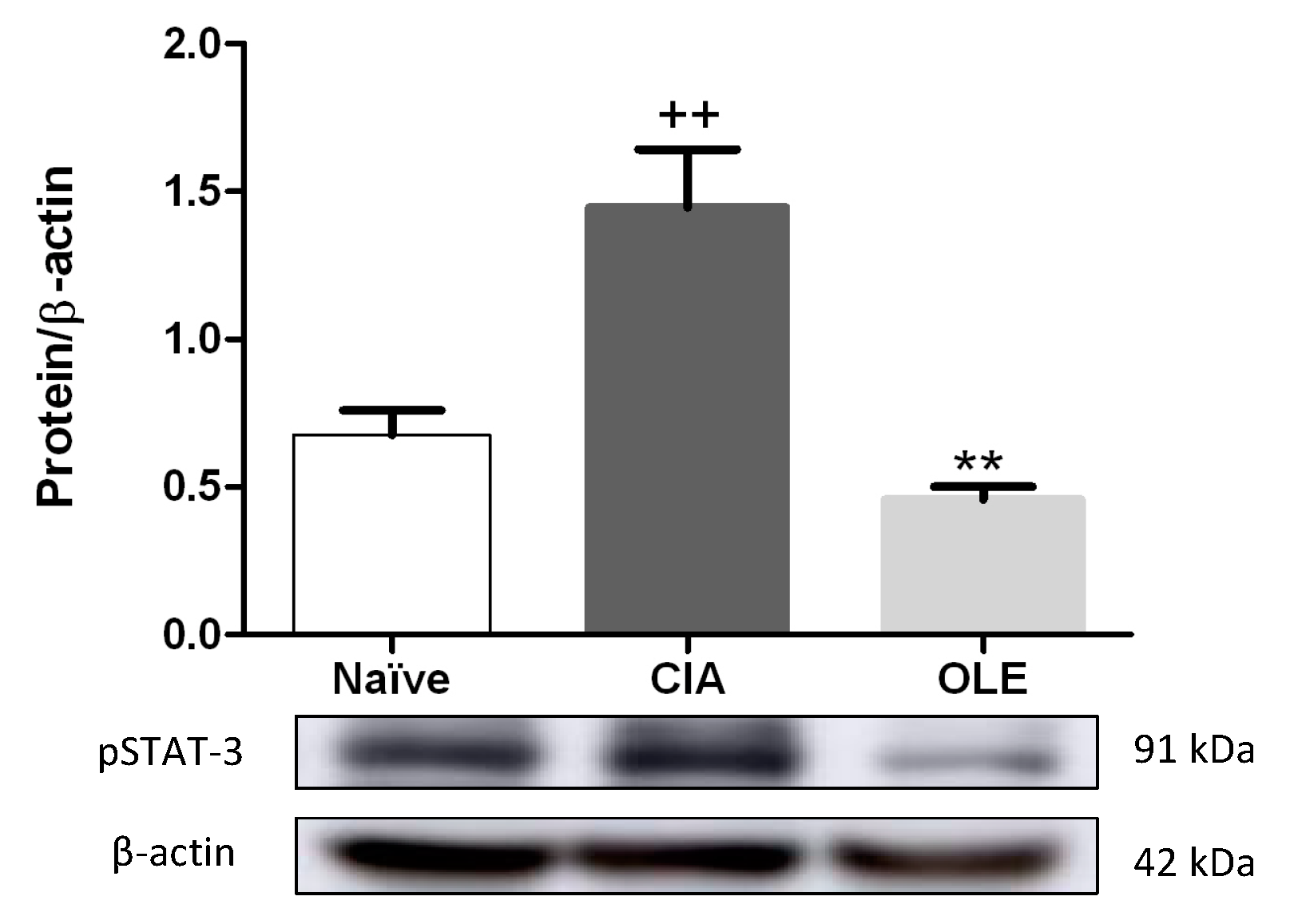
3.5. Effects of Dietary OLE on pSTAT-3 Protein Expression
3.6. Dietary OLE Induces Nrf-2/HO-1 Antioxidant Pathway Activation
3.7. OLE Diet Modulates MAPKs Signaling Pathway
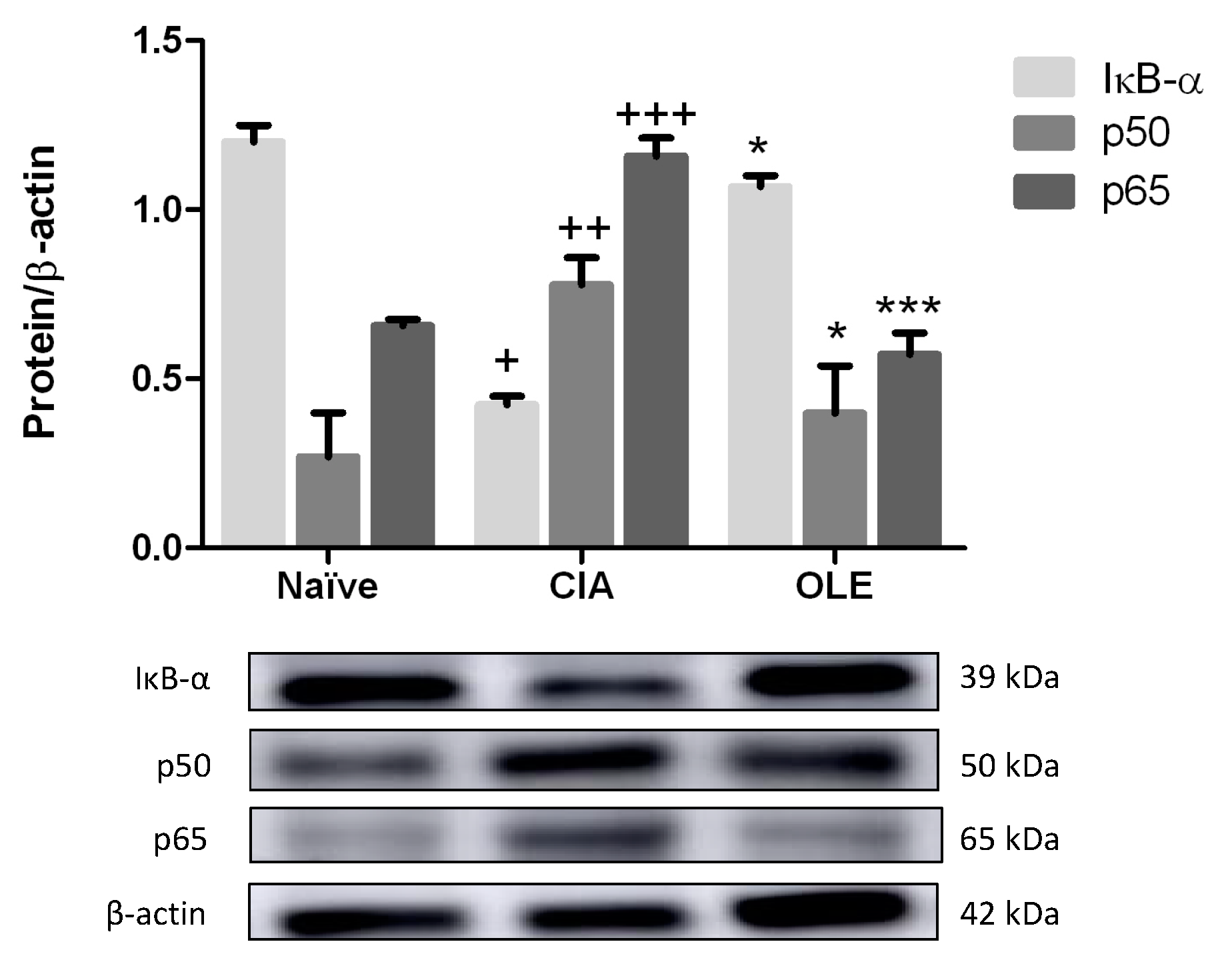
3.8. Effects of Dietary OLE on NF-κB Signaling Pathway
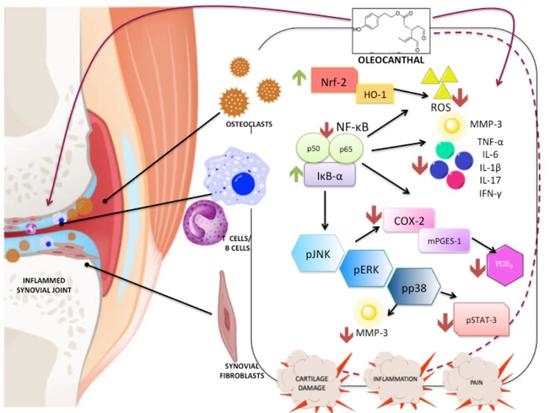
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-magnostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Paolino, S.; Pacini, G.; Patanè, M.; Alessandri, E.; Cattelan, F.; Goegan, F.; Pizzorni, C.; Gotelli, E.; Cutolo, M. Interactions between microbiota, diet/nutrients and immune/inflammatory response in rheumatic diseases: Focus on rheumatoid arthritis. Reumatologia 2019, 57, 151–157. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Malemud, C.J. Intracellular Signaling Pathways in Rheumatoid Arthritis. J. Clin. Cell. Immunol. 2013, 4, 160. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; De Caterina, R. Relevance of new drug discovery to reduce NF-κB activation in cardiovascular disease. Curr. Vasc. Pharmacol. 2012, 57, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Kunwar, B.; Mazhar, M.; Faizi, S.; Ahmed, D.; Raza, M.S.; Simjee, S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF- κB and iNOS activation. Int. Immunopharmacol. 2018, 59, 310–317. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Nacher-Juan, J.; Alcaraz, M.J. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346. [Google Scholar] [CrossRef]
- Wruck, C.J.; Fragoulis, A.; Gurzynski, A.; Brandenburg, L.-O.; Kan, Y.W.; Chan, K.; Hassenpflug, J.; Freitag-Wolf, S.; Varoga, D.; Lippross, S.; et al. Role of oxidative stress in rheumatoid arthritis: Insights from the Nrf2-knockout mice. Ann. Rheum. Dis. 2010, 70, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, A.-L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.L.; Montoya, T.; Alarcón-De-La-Lastra, C.; Sánchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nat. Cell Biol. 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Quintero, J.E.; Rosado, J.A. Anticancer molecular mechanisms of oleocanthal. Phytother. Res. 2020, 34, 2820–2834. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-yerena, A.; Rinaldi-de-Alvarenga, J.F.; Romero-del-Castillo-Alba, J.; Vallverdú-queralt, A.; Escribano-Ferrer, E.; Lamuela-raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Carretero, A.S.; Fernández-Gutiérrez, A. Exploratory analysis of human urine by LC–ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef]
- Silva, S.; Garcia-Aloy, M.; Figueira, M.E.; Combet, E.; Mullen, W.; Bronze, M.R. High Resolution Mass Spectrometric Analysis of Secoiridoids and Metabolites as Biomarkers of Acute Olive Oil Intake-An Approach to Study Interindividual Variability in Humans. Mol. Nutr. Food Res. 2017, 62, 1–11. [Google Scholar] [CrossRef]
- Suárez, M.; Valls, R.M.; Romero, M.-P.; Macià, A.; Fernández, S.; Giralt, M.; Solà, R.; Motilva, M.-J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011, 106, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Mete, M.; Aydemir, I.; Unsal, U.U.; Collu, F.; Vatandas, G.; Gurcu, B.; Duransoy, Y.K.; Taneli, F.; Tuglu, M.I.; Selcuki, M. Neuroprotective effects of oleocanthal, a compound in virgin olive oil, in a rat model of traumatic brain injury. Turk. Neurosurg. 2017, 28, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.; Lago, F.; Smith, A.S., III; Gualillo, O. Oleocanthal Inhibits Proliferation and MIP-1α Expression in Human Multiple Myeloma Cells. Curr. Med. Chem. 2013, 20, 2467–2475. [Google Scholar] [CrossRef]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B.; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B.; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Montoya, T.; Castejón, M.L.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; De-La-Lastra, C.A. Oleocanthal Modulates LPS-Induced Murine Peritoneal Macrophages Activation via Regulation of Inflammasome, Nrf-2/HO-1, and MAPKs Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5552–5559. [Google Scholar] [CrossRef]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-De-La-Lastra, C.; Ferrándiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.L.; Alarcón-de-la-Lastra, C.; Rosillo, M.A.; Montoya, T.; Fernández-Bolaños, J.G.; González-Benjumea, A.; Sánchez-Hidalgo, M. A new peracetylated oleuropein derivative ameliorates joint inflammation and destruction in a murine collagen-induced arthritis model via activation of the Nrf-2/Ho-1 antioxidant pathway and suppression of MAPKs and NF-κB activation. Nutrients 2021, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alves, C.H.; Farrell, E.; Vis, M.; Colin, E.M.; Lubberts, E. Animal Models of Bone Loss in Inflammatory Arthritis: From Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 27–47. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Grzybowska-Kowalczyk, A.; Wojtecka-Lukasik, E.; Maslinski, S. IL-23 in the Pathogenesis of Rheumatoid Arthritis. Scand. J. Immunol. 2010, 71, 134–145. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Matrix metalloproteinase gene activation resulting from disorded epigenetic mechanisms in rheumatoid arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef]
- Oike, T.; Sato, Y.; Kobayashi, T.; Miyamoto, K.; Nakamura, S.; Kaneko, Y.; Kobayashi, S.; Harato, K.; Saya, H.; Matsumoto, M.; et al. Stat3 as a potential therapeutic target for rheumatoid arthritis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2007, 47, 409–414. [Google Scholar] [CrossRef]
- Trentham, D.E.; Townes, A.S.; Kang, A.H. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 1977, 146, 857–868. [Google Scholar] [CrossRef]
- Schurgers, E.; Billiau, A.; Matthys, P. Collagen-Induced arthritis as an animal model for rheumatoid arthritis: Focus on interferon-γ. J. Interferon Cytokine Res. 2011, 31, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.A.; Langrish, C.L.; Chen, Y.; Blumenschein, W.; McClanahan, T.; Kastelein, R.A.; Sedgwick, J.D.; Cua, D.J. Divergent Pro- and Antiinflammatory Roles for IL-23 and IL-12 in Joint Autoimmune Inflammation. J. Exp. Med. 2003, 198, 1951–1957. [Google Scholar] [CrossRef]
- Zwerina, J.; Redlich, K.; Schett, G.; Smolen, J.S. Pathogenesis of Rheumatoid Arthritis: Targeting Cytokines. Ann. N. Y. Acad. Sci. 2005, 1051, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Veis, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Lubberts, E.; Alarcón-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagne-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Girardelli, M.; Bianco, A.M.R.; Marcuzzi, A.; Crovella, S. A comparative analysis of serologic parameters and oxidative stress in osteoarthritis and rheumatoid arthritis: Reply to Mishra and colleagues. Rheumatol. Int. 2012, 33, 2445–2446. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppresion by regulating ROS-dependent p38 pathway. Free Rad. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Allard-Chamard, H.; Carrier, N.; Dufort, P.; Durand, M.; De Brum-Fernandes, A.; Boire, G.; Komarova, S.; Dixon, S.; Harrison, R.; Manolson, M.; et al. Osteoclasts and their circulating precursors in rheumatoid arthritis: Relationships with disease activity and bone erosions. Bone Rep. 2020, 12, 100282. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nishikaku, F.; Nakatuka, M.; Koga, Y. Osteoclast-like cells in murine collagen induced arthritis. J. Rheumatol. 1998, 25, 1154–1160. [Google Scholar]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Anaparti, V.; Hitchon, C.; Mookherjee, N. Buprenorphine Alters Inflammatory and Oxidative Stress Molecular Markers in Arthritis. Mediat. Inflamm. 2017, 2017, 2515408. [Google Scholar] [CrossRef]
- McCoy, J.M.; Wicks, J.R.; Audoly, L.P. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Investig. 2002, 110, 651–658. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Sánchez-Fidalgo, S.; Aparicio-Soto, M.; Villegas, I.; Alarcón-de-la-Lastra, C. Dietary extra-virgin olive oil prevents inflammatory response and cartilage matrix degradation in murine collagen-induced arthritis. Eur. J. Nutr. 2016, 55, 315–325. [Google Scholar] [CrossRef]
- Carpi, S.; Scoditti, E.; Massaro, M.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Poli, G.; Tuccinardi, T.; Doccini, S.; et al. The Extra-Virgin Olive Oil Polyphenols Oleocanthal and Oleacein Counteract Inflammation-Related Gene and miRNA Expression in Adipocytes by Attenuating NF-κB Activation. Nutrients 2019, 11, 2855. [Google Scholar] [CrossRef]
- Mori, T.; Miyamoto, T.; Yoshida, H.; Asakawa, M.; Kawasumi, M.; Kobayashi, T.; Morioka, H.; Chiba, K.; Toyama, Y.; Yoshimura, A. IL-1β and TNF-α -initiated IL-6—STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Intern. Immunol. 2011, 23, 701–712. [Google Scholar] [CrossRef]
- Maicas, N.; Ferrándiz, M.L.; Brines, R.; Ibáñez, L.; Cuadrado, A.; Koenders, M.I.; Berg, W.B.V.D.; Alcaraz, M.J. Deficiency of Nrf2 Accelerates the Effector Phase of Arthritis and Aggravates Joint Disease. Antioxid. Redox Signal. 2011, 15, 889–901. [Google Scholar] [CrossRef]
- Naidu, S.; Vijayan, V.; Santoso, S.; Kietzmann, T.; Immenschuh, S. Inhibition and Genetic Deficiency of p38 MAPK Up-Regulates Heme Oxygenase-1 Gene Expression via Nrf2. J. Immunol. 2009, 182, 7048–7057. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Rosillo, M.Á.; González-Benjumea, A.; Alarcón-de-la-Lastra, C. Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants 2021, 10, 650. https://doi.org/10.3390/antiox10050650
Montoya T, Sánchez-Hidalgo M, Castejón ML, Rosillo MÁ, González-Benjumea A, Alarcón-de-la-Lastra C. Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants. 2021; 10(5):650. https://doi.org/10.3390/antiox10050650
Chicago/Turabian StyleMontoya, Tatiana, Marina Sánchez-Hidalgo, María Luisa Castejón, María Ángeles Rosillo, Alejandro González-Benjumea, and Catalina Alarcón-de-la-Lastra. 2021. "Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice" Antioxidants 10, no. 5: 650. https://doi.org/10.3390/antiox10050650
APA StyleMontoya, T., Sánchez-Hidalgo, M., Castejón, M. L., Rosillo, M. Á., González-Benjumea, A., & Alarcón-de-la-Lastra, C. (2021). Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants, 10(5), 650. https://doi.org/10.3390/antiox10050650