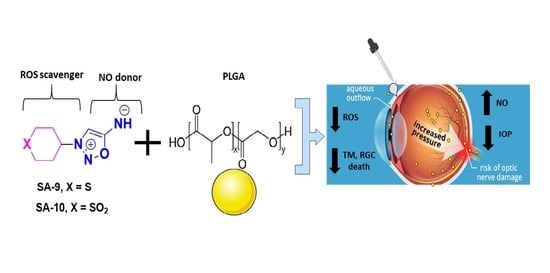
Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma
Abstract
1. Introduction
2. Methods
2.1. Materials and Reagents
2.2. Synthesis of SA-9 and SA-10

2.2.1. Synthesis of Compound SA-9
2.2.2. Synthesis of Compound SA-10
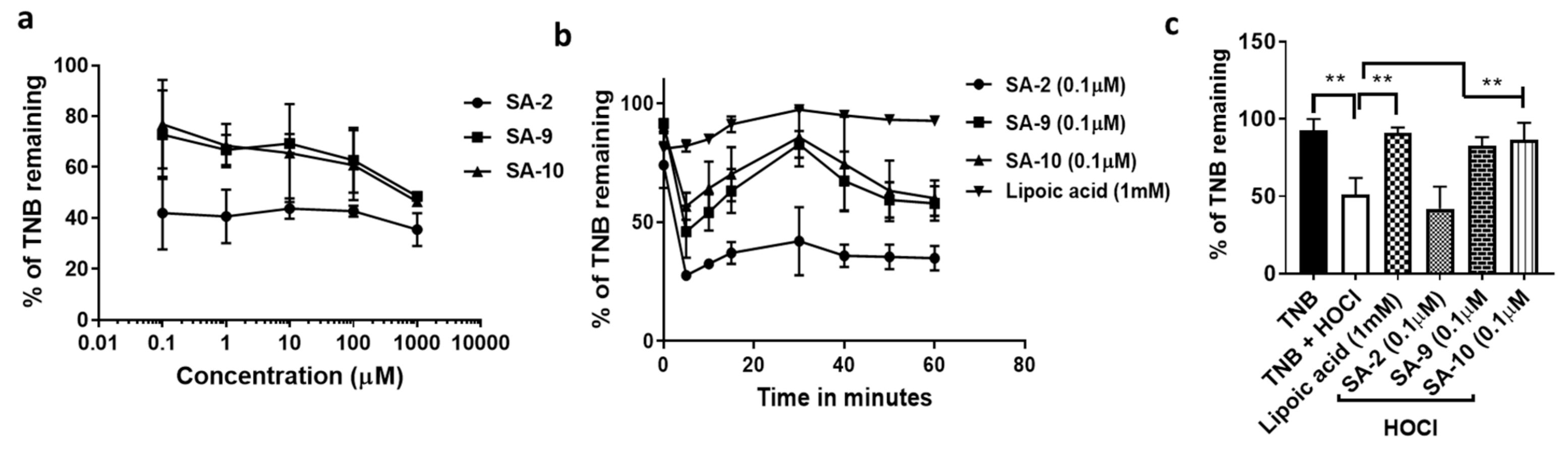
2.3. Superoxide Anion Radical Scavenging Assay
2.4. Hypochlorous Acid (HOCl) Radical Scavenging Assay
2.5. Measurement of Total Nitrite Release by Griess Assay
2.6. In Vitro Peroxynitrite Measurement Assay
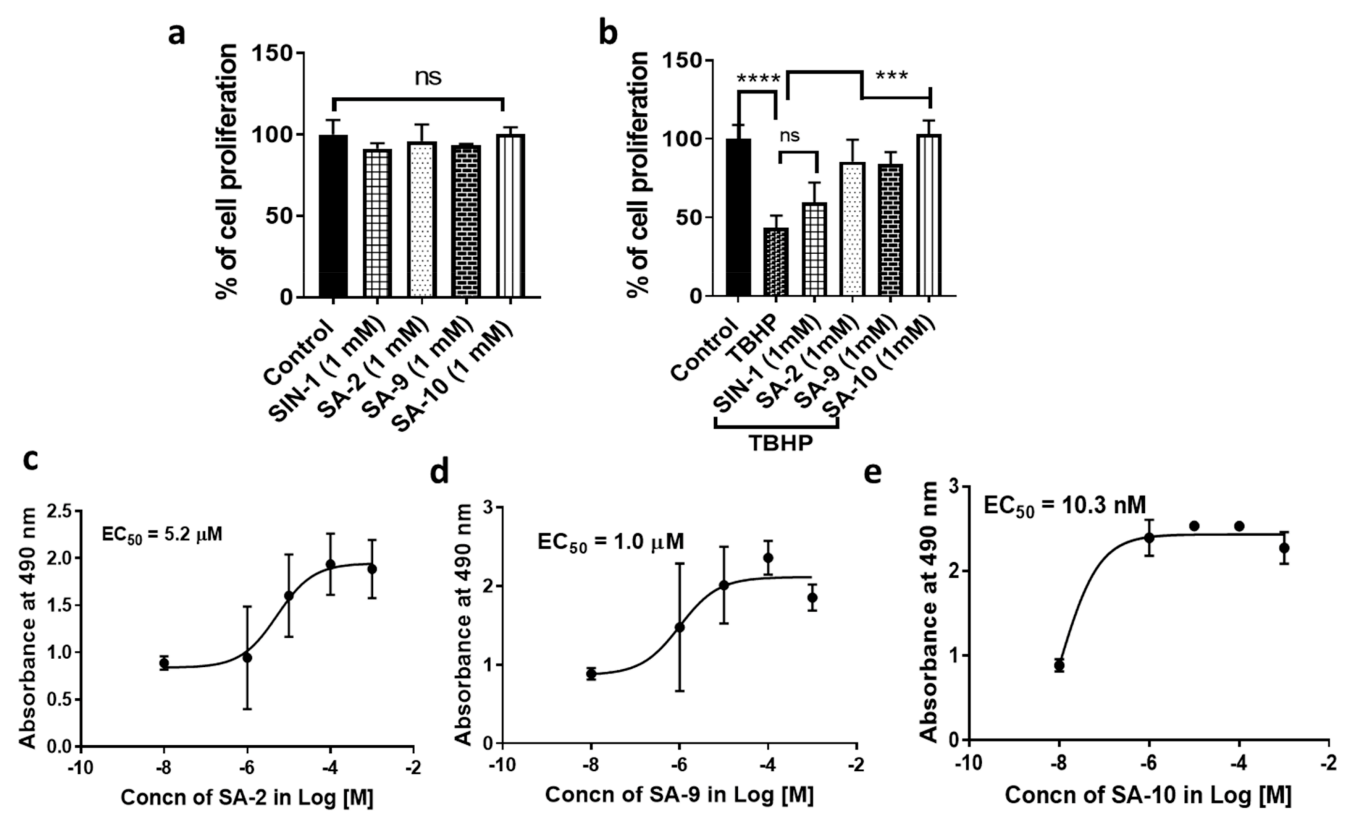
2.7. In Vitro Cell Viability Assay
2.8. In Vitro Cytoprotection Assay
2.9. Fabrication of SA-9NPs
2.10. Characterization of SA-9NPs
2.11. Animals
2.12. Formulation and Storage
2.13. Ocular Biodistribution after SA-9 and SA-9NPs Eye Drop
2.14. IOP Lowering Activity of SA-9 NPs in Dexamethasone Induced Ocular Hypertensive (OHT) Mouse Eyes
3. Results
3.1. Anti-Oxidant Activity of SA Compounds
3.2. Compounds SA-9 and SA-10 Release Nitric Oxide in Cells but Not in Buffer Formulation
3.3. Compounds SA-9 and SA-10 Dose Dependently Protect TM Cells from Oxidative Stress Induced Cell Death
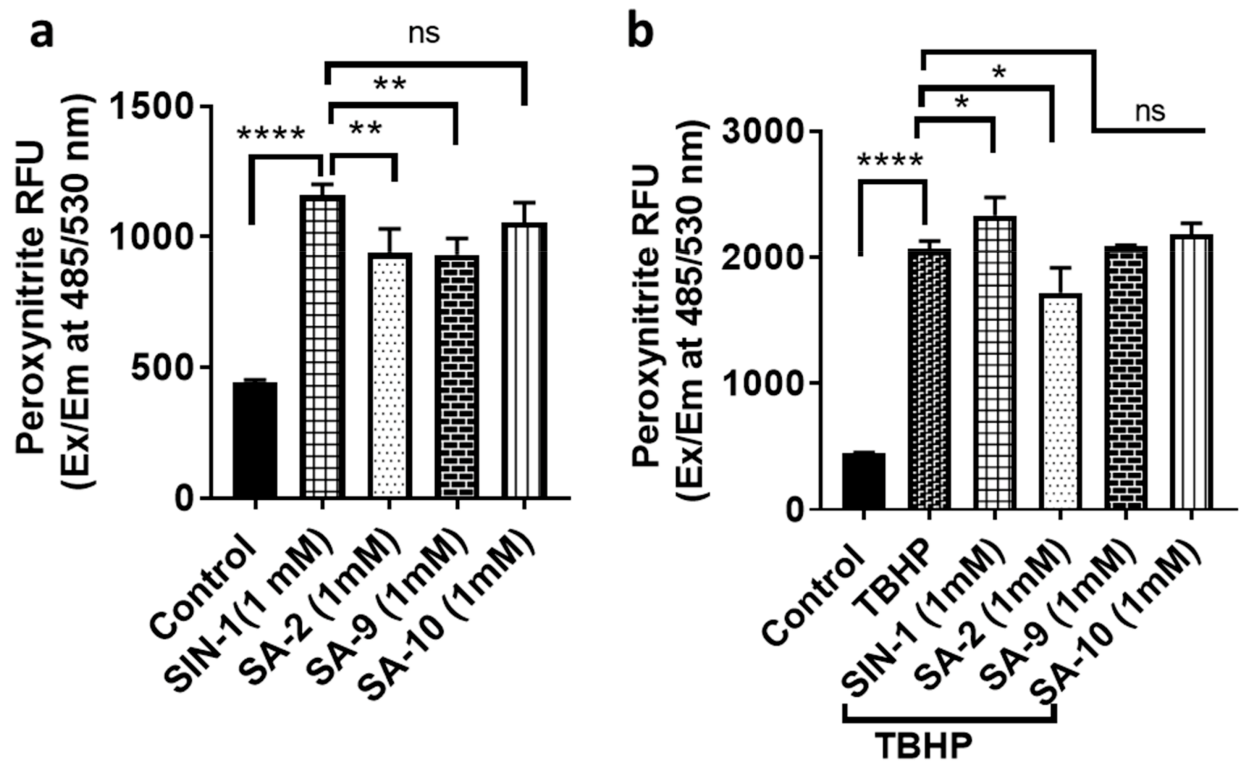
3.4. Effect of SA Compounds in Peroxinitrite Radical Formation in NTM5 Cells
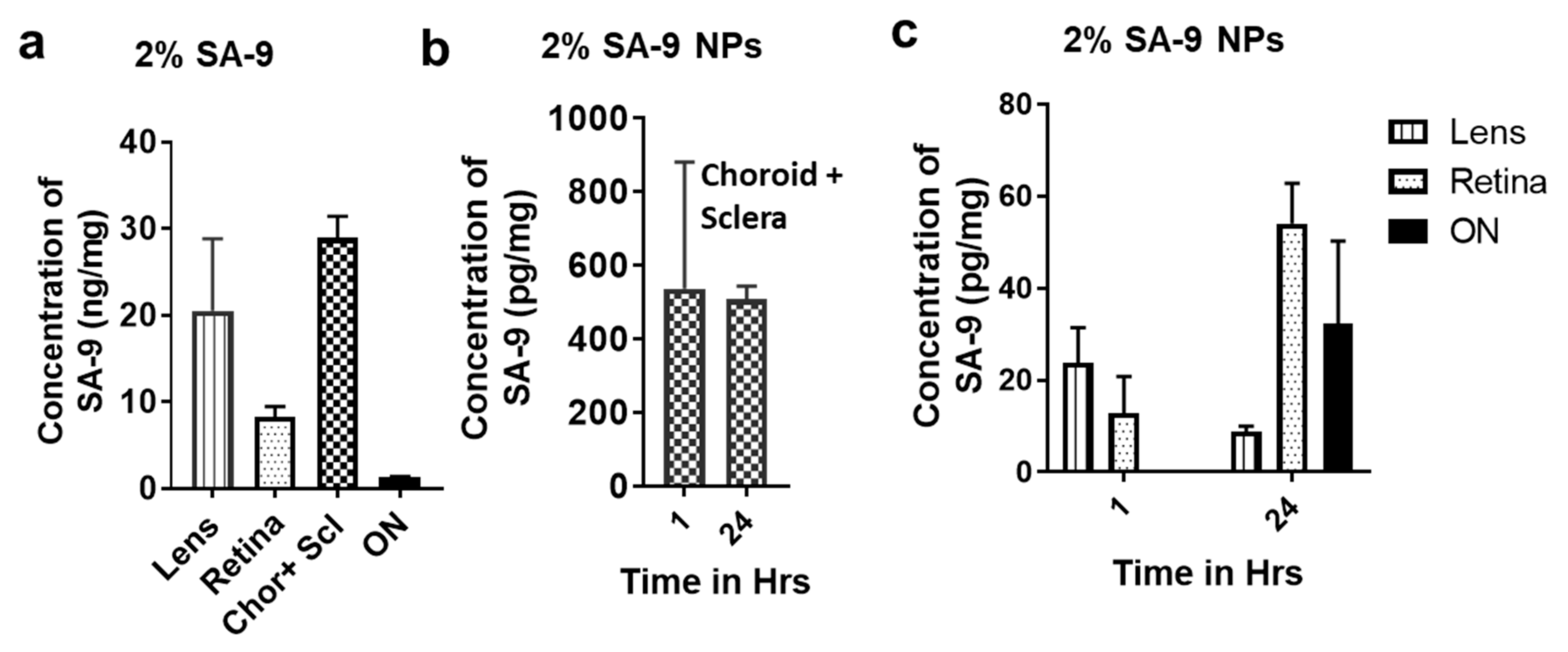
3.5. Bioavailability of SA-9 and SA-9-NPs on Mouse Eyes
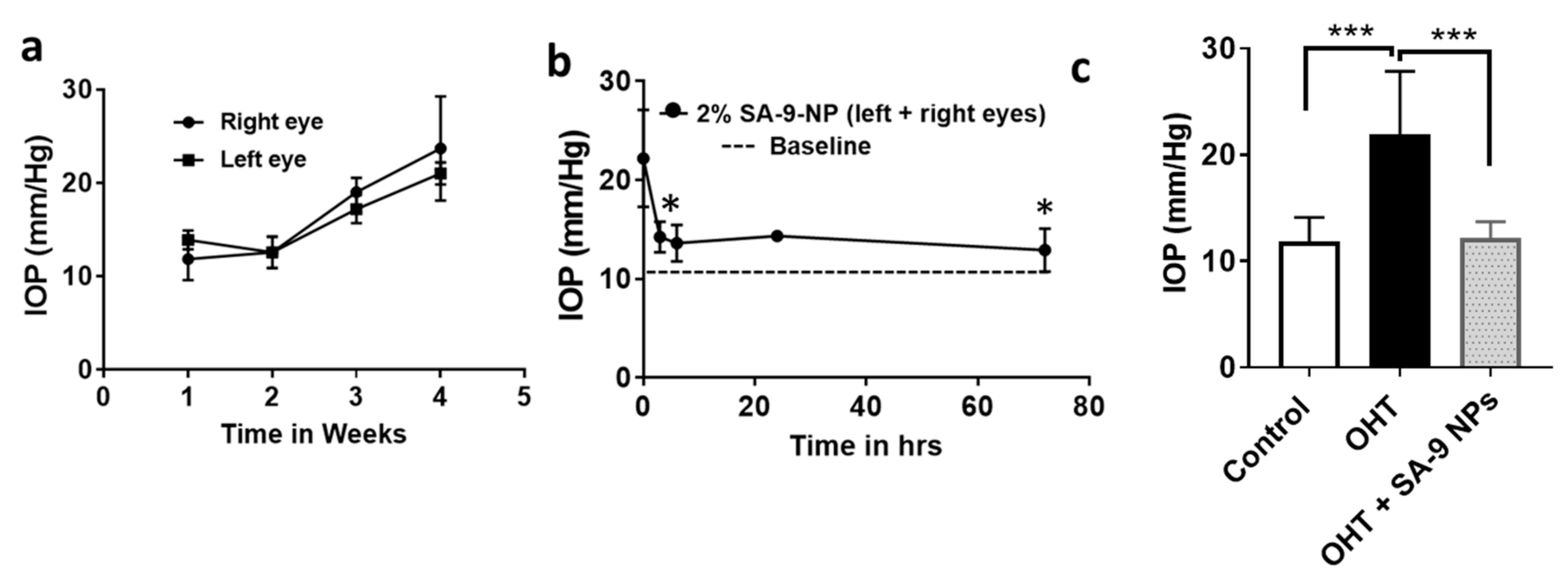
3.6. SA-9NPs Eye Drop Reduced IOP in OHT Mouse Eyes and Provided Longer Duration of Action
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krakau, C.E.T. Intraocular Pressure Elevation—Cause or Effect in Chronic Glaucoma? Ophthalmol. J. Int. D’Ophtalmologie Int. J. Ophthalmol. Z. Fur. Augenheilkd. 1981, 182, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A.; Arafa, L.F.; El-Baz, A. Oxidative stress markers in patients with primary open-angle glaucoma. Curr. Eye Res. 2010, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, C.M.; Schouten, J.S.; van de Borne, B.W.; Webers, C.A. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology 2005, 112, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; De Ieso, M.L.; Pasquale, L.R.; Madekurozwaa, M.; van Batenburg-Sherwooda, J.; Overby, D.R.; Stamer, W.D. The vital role for nitric oxide in intraocular pressure homeostasis. Prog. Retin. Eye Res. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.A.; Ngai, P.; Mosaed, S.; Lin, K.Y. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin. Ophthalmol. 2016, 10, 2035–2050. [Google Scholar] [CrossRef]
- Huang, S.S.; Lu, Y.J.; Huang, J.P.; Wu, Y.T.; Day, Y.J.; Hung, L.M. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J. Vasc. Surg. 2014, 59, 483–491. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Mou, R.-T.; Feng, D.-X.; Wang, Z.; Chen, G. The role of nitric oxide in stroke. Med. Gas Res. 2017, 7, 194–203. [Google Scholar]
- Erdurmus, M.; Yagci, R.; Atis, O.; Karadag, R.; Akbas, A.; Hepsen, I.F. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr. Eye Res. 2011, 36, 713–718. [Google Scholar] [CrossRef]
- Minhas, G.; Morishita, R.; Anand, A. Preclinical Models to Investigate Retinal Ischemia: Advances and Drawbacks. Front. Neurol. 2012, 3, 75. [Google Scholar] [CrossRef]
- Acharya, S.; Rogers, P.; Krishnamoorthy, R.R.; Stankowska, D.L.; Dias, H.V.R.; Yorio, T. Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy. Bioorganic Med. Chem. Lett. 2016, 26, 1490–1494. [Google Scholar] [CrossRef]
- Stankowska, D.L.; Dibas, A.; Li, L.; Zhang, W.; Krishnamoorthy, V.R.; Chavala, S.H.; Nguyen, T.P.; Yorio, T.; Ellis, D.Z.; Acharya, S. Hybrid Compound SA-2 is Neuroprotective in Animal Models of Retinal Ganglion Cell Death. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3064–3073. [Google Scholar] [CrossRef]
- Stankowska, D.L.; Millar, J.C.; Kodati, B.; Behera, S.; Chaphalkar, R.M.; Nguyen, T.; Nguyen, K.T.; Krishnamoorthy, R.R.; Ellis, D.Z.; Acharya, S. Nanoencapsulated hybrid compound SA-2 with long-lasting intraocular pressure lowering activity in rodent eyes. Mol. Vis. 2021, 27, 37–49. [Google Scholar]
- Ellis, D.Z.; Dismuke, W.M.; Chokshi, B.M. Characterization of Soluble Guanylate Cyclase in NO-Induced Increases in Aqueous Humor Outflow Facility and in the Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1808–1813. [Google Scholar] [CrossRef]
- Xu, C.; Liu, S.; Liu, Z.; Song, F.; Liu, S. Superoxide generated by pyrogallol reduces highly water-soluble tetrazolium salt to produce a soluble formazan: A simple assay for measuring superoxide anion radical scavenging activities of biological and abiological samples. Anal. Chim. Acta 2013, 793, 53–60. [Google Scholar] [CrossRef]
- Ching, T.L.; de Jong, J.; Bast, A. A method for screening hypochlorous acid scavengers by inhibition of the oxidation of 5-thio-2-nitrobenzoic acid: Application to anti-asthmatic drugs. Anal. Biochem. 1994, 218, 377–381. [Google Scholar] [CrossRef]
- Le, D.Q.; Kuriakose, A.E.; Nguyen, D.X.; Nguyen, K.T.; Acharya, S. Hybrid Nitric Oxide Donor and its Carrier for the Treatment of Peripheral Arterial Diseases. Sci. Rep. 2017, 7, 8692. [Google Scholar] [CrossRef]
- Tse, D.Y.; Kim, S.J.; Chung, I.; He, F.; Wensel, T.G.; Wu, S.M. The ocular toxicity and pharmacokinetics of simvastatin following intravitreal injection in mice. Int. J. Ophthalmol. 2017, 10, 1361–1369. [Google Scholar]
- Patel, G.C.; Phan, T.N.; Maddineni, P.; Kasetti, R.B.; Millar, J.C.; Clark, A.F.; Zode, G.S. Dexamethasone-Induced Ocular Hypertension in Mice: Effects of Myocilin and Route of Administration. Am. J. Pathol. 2017, 187, 713–723. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming growth factor β2 (TGFβ2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef]
- Stocker, R.; Huang, A.; Jeranian, E.; Hou, J.Y.; Wu, T.T.; Thomas, S.R.; Keaney, J.F., Jr. Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2028–2033. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, Q.; Liu, J.; Liao, J.; Peng, R.; Xi, Y.; Diwu, Z. Fluorescent real-time quantitative measurements of intracellular peroxynitrite generation and inhibition. Anal. Biochem. 2017, 520, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat. Commun. 2020, 11, 5594. [Google Scholar] [CrossRef] [PubMed]
- Messin, R.; Opolski, G.; Fenyvesi, T.; Carreer-Bruhwyler, F.; Dubois, C.; Famaey, J.P.; Géczy, J. Efficacy and safety of molsidomine once-a-day in patients with stable angina pectoris. Int. J. Cardiol. 2005, 98, 79–89. [Google Scholar] [CrossRef]
- Behar-Cohen, F.F.; Goureau, O.; D’Hermies, F.; Courtois, Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1711–1715. [Google Scholar]
- Biasetti, M.; Dawson, R., Jr. Effects of sulfur containing amino acids on iron and nitric oxide stimulated catecholamine oxidation. Amino Acids 2002, 22, 351–368. [Google Scholar] [CrossRef]
- Fernandes, E.; Toste, S.A.; Lima, J.L.; Reis, S. The metabolism of sulindac enhances its scavenging activity against reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2003, 35, 1008–1017. [Google Scholar] [CrossRef]
- Bogatyrenko, T.N.; Kandalintseva, N.V.; Sashenkova, T.E.; Mishchenko, D.V. Sulfur-containing phenolic antioxidants increasing antitumor efficiency of cyclophosphamide and its combination with nitric oxide donor. Russ. Chem. Bull. 2018, 67, 700–704. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Chmura, A.; Stasicka, Z. Interplay between iron complexes, nitric oxide and sulfur ligands: Structure, (photo)reactivity and biological importance. Coord. Chem. Rev. 2005, 249, 2408–2436. [Google Scholar] [CrossRef]
- Sánchez López, E. Recent advances in nanoscopic drug delivery systems for the treatment of glaucoma. Acta Ophthalmol. 2019, 97. [Google Scholar] [CrossRef]
- Warsi, M.H.; Anwar, M.; Garg, V.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: Pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf. B Biointerfaces 2014, 122, 423–431. [Google Scholar] [CrossRef]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amankwa, C.E.; Gondi, S.R.; Dibas, A.; Weston, C.; Funk, A.; Nguyen, T.; Nguyen, K.T.; Ellis, D.Z.; Acharya, S. Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma. Antioxidants 2021, 10, 575. https://doi.org/10.3390/antiox10040575
Amankwa CE, Gondi SR, Dibas A, Weston C, Funk A, Nguyen T, Nguyen KT, Ellis DZ, Acharya S. Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma. Antioxidants. 2021; 10(4):575. https://doi.org/10.3390/antiox10040575
Chicago/Turabian StyleAmankwa, Charles E., Sudershan R. Gondi, Adnan Dibas, Courtney Weston, Arlene Funk, Tam Nguyen, Kytai T. Nguyen, Dorette Z. Ellis, and Suchismita Acharya. 2021. "Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma" Antioxidants 10, no. 4: 575. https://doi.org/10.3390/antiox10040575
APA StyleAmankwa, C. E., Gondi, S. R., Dibas, A., Weston, C., Funk, A., Nguyen, T., Nguyen, K. T., Ellis, D. Z., & Acharya, S. (2021). Novel Thiol Containing Hybrid Antioxidant-Nitric Oxide Donor Small Molecules for Treatment of Glaucoma. Antioxidants, 10(4), 575. https://doi.org/10.3390/antiox10040575