Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Phytochemical Analysis
Identification and Quantification of Polyphenolic Compounds by HPLC-DAD-ESI MS
2.4. Animals and Experimental Design
2.5. Biochemical Serum Analysis
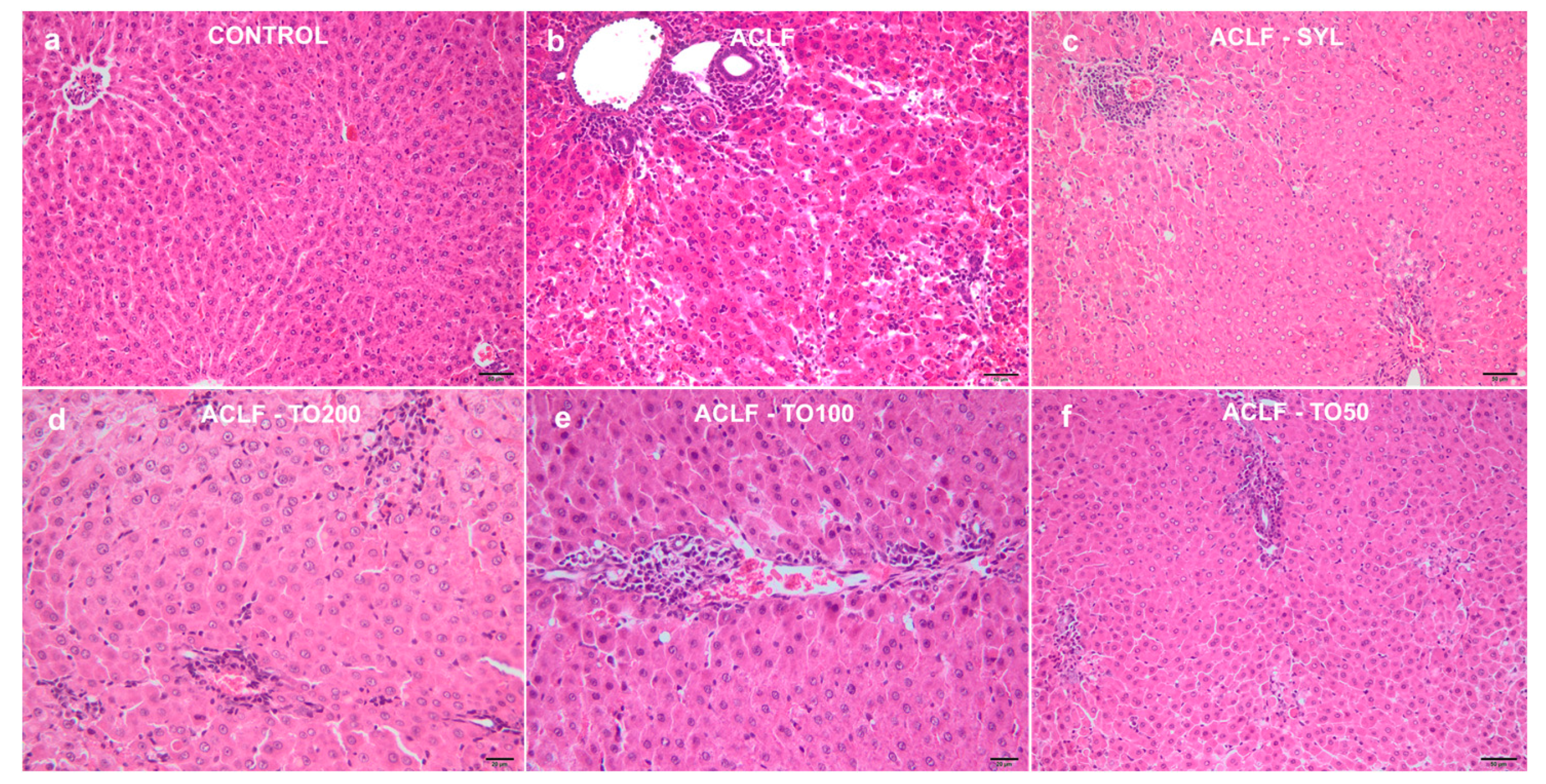
2.6. Histological Assessment
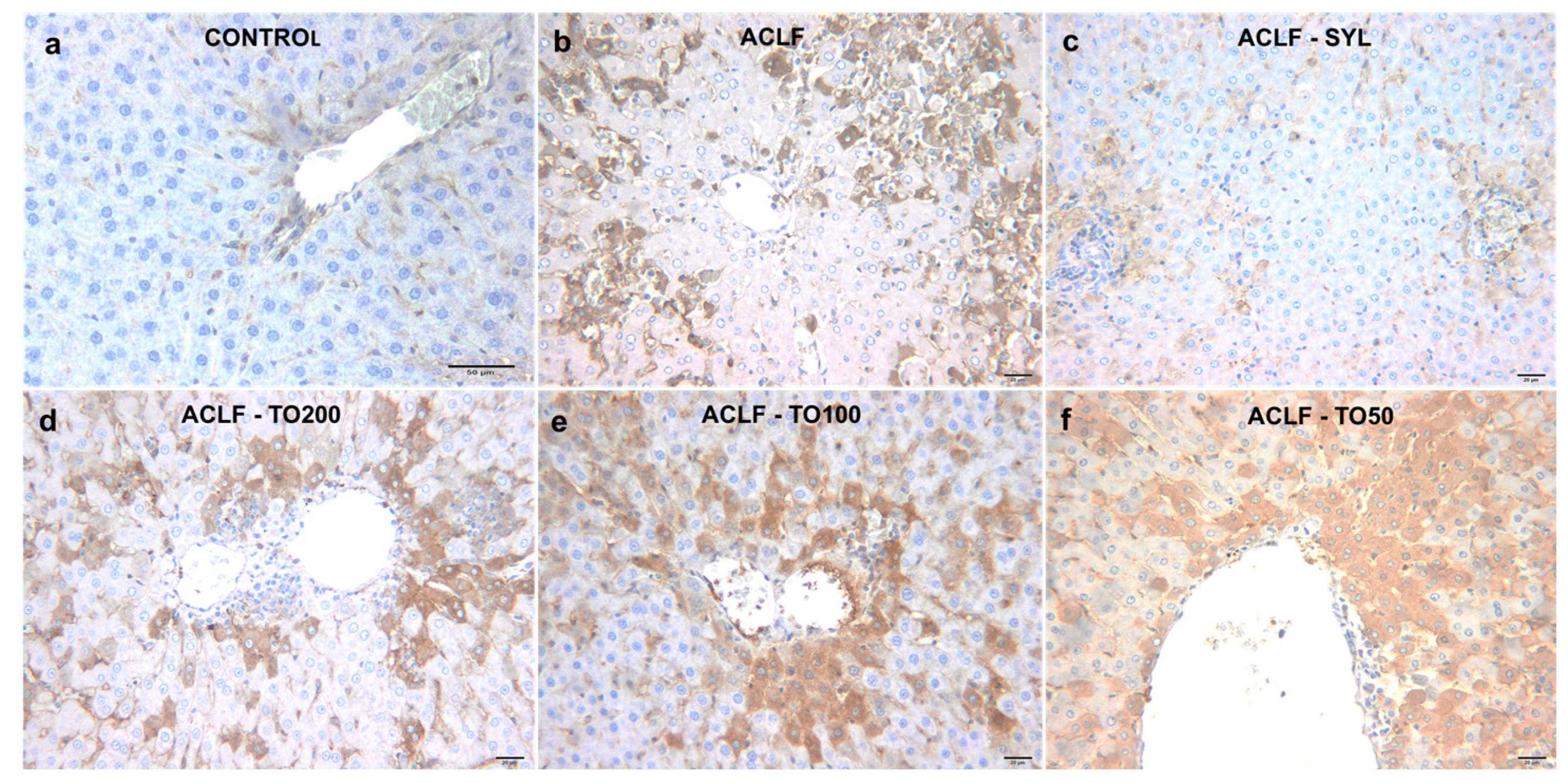
2.7. Immunohistochemical Analysis of 3-Nitrotyrosine
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Biochemical Serum Analysis
3.3. Histological Assessment
3.4. 3-Nitrityrosine Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, S.A.; Sharief, N.H.; Mohamed, Y.S. Hepatoprotective Activity of Some Medicinal Plants in Sudan. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-Chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver (APASL): An Update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Moreau, R.; Kamath, P.S.; Vargas, H.E.; Arroyo, V.; Reddy, K.R.; Szabo, G.; Tandon, P.; Olson, J.; Karvellas, C.; et al. Acute-on-Chronic Liver Failure: Getting Ready for Prime Time? Hepatology 2018, 68, 1621–1632. [Google Scholar] [CrossRef]
- Asrani, S.K.; Simonetto, D.A.; Kamath, P.S. Acute-on-Chronic Liver Failure. Clin. Gastroenterol. Hepatol. 2015, 13, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Ginès, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, D.J.; Alves, V.; Balabaud, C.; Bhathal, P.S.; Bioulac-Sage, P.; Colombari, R.; Crawford, J.M.; Dhillon, A.P.; Ferrell, L.; Gill, R.M.; et al. Acute-on-chronic liver failure 2018: A need for (urgent) liver biopsy? Expert Rev. Gastroenterol. Hepatol. 2018, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, D.; Dharod, M.V.; Chandnani, M.; Baijal, R.; Kumar, P.; Jain, M.; Patel, N.; Kamani, P.; Issar, S.; Shah, N.; et al. Acute-on-chronic liver failure: A prospective study to determine the clinical profile, outcome, and factors predicting mortality. Indian J. Gastroenterol. 2015, 34, 216–224. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Woollard, K.J.; McPhail, M.J.W.; Antoniades, C.G.; Possamai, L.A. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front. Immunol. 2018, 9, 2948. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Waseem Mumtaz, M.; Tayyab Akhtar, M.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef]
- Gerbino, A.; Russo, D.; Colella, M.; Procino, G.; Svelto, M.; Milella, L.; Carmosino, M. Dandelion root extract induces intracellular Ca2+ increases in HEK293 cells. Int. J. Mol. Sci. 2018, 19, 1112. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective effects of chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Zhang, L.; Xu, J.; Zhu, C.; Zhao, H.; Lv, G. Dandelion root extract suppressed gastric cancer cells proliferation and migration through targeting lncRNA-CCAT1. Biomed. Pharmacother. 2017, 93, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Meng, N.; Liu, Z.; Guo, L.; Dong, L.; Li, B.; Ye, Q. Neuroprotective effects of Taraxacum officinale wigg. extract on glutamate-induced oxidative stress in HT22 cells via HO-1/Nrf2 pathways. Nutrients 2018, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Noh, Y.; Kim, M.S.; Jang, A.; Lee, C.E.; Myung, S.C. Steroidogenic effects of Taraxacum officinale extract on the levels of steroidogenic enzymes in mouse Leydig cells. Anim. Cells Syst. 2018, 22, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef]
- Abdel-Magied, N.; Abdel Fattah, S.M.; Elkady, A.A. Differential effect of Taraxacum officinale L. (dandelion) root extract on hepatic and testicular tissues of rats exposed to ionizing radiation. Mol. Biol. Rep. 2019, 46, 4893–4907. [Google Scholar] [CrossRef]
- Al-Rasheed, N.; Faddah, L.; Sharaf, I.A.; Mohamed, A.M.; Al-Rasheed, N.; Abdel Baky, N.A. Assessment of the potential role of silymarin alone or in combination with vitamin E and/or curcumin on the carbon tetrachloride induced liver injury in rat. Braz. Arch. Biol. Technol. 2015, 58, 833–842. [Google Scholar] [CrossRef]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary characterization and Hepatoprotective effects of polysaccharides from dandelion Root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef]
- Pfingstgraf, I.O.; Taulescu, M.; Orasan, R.; Pop, R.M.; Laurian, V.; Toma, C. Alina Elena Parvu Effect of Taraxacum officinale L. (dandelion) root extract in experimental chronic liver failure. Rev. Rom. Med. Vet. 2020, 30, 85–91. [Google Scholar]
- Rusu, M.E.; Gheldiu, A.M.; Mocan, A.; Moldovan, C.; Popa, D.S.; Tomuta, I.; Vlase, L. Process optimization for improved phenolic compounds recovery from walnut (Juglans regia L.) Septum: Phytochemical profile and biological activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.M.; Najafzadeh, H.; Bahmei, S. Protective role of silymarin and D-penicillamine against lead-induced liver toxicity and oxidative stress. Toxicol. Ind. Health 2017, 33, 512–518. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, L.K.; Chen, H.; Fan, C.; Li, X.; He, C.M.; Gong, Z.J. Ethyl pyruvate protects against experimental acute-onchronic liver failure in rats. World J. Gastroenterol. 2012, 18, 5709–5718. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Lian, L.-H.; Jiang, Y.-Z.; Nan, J.-X. Hepatoprotective effects of salidroside on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J. Pharm. Pharmacol. 2009, 61, 1375–1382. [Google Scholar] [CrossRef]
- Li, F.; Miao, L.; Sun, H.; Zhang, Y.; Bao, X.; Zhang, D. Establishment of a new acute-on-chronic liver failure model. Acta Pharm. Sin. B 2017, 7, 326–333. [Google Scholar] [CrossRef]
- Pârvu, M.; Moţ, C.A.; Pârvu, A.E.; Mircea, C.; Stoeber, L.; Roşca-Casian, O.; Ţigu, A.B. Allium sativum extract chemical composition, antioxidant activity and antifungal effect against meyerozyma guilliermondii and rhodotorula mucilaginosa causing onychomycosis. Molecules 2019, 24, 3958. [Google Scholar] [CrossRef]
- Andreicut, A.-D.; Pârvu, A.E.; Mot, A.C.; Pârvu, M.; Fischer Fodor, E.; Cătoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical Analysis of Anti-Inflammatory and Antioxidant Effects of Mahonia aquifolium Flower and Fruit Extracts. Oxid. Med. Cell. Longev. 2018, 2018, 2879793. [Google Scholar] [CrossRef]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.M.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U.; et al. Revised guides for organ sampling and trimming in rats and mice—Part 1. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2003. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Ciobanu, L.; Taulescu, M.; Apostu, D.; Lucaciu, O.; Filip, G.A.; Feldrihan, V.; Licarete, E.; Ilea, A.; Piciu, A.; et al. Periodontal disease may induce liver fibrosis in an experimental study on Wistar rats. J. Periodontol. 2019, 90, 911–919. [Google Scholar] [CrossRef] [PubMed]
- García-Monzón, C.; Majano, P.L.; Zubia, I.; Sanz, P.; Apolinario, A.; Moreno-Otero, R. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J. Hepatol. 2000, 32, 331–338. [Google Scholar] [CrossRef]
- Lazarova, I.; Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Gevrenova, R.; Zheleva-Dimitrova, D.; Zengin, G.; Danchev, N.D. Hepatoprotective and antioxidant potential of Asphodeline lutea (L.) Rchb. roots extract in experimental models in vitro/in vivo. Biomed. Pharmacother. 2016, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Aremu, O.O.; Oyedeji, A.O.; Oyedeji, O.O.; Nkeh-Chungag, B.N.; Rusike, C.R.S. In Vitro and In Vivo Antioxidant Properties of Taraxacum officinale in Nω-Nitro-l-Arginine Methyl Ester (L-NAME)-Induced Hypertensive Rats. Antioxidants 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phyther. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef]
- Karakuş, A.; Değer, Y.; Yıldırım, S. Protective effect of Silybum marianum and Taraxacum officinale extracts against oxidative kidney injuries induced by carbon tetrachloride in rats. Ren. Fail. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yoon, K.D.; Kim, J. Chemical constituents from Taraxacum officinale and their α-glucosidase inhibitory activities. Bioorganic Med. Chem. Lett. 2018, 28, 476–481. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic Fractions from Dandelion Leaves and Petals as Modulators of the Antioxidant Status and Lipid Profile in an In Vivo Study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.; Kalavagunta, P.K.; Li, J.; He, Q.; Zhang, Y.; Ahmad, O.; Yin, H.; Wang, T.; Shang, J. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed. Pharmacother. 2018, 104, 679–685. [Google Scholar] [CrossRef]
- Massaad, C.; Iuliano, L.; Lizard, G. Oxysterols and phytosterols in human health. Chem. Phys. Lipids 2017, 207, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and Inflammation. Curr. Med. Chem. 2018, 26, 6724–6734. [Google Scholar] [CrossRef]
- Hovenkamp, E.; Demonty, I.; Plat, J.; Lütjohann, D.; Mensink, R.P.; Trautwein, E.A. Biological effects of oxidized phytosterols: A review of the current knowledge. Prog. Lipid Res. 2008, 47, 37–49. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C.; Snyder, J. Annual Review of Immunology Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ. Toxicol. 2016. [Google Scholar] [CrossRef]
- Macdonald, S.; Andreola, F.; Bachtiger, P.; Amoros, A.; Pavesi, M.; Mookerjee, R.; Zheng, Y.B.; Gronbaek, H.; Gerbes, A.L.; Sola, E.; et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology 2018, 67, 989–1002. [Google Scholar] [CrossRef]
- Hamza, A.A.; Mohamed, M.G.; Lashin, F.M.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 9. [Google Scholar] [CrossRef]
- Jin, L.; Gao, H.; Wang, J.P.; Yang, S.J.; Wang, J.; Liu, J.F.; Yang, Y.; Yan, T.T.; Chen, T.; Zhao, Y.; et al. Role and regulation of autophagy and apoptosis by nitric oxide in hepatic stellate cells during acute liver failure. Liver Int. 2017, 37, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, X.; Ding, X.; Teng, J. Analysis of the high incidence of acute kidney injury associated with acute-on-chronic liver failure. Hepatol. Int. 2018, 12, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Sheikh, M.F.; Lamb, E.; Agarwal, B.; Jalan, R. Acute kidney injury in acute-on-chronic liver failure: Where does hepatorenal syndrome fit? Kidney Int. 2017, 92, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Owatari, M.S.; Alves Jesus, G.F.; Brum, A.; Pereira, S.A.; Lehmann, N.B.; de Pádua Pereira, U.; Martins, M.L.; Pedreira Mouriño, J.L. Sylimarin as hepatic protector and immunomodulator in Nile tilapia during Streptococcus agalactiae infection. Fish Shellfish Immunol. 2018, 82, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Cai, F.F.; Chen, Q.L.; Song, Y.N.; Sun, Y.; Wei, B.; Li, X.Y.; Hu, Y.Y.; Liu, P.; Su, S.B. Chinese herbal formula Fuzheng Huayu alleviates CCl 4-induced liver fibrosis in rats: A transcriptomic and proteomic analysis. Acta Pharmacol. Sin. 2018, 39, 930–941. [Google Scholar] [CrossRef]
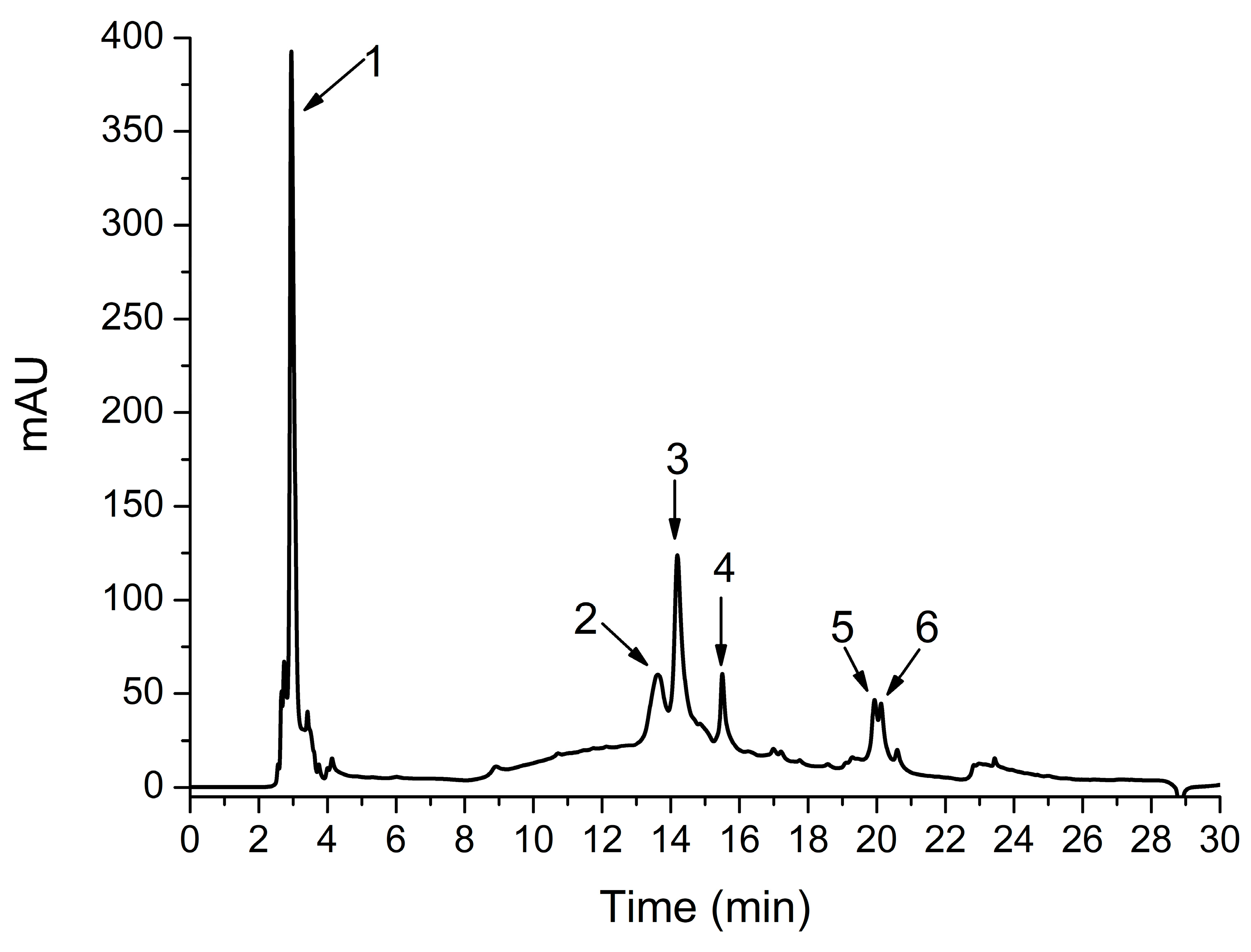
| No | Retention Time Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Tentative Identification | Concentration * mg CA/ g TOERE |
|---|---|---|---|---|---|
| 1 | 2.95 | 270 | 138 | Hydroxybenzoic acid | 3.65 ± 0.15 |
| 2 | 13.62 | 320 | 181, 163 | Caffeic acid | 1.09 ± 0.02 |
| 3 | 14.19 | 322 | 475, 312 | Chicoric acid | 1.95 ± 0.15 |
| 4 | 15.50 | 322 | 369 | Feruloylquinic acid | 0.6 ± 0.08 |
| 5 | 19.93 | 322 | 516, 181,163 | Dicaffeoylquinic acid | 0.53 ± 0.04 |
| 6 | 20.12 | 322 | 516, 181,163 | Dicaffeoylquinic acid isomer | 0.4 ± 0.03 |
| Groups | AST (U/L) | ALT (U/L) | TB (mg/dL) | ALP (mg/dL) | GGT (mg/dL) | Urea (mg/dL) | CR (mg/dL) |
|---|---|---|---|---|---|---|---|
| ACLF-TO200 | 81.12 a ± 5.27 | 71.64 a,b,c ± 11.32 | 2.27 a,b,c ± 0.37 | 328.45 a,b ± 14.72 | 60.42 a,b,c ± 9.20 | 67.14 a,b,c ± 4.21 | 1.75 a,b ± 0.21 |
| ACLF-TO100 | 82.14 a,b,c ± 4.20 | 54.08 b,c ± 12.37 | 1.30 b,c ± 0.27 | 310.38 a,b,c ± 11.19 | 49.97 b,c ± 8.37 | 78.93 a,b ± 5.18 | 1.78 a,b ± 0.14 |
| ACLF-TO50 | 84.24 a,b,c ± 8.06 | 144.93 a,b,c ± 19.79 | 2.02 a,b ± 0.51 | 329.61 a,b ± 37.89 | 107.34 a,b,c ± 18.33 | 110.30 a,b,c ± 7.89 | 2.15 a,b ± 0.40 |
| ACLF-SYL | 126.37 a,b ± 6.58 | 111.67 a,b ± 13.04 | 2.44 a,b ± 0.13 | 332.59 a ± 29.20 | 74.51 a,b ± 9.86 | 81.25 a,b ± 12.15 | 2.02 a,b ± 0.29 |
| ACLF | 222.65 a,c ± 11.08 | 174.08 a,c ± 15.16 | 3.74 a,c ± 0.53 | 358.94 a,c ± 13.55 | 117.71 a,c ± 15.47 | 255.49 a,c ± 19.48 | 3.53 a,c ± 0.28 |
| Control | 35.04 ± 6.63 | 47.55 ± 10.08 | 1.01 ± 0.11 | 263.75 ± 15.20 | 44.31 ± 4.58 | 39.16 ± 2.71 | 0.57 ± 0.04 |
| Groups | TOS (µM H2O2/L) | TAR (mM TROLOX/L) | OSI | MDA (nM/L) | NOx (µM/L) | 3NT (nmol/L) | SH (mM GSH/L) |
|---|---|---|---|---|---|---|---|
| ACLF-TO200 | 30.61 a,b,c ± 6.85 | 1.088 ± 0.001 | 31.57 a,b,c ± 6.13 | 3.05 a,b,c ± 0.28 | 21.92 b,c ± 3.74 | 769.36 a,b,c ± 78.46 | 0.48 a,b ± 0.03 |
| ACLF-TO100 | 35.50 a,b,c ± 7.27 | 1.089 ± 0.001 | 31.17 a,b,c ± 4.84 | 3.67 b ± 0.59 | 25.76 a,b,c ± 4.50 | 768.66 a,b,c ± 69.75 | 0.48 a,b ± 0.08 |
| ACLF-TO50 | 40.45 a,b,c ± 8.46 | 1.089 ± 0.001 | 31.82 a,b,c ± 9.39 | 3.95 a,b ± 0.47 | 30.52 a,b,c ± 7.60 | 820.20 a,b,c ± 48.43 | 0.48 a,b ± 0.02 |
| ACLF-SYL | 36.41 a,b ± 7.75 | 1.092 ± 0.003 | 37.03 a,b ± 8.27 | 3.83 a,b ± 0.34 | 36.56 a,b ± 6.76 | 971.07 a,b ± 68.34 | 0.52 a,b ± 0.02 |
| ACLF | 47.98 a,c ± 7.95 | 1.089 ± 0.001 | 40.40 a,c ± 8.60 | 5.37 a,c ± 0.08 | 51.49 a,c ± 7.32 | 1053.99 a,c ± 91.15 | 0.40 a,c ± 0.03 |
| Control | 21.18 ± 1.72 | 1.089 ± 0.001 | 21.59 ± 4.61 | 3.57 ± 0.36 | 19.98 ± 1.99 | 480.45 ± 56.62 | 0.59 ± 0.01 |
| Groups | Portal Inflammation | Periportal Degeneration/ Necrosis | Intralobular Degeneration/ Necrosis | Fibrosis | HAI | 3NT |
|---|---|---|---|---|---|---|
| ACLF-TO200 | 1.60 a,b,c ± 0.89 | 2.20 a,b,c ± 0.10 | 1.00 a,b,c ± 0.01 | 1.20 a,c ± 0.10 | 5.80 a,c ± 1.92 | 1.40 a,b,c ± 0.55 |
| ACLF-TO100 | 2.20 a,b,c ± 0.10 | 2.60 a,b,c ± 0.89 | 1.40 a,b,c ± 0.89 | 1.00 a,c ± 0.10 | 7.20 a,b,c ± 1.10 | 1.40 a,b,c ± 0.55 |
| ACLF-TO50 | 2.60 a,b,c ± 0.89 | 2.20 a,b,c ± 1.10 | 2.20 a,b,c ± 1.10 | 1.00 a,c ± 0.10 | 8.00 a,b,c ± 1.41 | 1.80 a,b,c ± 0.45 |
| ACLF-SYL | 1.00 a,b ± 0.00 | 0.60 a,b ± 0.55 | 0.80 a,b ± 0.45 | 0.40 a,b ± 0.55 | 2.80 a,b ± 0.45 | 1.20 a,b ± 0.45 |
| ACLF | 3.60 a,c ± 0.55 | 4.80 a,c ± 0.84 | 3.60 a,b ± 0.55 | 1.00 a,b ± 0.10 | 12.80 a,b ± 1.64 | 2.40 a,b ± 0.55 |
| Control | 0.40 ± 0.55 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.45 | 0.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. https://doi.org/10.3390/antiox10040504
Pfingstgraf IO, Taulescu M, Pop RM, Orăsan R, Vlase L, Uifalean A, Todea D, Alexescu T, Toma C, Pârvu AE. Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants. 2021; 10(4):504. https://doi.org/10.3390/antiox10040504
Chicago/Turabian StylePfingstgraf, Iulia Olimpia, Marian Taulescu, Raluca Maria Pop, Remus Orăsan, Laurian Vlase, Ana Uifalean, Doina Todea, Teodora Alexescu, Corina Toma, and Alina Elena Pârvu. 2021. "Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure" Antioxidants 10, no. 4: 504. https://doi.org/10.3390/antiox10040504
APA StylePfingstgraf, I. O., Taulescu, M., Pop, R. M., Orăsan, R., Vlase, L., Uifalean, A., Todea, D., Alexescu, T., Toma, C., & Pârvu, A. E. (2021). Protective Effects of Taraxacum officinale L. (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants, 10(4), 504. https://doi.org/10.3390/antiox10040504










