Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis
Abstract
1. Introduction
2. Natural-Compound-Based Treatments for OA Therapy
2.1. Alkaloids
Berberine
2.2. Flavonoids
2.2.1. Apigenin
2.2.2. Astragalin
2.2.3. Baicalein
2.2.4. Chrysin
2.2.5. Genistein
2.2.6. Icariin
2.2.7. Kaempferol
2.2.8. Luteolin
2.2.9. Naringin
2.2.10. Puerarin
2.2.11. Silibinin/Silymarin
2.2.12. Wogonin
2.3. Phenols
2.3.1. Curcumin
2.3.2. Gingerly/Ginger
2.3.3. Oleuropein
2.3.4. Resveratrol
2.3.5. Salvianolic Acid B
2.4. Polysaccharides
Achyranthes bidentata Extracts
2.5. Terpenoids
2.5.1. Andrographolide
2.5.2. Astaxanthin
2.5.3. Aucubin
2.5.4. Boswellia serrata
2.5.5. Celastrol
2.5.6. Ginsenoside
2.5.7. Harpagophytum procumbens
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brennan-Olsen, S.L.; Cook, S.; Leech, M.T.; Bowe, S.J.; Kowal, P.; Ackerman, N.; Page, R.S.; Hosking, S.M.; Pasco, J.A.; Mohebbi, M. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: Analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) Wave 1. BMC Musculoskelet. Disord. 2017, 18, 271. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef]
- Spetea, M. Opioid Receptors and Their Ligands in the Musculoskeletal System and Relevance for Pain Control. Curr. Pharm. Des. 2014, 19, 7382–7390. [Google Scholar] [CrossRef]
- Osani, M.C.; Bannuru, R.R. Efficacy and safety of duloxetine in osteoarthritis: A systematic review and meta-analysis. Korean J. Intern. Med. 2019, 34, 966–973. [Google Scholar] [CrossRef]
- Oo, W.M.; Liu, X.; Hunter, D.J. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opin. Drug Metab. Toxicol. 2019, 15, 1021–1032. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried Plum Polyphenols Inhibit Osteoclastogenesis by Downregulating NFATc1 and Inflammatory Mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009, 58, 899–908. [Google Scholar] [CrossRef]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Yang, Z.; Zheng, C.; Jing, J.; Chen, Y.; Ye, X.; Lian, X.; Qiu, W.; Yang, F.; et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+-nuclear fact. J. Bone Miner. Res. 2012, 27, 1298–1308. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [PubMed]
- Smith, C.; Patel, R.; Vannabouathong, C.; Sales, B.; Rabinovich, A.; McCormack, R.; Belzile, E.L.; Bhandari, M. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1974–1983. [Google Scholar] [CrossRef]
- Cao, P.; Li, Y.; Tang, Y.; Ding, C.; Hunter, D.J. Pharmacotherapy for knee osteoarthritis: Current and emerging therapies. Expert Opin. Pharmacother. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Berberine and musculoskeletal disorders: The therapeutic potential and underlying molecular mechanisms. Phytomedicine 2019, 152892. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Lee, H.P.; Hung, C.Y.; Tsai, C.H.; Li, T.M.; Tang, C.H. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharmacol. 2015, 289, 20–29. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, H.; Li, Y.; Deng, M.; He, B.; Xia, S.; Zhang, C.; Liu, S. Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur. J. Pharmacol. 2016, 789, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.-Q.; Yu, L.; He, B.; Wu, S.-H.; Zhao, Q.; Xia, S.-Q.; Mei, H.-J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015, 20, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.-Q.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ming, J.; Deng, M.; Li, Y.; Li, B.; Li, J.; Ma, Y.; Chen, Z.; Liu, S. Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-ĸB signaling. Pharmacol. Res. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Lee, H.W.; Suh, J.H.; Kim, H.N.; Kim, A.Y.; Park, S.Y.; Shin, C.S.; Choi, J.-Y.; Kim, J.B. Berberine Promotes Osteoblast Differentiation by Runx2 Activation With p38 MAPK. J. Bone Miner. Res. 2008, 23, 1227–1237. [Google Scholar] [CrossRef]
- Wei, P.; Jiao, L.; Qin, L.-P.; Yan, F.; Han, T.; Zhang, Q.-Y. Effects of berberine on differentiation and bone resorption of osteoclasts derived from rat bone marrow cells. J. Chin. Integr. Med. 2009, 7, 342–348. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. Int. Rev. J. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [CrossRef]
- Davidson, R.K.; Green, J.; Gardner, S.; Bao, Y.; Cassidy, A.; Clark, I.M. Identifying chondroprotective diet-derived bioactives and investigating their synergism. Sci. Rep. 2018, 8, 17173. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, D.K.; Shin, H.-D.; Lee, H.J.; Jo, H.S.; Jeong, J.H.; Choi, Y.L.; Lee, C.J.; Hwang, S.-C. Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes. Biomol. Ther. 2016, 24, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Ramos-Torrecillas, J.; De luna-Bertos, E.; Ruiz, C.; Garcia-Martinez, O. Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression. Nutrients 2019, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Zha, X.; Xu, Z.; Li, L.; Liu, Y.; Xu, L.; Cui, L.; Xu, D.; Zhu, B. Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Mol. Cell. Biochem. 2015, 407, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, D.; Qin, Y.; Xu, M.; Zhou, L.; Xu, W.; Liu, X.; Ye, L.; Yue, S.; Zheng, Q.; et al. Astragalin Promotes Osteoblastic Differentiation in MC3T3-E1 Cells and Bone Formation in vivo. Front. Endocrinol. 2019, 10, 228. [Google Scholar] [CrossRef]
- Ma, Z.; Piao, T.; Wang, Y.; Liu, J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int. Immunopharmacol. 2015, 25, 83–87. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, T.; Wang, X.; Xu, H.; Liu, Y.; Wang, Y.; Shi, Q.; Liang, Q. Astragalin Suppresses Inflammatory Responses and Bone Destruction in Mice With Collagen-Induced Arthritis and in Human Fibroblast-Like Synoviocytes. Front. Pharmacol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Chen, X.; Zhang, Y.; Zhang, Y.; Jia, Y.; Wang, H.; Liu, Y.; Xiao, L. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int. Immunopharmacol. 2014, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Song, X.; Bai, H.; Ma, T.; Zhang, Z.; Li, X.; Jiang, R.; Wang, G.; Fan, X.; et al. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget 2017, 8, 90781–90795. [Google Scholar] [CrossRef]
- Chen, W.-P.; Xiong, Y.; Hu, P.-F.; Bao, J.-P.; Wu, L.-D. Baicalein Inhibits MMPs Expression via a MAPK-Dependent Mechanism in Chondrocytes. Cell. Physiol. Biochem. 2015, 36, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Ryu, S.Y.; Bae, M.A.; Choi, J.-S.; Min, Y.K.; Kim, S.H. Baicalein inhibits osteoclast differentiation and induces mature osteoclast apoptosis. Food Chem. Toxicol. 2008, 46, 3375–3382. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.-J.; Chen, J.; Zhang, P.; Wang, T.; Chen, T.-Y.; Yan, B.; Huang, B.; Wang, L.; Huang, M.-J.; et al. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des. Devel. Ther. 2015, 9, 5169–5183. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Azimi-Nezhad, M. Protective Effects of Chrysin Against Drugs and Toxic Agents. Dose Response 2017, 15, 1559325817711782. [Google Scholar] [CrossRef]
- Zheng, W.; Tao, Z.; Cai, L.; Chen, C.; Zhang, C.; Wang, Q.; Ying, X.; Hu, W.; Chen, H. Chrysin Attenuates IL-1β-Induced Expression of Inflammatory Mediators by Suppressing NF-κB in Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1143–1154. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, W.; Huang, C.; Ding, Q.; Liang, C.; Wang, L.; Hou, Z.; Zhang, Z. Chrysin protects human osteoarthritis chondrocytes by inhibiting inflammatory mediator expression via HMGB1 suppression. Mol. Med. Rep. 2018, 19, 1222–1229. [Google Scholar] [CrossRef]
- Zeng, W.; Yan, Y.; Zhang, F.; Zhang, C.; Liang, W. Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell 2013, 4, 539–547. [Google Scholar] [CrossRef]
- Menon, A.H.; Soundarya, S.P.; Sanjay, V.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Sustained release of chrysin from chitosan-based scaffolds promotes mesenchymal stem cell proliferation and osteoblast differentiation. Carbohydr. Polym. 2018, 195, 356–367. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Claassen, H.; Briese, V.; Manapov, F.; Nebe, B.; Schünke, M.; Kurz, B. The phytoestrogens daidzein and genistein enhance the insulin-stimulated sulfate uptake in articular chondrocytes. Cell Tissue Res. 2008, 333, 71–79. [Google Scholar] [CrossRef]
- Tanamas, S.K.; Wijethilake, P.; Wluka, A.E.; Davies-Tuck, M.L.; Urquhart, D.M.; Wang, Y.; Cicuttini, F.M. Sex hormones and structural changes in osteoarthritis: A systematic review. Maturitas 2011, 69, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Wang, C.-C.; Lu, J.-W.; Lee, C.-H.; Chen, S.-C.; Ho, Y.-J.; Peng, Y.-J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, W.; Wu, N.; Jiang, S.; Li, W. Protective Effect of Genistein on Condylar Cartilage through Downregulating NF-κB Expression in Experimentally Created Osteoarthritis Rats. BioMed Res. Int. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Ming, L.-G.; Chen, K.-M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef]
- Kim, M.; Lim, J.; Lee, J.-H.; Lee, K.-M.; Kim, S.; Park, K.W.; Nho, C.W.; Cho, Y.S. Understanding the functional role of genistein in the bone differentiation in mouse osteoblastic cell line MC3T3-E1 by RNA-seq analysis. Sci. Rep. 2018, 8, 3257. [Google Scholar] [CrossRef]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Levy, R.M. Combination of alendronate and genistein synergistically suppresses osteoclastic differentiation of RAW267.4 cells in vitro. Exp. Ther. Med. 2017, 14, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, F.; He, Q.; Wang, J.; Shiu, H.T.; Shu, Y.; Tsang, W.P.; Liang, S.; Zhao, K.; Wan, C. Flavonoid Compound Icariin Activates Hypoxia Inducible Factor-1α in Chondrocytes and Promotes Articular Cartilage Repair. PLoS ONE 2016, 11, e0148372. [Google Scholar] [CrossRef]
- Liu, M.-H.; Sun, J.-S.; Tsai, S.-W.; Sheu, S.-Y.; Chen, M.-H. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr. Res. 2010, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Wang, J.; Liu, Y.; Liu, J.; Hu, L.; Panayi, A.C.; Liu, G.; Zhou, W. Icariin Activates Autophagy via Down-Regulation of the NF-κB Signaling-Mediated Apoptosis in Chondrocytes. Front. Pharmacol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Zou, W.; Wu, R.M.; Yang, J.; Fan, J.N.; Zhao, X.K.; Li, H.Y. Icariin Alleviates IL-1β-Induced Matrix Degradation By Activating The Nrf2/ARE Pathway In Human Chondrocytes. Drug Des. Devel. Ther. 2019, 13, 3949–3961. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Wang, J.; Liu, X.; Wei, H.; Han, Y.; Yang, S.; Wang, X. Icariin regulates the osteoblast differentiation and cell proliferation of MC3T3-E1 cells through microRNA-153 by targeting Runt-related transcription factor 2. Exp. Ther. Med. 2018, 15, 5159–5166. [Google Scholar] [CrossRef]
- Ma, H.-P.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Zhou, J.; Gao, Y.-H.; Xian, C.J.; Chen, K.-M. Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif. 2014, 47, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wang, S.; Wang, Q. Effect of icariin on serum bone turnover markers expressions and histology changes in mouse osteoarthritis model. Chin. J. Reparative Reconstr. Surg. 2017, 31, 963–969. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases (Review). Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. Med. Sci. Monit. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, Q.; Mao, Z.; Wang, P.; Zhang, R.; Ma, X.; Chen, J.; You, H. Kaempferol inhibits interleukin-1β stimulated matrix metalloproteinases by suppressing the MAPK-associated ERK and P38 signaling pathways. Mol. Med. Rep. 2018, 18, 2697–2704. [Google Scholar] [CrossRef]
- Kim, I.-R.; Kim, S.-E.; Baek, H.-S.; Kim, B.-J.; Kim, C.-H.; Chung, I.-K.; Park, B.-S.; Shin, S.-H. The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells. BMC Complement. Altern. Med. 2016, 16, 333. [Google Scholar] [CrossRef]
- Sharma, A.R.; Nam, J.-S. Kaempferol stimulates WNT/β-catenin signaling pathway to induce differentiation of osteoblasts. J. Nutr. Biochem. 2019, 74, 108228. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Xu, B.; Yuan, Z.; Leng, Y.; Min, J.; Lan, X.; Luo, J. Kaempferol promotes bone formation in part via the mTOR signaling pathway. Mol. Med. Rep. 2019, 20, 5197–5207. [Google Scholar] [CrossRef]
- Kim, C.-J.; Shin, S.-H.; Kim, B.-J.; Kim, C.-H.; Kim, J.-H.; Kang, H.-M.; Park, B.-S.; Kim, I.-R. The Effects of Kaempferol-Inhibited Autophagy on Osteoclast Formation. Int. J. Mol. Sci. 2018, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Q.; Han, T.; Zhang, X.; Wu, J.-Z.; Rahman, K.; Qin, L.-P.; Zheng, C.-J. Kaempferitrin prevents bone lost in ovariectomized rats. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 1159–1162. [Google Scholar] [CrossRef]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.T.; Mishra, P.R.; Trivedi, R. Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Moncada-Pazos, A.; Obaya, A.J.; Viloria, C.G.; López-Otín, C.; Cal, S. The nutraceutical flavonoid luteolin inhibits ADAMTS-4 and ADAMTS-5 aggrecanase activities. J. Mol. Med. 2011, 89, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-J.; Ryu, J.; Lee, C.J.; Hwang, S.-C. Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee. Biomol. Ther. 2014, 22, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, H.; Li, L.; Xu, D.; Gao, Y.; Guan, Y.; Chen, Q. 5,7,3′,4′-Tetramethoxyflavone protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1α pathway. Connect. Tissue Res. 2018, 59, 157–166. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Z.; Shi, W.; Zhang, R.; Li, L.; Liu, H.; Wu, L. TMF inhibits miR-29a/Wnt/β-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes. Drug Des. Dev. Ther. 2019, 13, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-M. Modulatory effects of luteolin on osteoblastic function and inflammatory mediators in osteoblastic MC3T3-E1 cells. Cell Biol. Int. 2007, 31, 870–877. [Google Scholar] [CrossRef]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Q.; Ahn, J.H.; Kim, S.B.; Kim, Y.C.; Sung, S.H.; Hwang, B.Y.; Lee, M.K. Luteolin downregulates IL-1β-induced MMP-9 and -13 expressions in osteoblasts via inhibition of ERK signalling pathway. J. Enzyme Inhib. Med. Chem. 2012, 27, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Khosravi, A.; Aidy, A.; Shafiei, M. Biphasic Response to Luteolin in MG-63 Osteoblast-Like Cells under High Glucose-Induced Oxidative Stress. Iran. J. Med. Sci. 2016, 41, 118–125. [Google Scholar]
- Jing, Z.; Wang, C.; Yang, Q.; Wei, X.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Ma, X.; Liu, K.; et al. Luteolin attenuates glucocorticoid-induced osteoporosis by regulating ERK/Lrp-5/GSK-3β signaling pathway in vivo and in vitro. J. Cell. Physiol. 2019, 234, 4472–4490. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.-J.; Kim, S.-Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef]
- Song, F.; Wei, C.; Zhou, L.; Qin, A.; Yang, M.; Tickner, J.; Huang, Y.; Zhao, J.; Xu, J. Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 2017, 233, 1723–1735. [Google Scholar] [CrossRef]
- Su, Y.-X.; Yan, H.; Chen, B.-J.; Zahn, Q.; Wang, Y.-R.; Lu, M.L.; Wang, W.-T.; He, Z.; Sheng, L. Effect of naringin of Drynaria Rhizome, a Chinese medical component of Zhuanggu Jianxi Recipe containing serum on caveolin-p38MAPK signal pathway in IL-1β induced rabbit degenerated chondrocytes. Chin. J. Integr. Tradit. West. Med. 2014, 34, 1492–1498. [Google Scholar]
- Zhao, Y.; Li, Z.; Wang, W.; Zhang, H.; Chen, J.; Su, P.; Liu, L.; Li, W. Naringin Protects Against Cartilage Destruction in Osteoarthritis Through Repression of NF-κB Signaling Pathway. Inflammation 2016, 39, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz. J. Med. Biol. Res. 2017, 50, e5714. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.-F.; Sun, W.-X. Effect of Naringin on Monosodium Iodoacetate-Induced Osteoarthritis Pain in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3746–3751. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Wooley, P.H.; Xu, Z.; Yang, S.-Y. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2013, 18, 478–485. [Google Scholar] [CrossRef]
- Wang, D.; Ma, W.; Wang, F.; Dong, J.; Wang, D.; Sun, B.; Wang, B. Stimulation of Wnt/β-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell. Physiol. Biochem. 2015, 36, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, J.; Fan, Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARγ. Mol. Med. Rep. 2015, 12, 4759–4765. [Google Scholar] [CrossRef]
- Zhai, Y.-K.; Niu, Y.-B.; Pan, Y.-L.; Li, C.-R.; Wu, X.-L.; Mei, Q.-B. Effects of naringin on proliferation, differentiation and maturation of rat calvarial osteoblasts in vitro. Chin. J. Integr. Tradit. West. Med. 2013, 38, 105–111. [Google Scholar]
- Xu, T.; Wang, L.; Tao, Y.; Ji, Y.; Deng, F.; Wu, X.-H. The Function of Naringin in Inducing Secretion of Osteoprotegerin and Inhibiting Formation of Osteoclasts. Evid.-Based Complement. Altern. Med. 2016, 8981650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects: ACTIVITY OF PUERARIN. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shan, H.; Wang, B.; Wang, N.; Zhou, Z.; Pan, C.; Wang, F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-γ Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology 2018, 102, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xie, Z.; Pei, J.; Wang, B.; Gao, Y.; Qu, Y. Puerarin alters the function of monocytes/macrophages and exhibits chondroprotection in mice. Mol. Med. Rep. 2019, 19, 2876–2882. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-P.; Zhu, X.-F.; Yang, L.; Liang, H.; Feng, S.-W.; Zhang, R.-H. Puerarin stimulates osteoblasts differentiation and bone formation through estrogen receptor, p38 MAPK, and Wnt/β-catenin pathways. J. Asian Nat. Prod. Res. 2012, 14, 897–905. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Zhou, S.; Gong, X.; Dai, Q.; Zhang, P.; Jiang, L. Puerarin Stimulates Osteogenic Differentiation and Bone Formation Through the ERK1/2 and p38-MAPK Signaling Pathways. Curr. Mol. Med. 2018, 17, 488–496. [Google Scholar] [CrossRef]
- Zhan, X.-Q.; Zeng, W.-W.; Zhang, Y.-Y.; Feng, Q.; Zhao, F.-M.; Jiang, Z.-Q.; Sun, C. Puerarin promotes the viability and differentiation of MC3T3-E1 cells by miR-204-regulated Runx2 upregulation. Mol. Med. Rep. 2017, 16, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Feng, Q.; Zhao, F.; Sun, C.; Zhou, T.; Yang, J.; Zhan, X. Puerarin inhibits TRPM3/miR-204 to promote MC3T3-E1 cells proliferation, differentiation and mineralization. Phytother. Res. 2018, 32, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Cheng, S.-Y.; Yang, R.; Zeng, X.-W.; Zhao, F.-M.; Zhan, X.-Q. Puerarin promotes the viability and differentiation of MC3T3-E1 cells by enhancing LC3B-mediated autophagy through downregulation of miR-204. Exp. Ther. Med. 2019, 19, 883–890. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Jian, S.; Li, B. Puerarin and zinc additively prevent mandibular bone loss through inhibiting osteoclastogenesis in ovariectomized rats. Histol. Histopathol. 2017, 32, 851–860. [Google Scholar] [CrossRef]
- Park, K.H.; Gu, D.R.; Jin, S.H.; Yoon, C.-S.; Ko, W.; Kim, Y.C.; Lee, S.H. Pueraria lobate Inhibits RANKL-Mediated Osteoclastogenesis Via Downregulation of CREB/PGC1β/c-Fos/NFATc1 Signaling. Am. J. Chin. Med. 2017, 45, 1725–1744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, M.; Yu, Q.; Yang, P.; Zhang, H.; Sun, Y.; Zhang, Z.; Gao, Y. Puearin prevents LPS-induced Osteoclast formation and bone loss via inhibition of akt activation. Biol. Pharm. Bull. 2016, 39, 2028–2035. [Google Scholar] [CrossRef]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, Transport and Drug-Drug Interactions of Silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.L.; Conti, F.; Maselli, A.; Pagano, M.-T.; Ruggieri, A.; Anticoli, S.; Fragale, A.; Gabriele, L.; Gagliardi, M.C.; Sanchez, M.; et al. The Natural Agonist of Estrogen Receptor β Silibinin Plays an Immunosuppressive Role Representing a Potential Therapeutic Tool in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1903. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Jassim, N.A.; Numan, I.T.; Al-Khalifa, I.I.; Abdullah, T.A. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. A comparative study with piroxicam and meloxicam. Saudi Med. J. 2009, 30, 98–103. [Google Scholar]
- Ashkavand, Z.; Malekinejad, H.; Amniattalab, A.; Rezaei-Golmisheh, A.; Vishwanath, B.S. Silymarin potentiates the anti-inflammatory effects of Celecoxib on chemically induced osteoarthritis in rats. Phytomedicine 2012, 19, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; Lou, Y.; Chen, C.; Zhang, C.; Tao, Z.; Li, H.; Cheng, L.; Ying, X. Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget 2017, 8, 99649–99665. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Jin, H.M.; Song, I.; Youn, B.U.; Lee, J.; Kim, N. Silibinin inhibits osteoclast differentiation mediated by TNF family members. Mol. Cells 2009, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-L.; Kang, S.-W.; Kang, M.-K.; Gong, J.-H.; Lee, E.-S.; Han, S.J.; Kang, Y.-H. Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J. Cell. Biochem. 2012, 113, 247–259. [Google Scholar] [CrossRef]
- Kim, J.-L.; Park, S.-H.; Jeong, D.; Nam, J.-S.; Kang, Y.-H. Osteogenic activity of silymarin through enhancement of alkaline phosphatase and osteocalcin in osteoblasts and tibia-fractured mice. Exp. Biol. Med. 2012, 237, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Sun, L.; Chen, X.; Xu, H.; Guo, X.; Chen, H.; Hong, J.; Cheng, S.; Peng, L. Silibinin promotes osteoblast differentiation of human bone marrow stromal cells via bone morphogenetic protein signaling. Eur. J. Pharmacol. 2013, 721, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.X.; Cai, W.J.; Sun, X.Y.; Dai, P.P.; Li, X.M.; Wang, Q.; Huang, X.L.; He, B.; Wang, P.P.; Wu, G.; et al. RAGE-dependent mitochondria pathway: A novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products. Cell Death Dis. 2018, 9, 674. [Google Scholar] [CrossRef]
- Kim, J.-L.; Kim, Y.-H.; Kang, M.-K.; Gong, J.-H.; Han, S.-J.; Kang, Y.-H. Antiosteoclastic activity of milk thistle extract after ovariectomy to suppress estrogen deficiency-induced osteoporosis. BioMed Res. Int. 2013, 919374. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Salmani, J.M.M.; Fu, R.; Chen, B. Advances of wogonin, an extract from Scutellaria baicalensis, for the treatment of multiple tumors. OncoTargets Ther. 2016, 9, 2935–2943. [Google Scholar] [CrossRef]
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.F.; Xue, H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev. 2005, 11, 141–150. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on matrix metalloproteinase-13 expression of interleukin-1β-treated articular chondrocytes and their cellular mechanisms: Inhibition of c-Fos/AP-1 and JAK/STAT signaling pathways. J. Pharmacol. Sci. 2011, 116, 221–231. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci. Rep. 2017, 7, 43789. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, H.J.; Lee, D.Y.; Jo, H.S.; Jeong, J.H.; Kim, D.H.; Nam, D.C.; Lee, C.J.; Hwang, S.-C. Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo. Biomol. Ther. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef]
- Sirong, S.; Yang, C.; Taoran, T.; Songhang, L.; Shiyu, L.; Yuxin, Z.; Xiaoru, S.; Tao, Z.; Yunfeng, L.; Xiaixiao, C. Effects of tetrahedral framework nucleic acid/wogonin complexes on osteoarthritis. Bone Res. 2020, 8, 6. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, X.; Wang, J.; Xu, L.; Zhou, L.; Yu, W.; Tao, Y.; Zhu, J.; Hu, B.; Liang, C.; et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int. Immunopharmacol. 2018, 65, 539–549. [Google Scholar] [CrossRef]
- Zhao, P.; Cheng, J.; Geng, J.; Yang, M.; Zhang, Y.; Zhang, Q.; Wang, Y.; Lu, B. Curcumin protects rabbit articular chondrocytes against sodium nitroprusside-induced apoptosis in vitro. Eur. J. Pharmacol. 2018, 828, 146–153. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, C.-K.; Song, J.-H.; Yun, J.-H.; Lee, A.; Park, H.-J. Highly bioavailable curcumin powder suppresses articular cartilage damage in rats with mono-iodoacetate (MIA)-induced osteoarthritis. Food Sci. Biotechnol. 2020, 29, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Ba, G.; Yu, D.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis by protecting osteoblasts from apoptosis in vivo and in vitro. Clin. Exp. Pharmacol. Physiol. 2016, 43, 268–276. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, J.; Shen, T.; Mu, S.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Mao, Y.; Sun, X.; Li, X.; Muhammad, I.; Gu, W.; Zhang, D.; Zhou, Y.; Ni, Z.; Ma, J.; et al. Attenuation of Oxidative Stress-Induced Osteoblast Apoptosis by Curcumin is Associated with Preservation of Mitochondrial Functions and Increased Akt-GSK3β Signaling. Cell. Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Gupte, P.A.; Giramkar, S.A.; Harke, S.M.; Kulkarni, S.K.; Desmukh, A.P.; Hingorani, L.L.; Mahajan, M.P.; Bhalerao, S.S. Evaluation of the efficacy and safety of Capsule Longvida® Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: A pilot clinical study. J. Inflamm. Res. 2019, 12, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jung, E.; Hyeon, H.; Seon, S.; Lee, D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102104. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hunter, D.J.; Eyles, J.; McLachlan, A.J.; Adiwidjaja, J.; Eagles, S.K.; Wang, X.S. Pharmacokinetic assessment of constituents of Boswellia serrata, pine bark extracts, curcumin in combination including methylsulfonylmethane in healthy volunteers. J. Pharm. Pharmacol. 2020, 72, 121–131. [Google Scholar] [CrossRef]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation ‘turmeric extract, black pepper, and ginger’ versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Liu, X.; Robbins, S.; Eyles, J.; Fedorova, T.; Virk, S.; Deveza, L.A.; McLachlan, A.; Hunter, D. Efficacy and safety of a supplement combination for hand osteoarthritis pain: Protocol for an internet-based randomised placebo-controlled trial (The RADIANT study). BMJ Open 2020, 10, e035672. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Araya-Quintanilla, F.; Gutierrez-Espinoza, H.; Munoz-Yanez, M.J.; Sanchez-Montoya, U.; Lopez-Jeldes, J. Effectiveness of Ginger on Pain and Function in Knee Osteoarthritis: A PRISMA Systematic Review and Meta-Analysis. Pain Physician 2020, 23, E151–E161. [Google Scholar]
- Hosseinzadeh, A.; Juybari, K.B.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1β in Cultured Chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Abusarah, J.; Benabdoune, H.; Shi, Q.; Lussier, B.; Martel-Pelletier, J.; Malo, M.; Fernandes, J.C.; Pereira de Souza, F.; Fahmi, H.; Benderdour, M. Elucidating the Role of Protandim and 6-Gingerol in Protection Against Osteoarthritis. J. Cell. Biochem. 2017, 118, 1003–1013. [Google Scholar] [CrossRef]
- Fan, J.Z.; Yang, X.; Bi, Z.G. The effects of 6-gingerol on proliferation, differentiation, and maturation of osteoblast-like MG-63 cells. Braz. J. Med. Biol. Res. 2015, 48, 637–643. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Kim, T.; Kim, R.; Ha, H. The Natural Product 6-Gingerol Inhibits Inflammation-Associated Osteoclast Differentiation via Reduction of Prostaglandin E2 Levels. Int. J. Mol. Sci. 2018, 19, 2068. [Google Scholar] [CrossRef]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef]
- Amorndoljai, P.; Taneepanichskul, S.; Niempoog, S.; Nimmannit, U. A Comparative of Ginger Extract in Nanostructure Lipid Carrier (NLC) and 1% Diclofenac Gel for Treatment of Knee Osteoarthritis (OA). J. Med. Assoc. Thai. 2017, 100, 447–456. [Google Scholar] [PubMed]
- Bolognesi, G.; Belcaro, G.; Feragalli, B.; Cornelli, U.; Cotellese, R.; Hu, S.; Dugall, M. Movardol® (N-acetylglucosamine, Boswellia serrata, ginger) supplementation in the management of knee osteoarthritis: Preliminary results from a 6-month registry study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5198–5204. [Google Scholar] [PubMed]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Agosti, I.D.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychoden, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Pang, K.-L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.-N.; Sanchez, C.; Membrez-Calfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.-F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive polyphenol hydroxytyrosol prevents bone loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sanchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Montserrat-de-la-Paz, S.; Abia, R.; Castejon, M.L.; Millan-Linares, M.C.; Alarcon-de-la-Lastra, C.; Fernadez-Bolanos, J.G.; Muriana, F.J.G. Oleuropein and its peracetylated derivative negatively regulate osteoclastogenesis by controlling the expression of genes involved in osteoclast differentiation and function. Food Funct. 2020, 11, 4038–4048. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Mathey, J.; Agalias, A.; Kati-Coulibaly, S.; Mardon, J.; Obled, C.; Davicco, M.-J.; Lebecque, P.; Horcajada, M.-N.; Skaltsounis, A.L.; et al. Dose-response study of effect of oleuropein, an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin. Nutr. 2006, 25, 859–868. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.-J.; Coxam, V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging 2015, 19, 77–86. [Google Scholar] [CrossRef]
- Nguyen, C.; Savouret, J.-F.; Widerak, M.; Corvol, M.-T.; Rannou, F. Resveratrol, Potential Therapeutic Interest in Joint Disorders: A Critical Narrative Review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Im, H.-J.; Li, X.; Chen, D.; Yan, D.; Kim, J.; Ellman, M.B.; Stein, G.S.; Cole, B.; Ranjan, K.C.; Cs-Szabo, G.; et al. Biological effects of the plant-derived polyphenol resveratrol in human articular cartilage and chondrosarcoma cells. J. Cell. Physiol. 2012, 227, 3488–3497. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.-S.; Chen, J.-W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.-G.; Xiao, D.-M.; Fan, M.; Wang, D.-P.; Xiong, J.-Y.; Chen, Y.; Dong, Y.; Liu, S.-L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Kang, D.-G.; Lee, H.J.; Lee, C.J.; Park, J.S. Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway. Biomol. Ther. 2018, 26, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α: Intrapoap-Articular Resveratrol Injection Prevents Osteoarthritis Progression. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective Effect of Resveratrol against IL-1β-Induced Inflammatory Response on Human Osteoarthritic Chondrocytes Partly via the TLR4/MyD88/NF-κB Signaling Pathway: An “in Vitro Study”. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet. Mediat. Inflamm. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.-H.; Jeong, J.-K.; Lee, Y.-J.; Seol, J.-W.; Jackson, C.J.; Park, S.-Y. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Hu, B.; Zhang, M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp. Ther. Med. 2017, 14, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2017, 17, 1493–1498. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; You, W.; Tang, X.; Li, X.; Gong, Z. Therapeutic effect of Resveratrol in the treatment of osteoarthritis via the MALAT1/miR-9/NF-κB signaling pathway. Exp. Ther. Med. 2020, 19, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol Mediated Modulation of Sirt-1/Runx2 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells: Potential Role of Runx2 Deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.-M.; Guo, C.; Han, J.-F. Resveratrol promotes osteoblastic differentiation in a rat model of postmenopausal osteoporosis by regulating autophagy. Nutr. Metab. 2020, 17, 29. [Google Scholar] [CrossRef]
- Huang, Y.; Hui, J.; Liu, F.Q.; Liu, J.; Zhang, X.J.; Guo, C.H.; Song, L.H. Resveratrol Promotes in vitro Differentiation of Osteoblastic MC3T3-E1 Cells via Potentiation of the Calcineurin/NFATc1 Signaling Pathway. Biochemistry 2019, 84, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 Interactions with p300 Modulate Receptor Activator of NF-κB Ligand (RANKL) Activation of NF-κB Signaling and Inhibit Osteoclastogenesis in Bone-derived Cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef]
- Kim, H.-N.; Han, L.; Iyer, S.; De Cabo, R.; Zhao, H.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs. Mol. Endocrinol. 2015, 29, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Miao, L.; Lu, Y.; Wang, L. Sirtuin 1 inhibits TNF-α-mediated osteoclastogenesis of bone marrow-derived macrophages through both ROS generation and TRPV1 activation. Mol. Cell. Biochem. 2019, 455, 135–145. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Evans, H.M.; Howe, P.R.C. Resveratrol supplementation reduces pain experience by postmenopausal women. Menopause 2017, 24, 916–922. [Google Scholar] [CrossRef]
- Hussain, S.; Marouf, B.; Ali, Z.; Ahmmad, R. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Cao, W.; Guo, X.-W.; Zheng, H.-Z.; Li, D.-P.; Jia, G.-B.; Wang, J. Current progress of research on pharmacologic actions of salvianolic acid B. Chin. J. Integr. Med. 2012, 18, 316–320. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Li, S.; Wang, P.; Zhu, W.; Liang, P.; Tan, J.; Cui, S. Salvianolic acid B regulates gene expression and promotes cell viability in chondrocytes. J. Cell. Mol. Med. 2017, 21, 1835–1847. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, C.; Zheng, W.; Tang, Q.; Chen, Y.; Zhang, X.; Guo, X.; Wang, J. Salvianolic acid B inhibits IL-1β-induced inflammatory cytokine production in human osteoarthritis chondrocytes and has a protective effect in a mouse osteoarthritis model. Int. Immunopharmacol. 2017, 46, 31–37. [Google Scholar] [CrossRef]
- Cui, L.; Li, T.; Liu, Y.; Zhou, L.; Li, P.; Xu, B.; Huang, L.; Chen, Y.; Liu, Y.; Tian, X.; et al. Salvianolic Acid B Prevents Bone Loss in Prednisone-Treated Rats through Stimulation of Osteogenesis and Bone Marrow Angiogenesis. PLoS ONE 2012, 7, e34647. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, L.; Li, J.; Xu, B.; Wu, T.; Fan, H.; Xu, W.; Yao, W.; Yang, Y.; Liu, Y.; et al. Salvianolic acid B and danshensu induce osteogenic differentiation of rat bone marrow stromal stem cells by upregulating the nitric oxide pathway. Exp. Ther. Med. 2017, 14, 2779–2788. [Google Scholar] [CrossRef][Green Version]
- He, X.; Shen, Q. Salvianolic acid B promotes bone formation by increasing activity of alkaline phosphatase in a rat tibia fracture model: A pilot study. BMC Complement. Altern. Med. 2014, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, Y.; Li, Z.; Liu, C.; Xue, P. Sal B targets TAZ to facilitate osteogenesis and reduce adipogenesis through MEK-ERK pathway. J. Cell. Mol. Med. 2019, 23, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yu, T.; Peng, L.; Wang, L.; Liao, Z.; Xu, W. PIM1, CYP1B1, and HSPA2 Targeted by Quercetin Play Important Roles in Osteoarthritis Treatment by Achyranthes bidentata. Evid. Based Complement. Alternat. Med. 2019, 1–10. [Google Scholar] [CrossRef]
- Weng, X.; Lin, P.; Liu, F.; Chen, J.; Li, H.; Huang, L.; Zhen, C.; Xu, H.; Liu, X.; Ye, H.; et al. Achyranthes bidentata polysaccharides activate the Wnt/β-catenin signaling pathway to promote chondrocyte proliferation. Int. J. Mol. Med. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Xiao, W.; Peng, L.; Wang, L.; Liao, Z.; Hu, L. Achyranthes bidentata extract protects chondrocytes functions through suppressing glycolysis and apoptosis via MAPK/AKT signaling axis. Am. J. Transl. Res. 2020, 12, 142–152. [Google Scholar] [PubMed]
- Zhang, D.; Wang, C.; Hou, X.; Yan, C. Structural characterization and osteoprotective effects of a polysaccharide purified from Achyranthes bidentata. Int. J. Biol. Macromol. 2019, 139, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Guo, W.; Lou, Z.; Zhang, H. Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell Biochem. Biophys. 2014, 70, 467–473. [Google Scholar] [CrossRef]
- Song, D.; Cao, Z.; Huang, S.; Tickner, J.; Li, N.; Qiu, H.; Chen, X.; Wang, C.; Chen, K.; Sun, Y.; et al. Achyranthes bidentata polysaccharide suppresses osteoclastogenesis and bone resorption via inhibiting RANKL signaling. J. Cell. Biochem. 2018, 119, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, S.; Wang, C.; Zhang, Q. A fructooligosaccharide from Achyranthes bidentata inhibits osteoporosis by stimulating bone formation. Carbohydr. Polym. 2019, 210, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes. Antioxidants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress. Antioxidants 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Villedieu-Percheron, E.; Ferreira, V.; Campos, J.F.; Destandau, E.; Pichon, C.; Berteina-Raboin, S. Quantitative determination of Andrographolide and related compounds in Andrographis paniculata extracts and biological evaluation of their Anti-Inflammatory Activity. Foods 2019, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Hancke, J.L.; Srivastav, S.; Cáceres, D.D.; Burgos, R.A. A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin®) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 2019, 33, 1469–1479. [Google Scholar] [CrossRef]
- Ding, Q.; Ji, X.; Cheng, Y.; Yu, Y.; Qi, Y.; Wang, X. Inhibition of matrix metalloproteinases and inducible nitric oxide synthase by andrographolide in human osteoarthritic chondrocytes. Mod. Rheumatol. 2013, 23, 1124–1132. [Google Scholar] [CrossRef]
- Li, B.; Jiang, T.; Liu, H.; Miao, Z.; Fang, D.; Zheng, L.; Zhao, J. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-Are signaling pathway. J. Cell. Physiol. 2018, 234, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Luo, Z.; Chen, X. Andrographolide mitigates cartilage damage via miR-27-3p-modulated matrix metalloproteinase13 repression. J. Gene Med. 2020, 22, e3187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, B.; Huang, L.; Wu, H.; Huang, J.; Liang, T.; Liu, H.; Zheng, L.; Zhao, J. Andrographolide Exerts Pro-Osteogenic Effect by Activation of Wnt/β-Catenin Signaling Pathway in Vitro. Cell. Physiol. Biochem. 2015, 36, 2327–2339. [Google Scholar] [CrossRef]
- Li, B.; Hu, R.-Y.; Sun, L.; Luo, R.; Lu, K.-H.; Tian, X.-B. Potential role of andrographolide in the proliferation of osteoblasts mediated by the ERK signaling pathway. Biomed. Pharmacother. 2016, 83, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef]
- Han, J.H.; Ju, J.H.; Lee, Y.S.; Park, J.H.; Yeo, I.J.; Park, M.H.; Roh, Y.S.; Han, S.B.; Hong, J.T. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci. Rep. 2018, 8, 14090. [Google Scholar] [CrossRef]
- Kumar, A.; Dhaliwal, N.; Dhaliwal, J.; Dharavath, R.N.; Chopra, K. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol. Rep. 2020, 72, 104–114. [Google Scholar] [CrossRef]
- Park, M.H.; Jung, J.C.; Hill, S.; Cartwright, E.; Dohnalek, M.H.; Yu, M.; Jun, H.J.; Han, S.B.; Hong, J.T.; Son, D.J. FlexPro MD®, a Combination of Krill Oil, Astaxanthin and Hyaluronic Acid, Reduces Pain Behavior and Inhibits Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Nutrients 2020, 12, 956. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- Huang, L.; Chen, W.-P. Astaxanthin ameliorates cartilage damage in experimental osteoarthritis. Mod. Rheumatol. 2015, 25, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Kim, K.-J.; Kim, S.-J.; Min, S.-K.; Hong, S.-G.; Son, Y.-J.; Yee, S.-T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2018, 53, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Wang, S.-N.; Xie, G.-P.; Qin, C.-H.; Chen, Y.-R.; Zhang, K.-R.; Li, X.; Wu, Q.; Dong, W.-Q.; Yang, J.; Yu, B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int. Immunopharmacol. 2015, 24, 408–415. [Google Scholar] [CrossRef]
- Young, I.-C.; Chuang, S.-T.; Hsu, C.-H.; Sun, Y.-J.; Liu, H.-C.; Chen, Y.-S.; Lin, F.-H. Protective effects of aucubin on osteoarthritic chondrocyte model induced by hydrogen peroxide and mechanical stimulus. BMC Complement. Altern. Med. 2017, 17, 91. [Google Scholar] [CrossRef]
- Huang, T.-L.; Yang, S.-H.; Chen, Y.-R.; Liao, J.-Y.; Tang, Y.; Yang, K.-C. The therapeutic effect of aucubin-supplemented hyaluronic acid on interleukin-1beta-stimulated human articular chondrocytes. Phytomedicine 2019, 53, 1–8. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, Y.; Yao, Z.-I.; Chen, P.; Yu, B.; Wang, S. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model. Drug Des. Devel. Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell. Biochem. 2011, 354, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Blain, E.J.; Ali, A.Y.; Duance, V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phytother. Res. 2010, 24, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, X.; Wong, H.H.; Wagner, C.A.; Lahey, L.J.; Robinson, W.H.; Sokolove, J. Oral and topical boswellic acid attenuates mouse osteoarthritis. Osteoarthr. Cartil. 2014, 22, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ichikawa, H.; Badmaev, V.; Aggarwal, B.B. Acetyl-11-Keto-β-Boswellic Acid Potentiates Apoptosis, Inhibits Invasion, and Abolishes Osteoclastogenesis by Suppressing NF-κB and NF-κB-Regulated Gene Expression. J. Immunol. 2006, 176, 3127–3140. [Google Scholar] [CrossRef]
- Bai, F.; Chen, X.; Yang, H.; Xu, H.-G. Acetyl-11-Keto-β-Boswellic Acid Promotes Osteoblast Differentiation by Inhibiting Tumor Necrosis Factor-α and Nuclear Factor-κB Activity. J. Craniofac. Surg. 2018, 29, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jiang, L.; Zhu, M.; Li, Y.; Luo, M.; Jiang, P.; Tong, S.; Zhang, H.; Yan, J. The genus Tripterygium: A phytochemistry and pharmacological review. Fitoterapia 2019, 137, 104190. [Google Scholar] [CrossRef]
- Ding, Q.-H.; Cheng, Y.; Chen, W.-P.; Zhong, H.-M.; Wang, X.-H. Celastrol, an inhibitor of heat shock protein 90β potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013, 708, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Ha, C.; Lin, T.; Wang, D.; Wang, Y.; Gong, M. Celastrol attenuates pain and cartilage damage via SDF-1/CXCR4 signalling pathway in osteoarthritis rats. J. Pharm. Pharmacol. 2018, 70, 81–88. [Google Scholar] [CrossRef]
- Feng, K.; Chen, H.; Xu, C. Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition. Inflamm. Res. 2020, 69, 385–400. [Google Scholar] [CrossRef]
- Cascão, R.; Vidal, B.; Finnila, M.A.J.; Lopes, I.P.; Teixeira, R.L.; Saarakkala, S.; Moita, L.F.; Fonseca, J.E. Effect of celastrol on bone structure and mechanics in arthritic rats. RMD Open 2017, 3, e000438. [Google Scholar] [CrossRef]
- Gan, K.; Xu, L.; Feng, X.; Zhang, Q.; Wang, F.; Zhang, M.; Tan, W. Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int. Immunopharmacol. 2015, 24, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.; Shehzad, O.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur. J. Pharmacol. 2014, 724, 145–151. [Google Scholar] [CrossRef]
- Kim, S.-H.; Na, J.-Y.; Song, K.-B.; Choi, D.-S.; Kim, J.-H.; Kwon, Y.-B.; Kwon, J. Protective Effect of Ginsenoside Rb1 on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J. Ginseng Res. 2012, 36, 161–168. [Google Scholar] [CrossRef]
- Na, J.-Y.; Kim, S.; Song, K.; Lim, K.-H.; Shin, G.-W.; Kim, J.-H.; Kim, B.; Kwon, Y.-B.; Kwon, J. Anti-apoptotic Activity of Ginsenoside Rb1 in Hydrogen Peroxide-treated Chondrocytes: Stabilization of Mitochondria and the Inhibition of Caspase-3. J. Ginseng Res. 2012, 36, 242–247. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Fan, W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol. Cell. Biochem. 2014, 392, 249–257. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, D.; Zuo, Q.; Wang, Z.; Fan, W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int. Orthop. 2013, 37, 2065–2070. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, L.; Wang, Z.; Zhang, S.; Rong, X.-F.; Li, R.-H. Ginsenoside Rb1 inhibits matrix metalloproteinase 13 through down-regulating Notch signaling pathway in osteoarthritis. Exp. Biol. Med. 2015, 240, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, S.; Sun, Y.; Pan, X.; Xiao, L.; Zou, L.; Ho, K.W.; Li, G. Translational potential of ginsenoside Rb1 in managing progression of osteoarthritis. J. Orthop. Transl. 2016, 6, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Jing, J.; Wang, Z.; Wu, D.; Huang, Y. Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection. Nutrients 2017, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Ginsenoside-Rg5 treatment inhibits apoptosis of chondrocytes and degradation of cartilage matrix in a rat model of osteoarthritis. Oncol. Rep. 2017, 37, 1497–1502. [Google Scholar] [CrossRef]
- Xie, J.-J.; Chen, J.; Guo, S.-K.; Gu, Y.-T.; Yan, Y.-Z.; Guo, W.-J.; Yao, C.-L.; Jin, M.-Y.; Xie, C.-L.; Wang, X.; et al. Panax quinquefolium saponin inhibits endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and attenuates the progression of osteoarthritis in rat. Biomed. Pharmacother. 2018, 97, 886–894. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Han, G.; Zhou, S.; Li, J.; Chen, M.; Li, H. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int. J. Mol. Med. 2020, 45, 1225–1236. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.-J.; Veerappan, K.; Yang, D.-U.; Yang, D.-C. Stimulative Effect of Ginsenosides Rg5:Rk1 on Murine Osteoblastic MC3T3-E1 Cells. Phytother. Res. 2014, 10, 1447–1455. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kim, Y.-J.; Yang, D.C. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway: Rh1, osteoblast growth and differentiation. J. Pharm. Pharmacol. 2014, 66, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Siddiqi, M.H.; Kim, Y.-J.; Jin, Y.; Huq, M.A.; Yang, D.C. Effect of Fermented Red Ginseng Extract Enriched in Ginsenoside Rg3 on the Differentiation and Mineralization of Preosteoblastic MC3T3-E1 Cells. J. Med. Food 2015, 18, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Bei, J.; Zhang, X.; Wu, J.; Hu, Z.; Xu, B.; Lin, S.; Cui, L.; Wu, T.; Zou, L. Ginsenoside Rb1 does not halt osteoporotic bone loss in ovariectomized rats. PLoS ONE 2018, 13, e0202885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Li, J.; Du, J.; Lv, X.; Weng, L.; Ling, C. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB and MAPKs pathways. Food Chem. Toxicol. 2012, 50, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.; Wei, B.; Liu, X.; Lei, Z.; Bai, X. Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem. Biol. Interact. 2016, 256, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Conradt, C.; Roufogalis, B.D. Effectiveness of Harpagophytum extracts and clinical efficacy. Phytother. Res. 2004, 18, 187–189. [Google Scholar] [CrossRef]
- Harpagophytum procumbens (devil’s claw). Monograph. Altern. Med. Rev. 2008, 13, 248–252.
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Kim, W.K.; Park, H.J.; Cho, L.; Kim, M.-R.; Kim, M.J.; Shin, J.-S.; Lee, J.H.; Ha, I.-H.; Lee, S.K. Anti-osteoporotic activity of harpagide by regulation of bone formation in osteoblast cell culture and ovariectomy-induced bone loss mouse models. J. Ethnopharmacol. 2016, 179, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wegener, T.; Lüpke, N.-P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil’s claw (Harpagophytum procumbens DC). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
| Compound (Source) | Category | Structure | Therapeutic Target | Treatment | Ref. |
|---|---|---|---|---|---|
| Berberine (Berberis vulgaris) | Benzyl isoquinolin alkaloid | 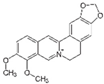 | Activation of AMPK signaling and inhibition of p38 MAPK/NF-κB pathways in chondrocytes. Activation of p38 MAPK signaling in osteoblasts. | Anti-inflammatory, anti-apoptotic, and anti-degradation in cartilage; Induction of bone formation. | [22,23,24,25,26,27,28] |
| Compound (Source) | Category | Structure | Therapeutic Target | Treatment | Ref. |
|---|---|---|---|---|---|
| Apigenin (chamomile, thyme, tea, extra virgin oil) | 4′,5,7-Trihydroxyflavone | 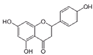 | Inhibition of IL-1β-induced effects and NF-κB, Hif-2α, and TGFβ/Smad2/3 pathways in chondrocytes. | Anti-inflammatory effect, prevent cartilage degradation. | [32,33] |
| Increases BMP-2, BMP-7, APL, and Col I in osteoblasts. Induces JNK and p38 MAPK pathways in osteoblasts. | Promotes osteoblastic differentiation. | [34,35] | |||
| Astragalin (Cuscuta chinensis) | Kaempferol 3-glucoside | 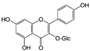 | Inhibition of IL-1β/NF-κB and MAPK in chondrocytes. | Anti-inflammatory effect, suppresses bone destruction. | [38,39] |
| Baicalein (Scutellaria baicalensis) | 5,6,7-Trihydroxyflavone | 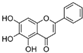 | Inhibition of IL-1β-induced effects in chondrocytes. Increases secretion of GAG and Col II. | Anti-catabolic and anti-apoptotic effects. | [42,43,44] |
| Increases osteoblast differentiation and inhibits osteoclast differentiation. | Attenuated OA in pre-clinical models. Inhibition of bone loss. | [45,46] | |||
| Chrysin (Passiflora caerulea, Scutellaria baicalensis) | 5,7-Dihydroxyflavone | 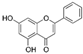 | Inhibition of IL-1β/NF-κB induction. Dowregulates the expression of iNOS, COX-2, MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, and HMGB-1 in chondrocytes. The level of NO, PGE2 decreases. | Anti-inflammatory and anti-apoptotic effects. | [48,49] |
| Activation of ERK/MAPK signaling in osteoblasts and upregulation of Runx-2 and Osx. | Induction of osteoblast differentiation. | [50,51] | |||
| Genistein (Genista tinctoria) | Isoflavone | 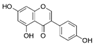 | Inhibition of IL-1β-induced effects via the activation of Nrf2/HO-1 signaling in chondrocytes. | Anti-catabolic effect. Attenuated OA in pre-clinical models. | [58,59] |
| Increases osteoblast differentiation via MAPK activation and inhibits osteoclast differentiation via NF-κB inhibition. | Inhibition of bone loss. | [60,61,62,63] | |||
| Icariin (Epimedium) | Flavonoid glycoside | 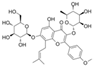 | Inhibition of IL-1β/TNF-α/LPS-induced effects via the inhibition of NF-κB and the activation of Nrf2/HO-1 signaling in chondrocytes. Increases the secretion of ACAN and Col II. Decreases the expression of MMP-1, 3, 9, 13, COX-2, and iNOS. | Anti-inflammatory and anti-catabolic effects. Increased cartilage repair in pre-clinical OA models. | [64,65,66,67,68] |
| Increases osteoblast differentiation via the activation of ERK, JUNK, and miR-153/Runx2 signaling. Increases the secretion of Col I APL. | Inhibition of bone loss. Improved bone remodeling in pre-clinical models. | [69,70,71,72] | |||
| Kaempferol (Kaempferia galanga) | 3,4′,5,7-Tetrahydroxyflavone | 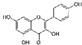 | Attenuation of IL-1β-induced effects by inhibiting p38 MAPK/NF-κB pathways in chondrocytes. | Anti-inflammatory effect. | [74,75] |
| Increases osteoblast differentiation via the activation of Wnt/β-catenin and mTOR signaling, increasing BMP-2, Rux-2, Osx, and Col I expression. Inhibits osteoclastogenesis by downregulating MAPK, c-Fos, and NFATc1. | Inhibition of bone loss and stimulation of bone formation. | [76,77,78,79,80,81] | |||
| Luteolin (Salvia tomentosa, Artemisia asiatica) | 3,4′,5,7-Tetrahydroxyflavone | 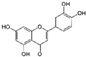 | Attenuation of IL-1β-induced effects by inhibiting NF-κB pathways and the activation of Foxo3a in chondrocytes. Decreases the expression of COX-2, iNOS, MMPs, and ADAMTS-4,5. Attenuates cartilage degradation and increases Col II secretion. | Anti-inflammatory and anti-catabolic effects. Attenuation of cartilage degradation. | [83,84,85,86,87] |
| Increases osteoblast differentiation via the regulation of ERK/Lrp-5/GSK-3β signaling, increasing BMP-7, Rux-2, Osx, Osc, APL, TGF-β1, and Col I expression. Inhibition of osteoclast differentiation. | Inhibition of bone loss and stimulation of bone formation. | [34,88,89,90,91,92,93,94] | |||
| Naringin (Citrus × paradisi) | Flavanone-7-O-glycoside | 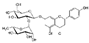 | Alleviation of IL-1β/TNFα/LPS-induced effects via inhibiting MAPK p38 and NF-κB signaling and the activation of Foxo3a in chondrocytes. Decreases the expression of MMPs and ADAMTS-4,5. Attenuates cartilage degradation. | Anti-inflammatory and anti-catabolic effects. Attenuation of cartilage degradation. | [95,96,97,98] |
| Increases osteoblast proliferation and differentiation. Increases the expression of Rux-2, Osx, Osc, BMP-2, OPN, and Col I expression. Inhibits osteoclast differentiation. | Inhibition of bone loss and promotes bone formation. | [99,100,101,102,103] | |||
| Puerarin (Pueraria lobate) | Isoflavone | 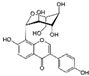 | Blocks the anti-catabolic effects in chondrocytes via the action of the AMPK/PGC-1α signaling pathway. Attenuates cartilage degradation. | Anti-inflammatory and anti-catabolic effects. Attenuation of cartilage degradation. | [105,106] |
| Promotes bone formation via the activation of p38 MAPK, ERK1/2-Runx2, and Wnt/β-catenin signaling and by inhibiting TRPM3/miR-204 expression. Inhibits osteoclastogenesis by downregulating CREB/PGC1β/c-Fos/NFATc1 signaling. | Inhibition of bone loss and promotes bone formation. | [107,108,109,110,111,112,113,114] | |||
| Silibinin/Silymarin (Silybum marianum) | Flavone | 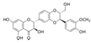 | Inhibition of IL-1β-induced effects by inhibiting PI3K/Akt and NF-κB signaling. Decreases the expression of iNOS, MMPs, and ADAMTS-4,5. Diminishes the secretion of NO, PEG2, TNF-α, and IL-6. Attenuates cartilage degradation and synovitis in vivo. | Anti-inflammatory, anti-oxidant, and anti-catabolic effects. Attenuation of cartilage degradation and synovitis. | [118,119,120] |
| Induces osteoblast differentiation, increasing the expression of Runx-2, BMP-2, ALP, and Col I. Inhibits osteoclastogenesis by disturbing TRAF6-c-Src signaling. | Anti-oxidant and anti-apoptotic effects in osteoblasts. Inhibition of bone loss. | [121,122,123,124,125,126] | |||
| Wogonin (Scutellaria baicalensis) | O-methylated flavone | 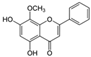 | Inhibition of IL-1β-induced effects by inhibiting c-Fos/AP-1 and JAK/STAT signaling and activating ROS/ERK/Nrf2 signaling. Decreases the expression of iNOS, MMPs, and ADAMTS-4,5. Diminishes the secretion of NO, PEG2, TNF-α, and IL-6. Attenuates cartilage degradation and synovitis in vivo. | Anti-inflammatory, anti-oxidant, and anti-catabolic effects. Attenuation of cartilage degradation and synovitis. | [129,130,131,132,133,134] |
| Compound | Category | Structure | Therapeutic Target | Treatment | Ref. |
|---|---|---|---|---|---|
| Curcumine (Curcuma longa) | Diferuloyl-methane | 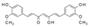 | Inhibits the expression of IL-6, iNOS, COX-2, MMPs, and ADAMTS4,5 and increases the expression of SOX-9 and Col II by inhibiting 5-LOX/NF-κB signaling and activating Nrf2/ARE signaling. | Anti-inflammatory, antioxidant, anti-apoptotic, and anti-catabolic effects. | [12,135,136,137,138] |
| Induces osteoblast differentiation, increasing the expression of Runx-2, Osx, Osc, and Col I by regulating Wnt signaling. | Attenuation of cartilage degradation and synovitis. Bone protection. | [139,140,141,142,143,144,145,146] | |||
| Gingerly/ginger (Zingier officinal) | 6-Gingerol | 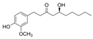 | Inhibits IL-1β-induced effects via the activation of Nrf2 signaling in chondrocytes. | Anti-apoptotic, antioxidant, and anti-inflammatory effects. | [149,150] |
| Induces osteoblasts differentiation and inhibits osteoclast differentiation. | Inhibition of bone loss. | [151,152] | |||
| Oleuropein (Olea europea) | Secoiridoid glycoside | 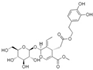 | Inhibits of IL-1β-induced effects by suppressing NF-κB and MAPK signaling. Decreases the expression of COX-2, iNOS, MMP-1, MMP-13, and ADAMTS-5. | Anti-inflammatory effects. Decreases synovitis, cartilage degradation, and osteophyte formation. | [160,161] |
| Increases calcium deposits and inhibits osteoclast formation and differentiation. | Bone protection. | [162,163,164,165,166] | |||
| Resveratrol (red grapes, blueberries, raspberries, mulberries) | 3,5,4′-Trihydroxy-trans-stilbene | 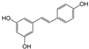 | Inhibits IL-1β-induced effects by suppressing NF-κB signaling and increasing SIRT-1 expression via the AMPK/mTOR pathway. It decreases the expression of iNOS, MMP1, MMP-3, MMP-13, and ADAMTS-4,5. | Anti-inflammatory and anti-apoptotic effects. Prevents cartilage degradation and maintains the homeostasis of cartilage and bone. | [171,172,173,174,175,176,177,178,179,180] |
| Induces osteoblast differentiation by modulating Sirt-1/Runx-2/Fox-1 and PI3K/AKT/mTOR signaling. Inhibits osteoclastogenesis via the activation of SIRT-1 and FoxOs. | Bone protection. | [181,182,183,184,185,186,187,188] | |||
| Salvianolic acid B (Radix salvia miltiorrhiza) | Polyphenol | 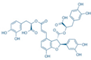 | Inhibits IL-1β-induced effects by suppressing NF-κB signaling. Decreases the expression of iNOS, COX-2, MMP-13, and ADAMTS-5. | Anti-inflammatory and anti-catabolic effects. Reduces cartilage degradation. | [192,193] |
| Induces osteoblast differentiation through the activation of ERK signaling, upregulating the expression of Runx-2, OPN, and Osx. | Bone protection and induces bone formation | [194,195,196,197] |
| Compound (Source) | Category | Therapeutic Target | Treatment | Ref. |
|---|---|---|---|---|
| Achyranthes bidentata extracts | Various polysaccharides | Induces chondrocyte proliferation, Wnt/β-catenin pathway activation, and inhibits apoptosis via MAPK/Akt signaling. | Anti-apoptotic effect and induces proliferation. | [199,200] |
| Promotes bone formation and inhibits osteoclastogenesis via the inhibition of RANK. | Bone formation. | [201,202,203] |
| Compound (Source) | Category | Structure | Therapeutic Target | Treatment | Ref. |
|---|---|---|---|---|---|
| Andrographolide (Andrographis paniculate) | Diterpenoid | 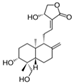 | Reduces oxidative stress and inhibits MMP-13 expression. Attenuates cartilage degradation via miR-27-3p/MMP13 signaling. | Anti-oxidant effects. Reduces the degradation of cartilage. | [210,211,212,213,214,215,216,217,218,219,220,221,222] |
| Promotes bone formation by inhibiting NF-κB signaling. | Bone formation | [213,214] | |||
| Astaxanthin (Haematococcus pluvialis) | Tetraterpenoid | 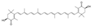 | Anti-catabolic effects via the activation of Nrf2–ARE signaling. Reduces cartilage degradation via MAPK signaling inhibition. | Antioxidant and anti-inflammatory effects. Attenuates degradation of cartilage | [221,222] |
| Aucubin (Aucuba japonica) | Iridoid glycoside | 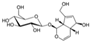 | Inhibits IL-1β-induced effects. Inhibits iNOS expression and NO production | Anti-inflammatory and antioxidant effects. Prevents OA progression. | [226,227,228,229] |
| Boswellia serrata | 11-Keto-β-boswellic, acetyl-11-keto-β-boswellic acid | 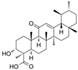 | Inhibits IL-1β/oncostatin-M-induced effect, decreasing the expression of MMP-9, MMP-13, and COX-2 and reducing the production of NO and PGE2. Inhibits 5-LOX and TNF-α. | Anti-inflammatory and antioxidant effects. | [15,232,233,234] |
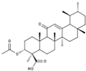 | Promotes osteoblast differentiation and suppresses osteoclastogenesis by inhibiting TNF-α and NF-κB signaling. | Bone protection. | [235,236] | ||
| Celastrol (Celastrus regelii, Tripterygium wilfordii) | Triterpenoid | 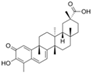 | Diminishes the IL-1β-induced catabolic effect, decreasing the expression of MMP-1, MMP-3, MMP-13, iNOS, and COX-2. Reduces cartilage degradation by inhibiting NF-κB signaling and activating SDF-1/CXCR4 signaling. | Anti-inflammatory and anti-catabolic effects. | [238,239,240] |
| Suppresses osteoclastogenesis by inhibiting MAPK and NF-κB signaling. | Bone protection. | [241,242] | |||
| Ginsenoside (Panax) | Terpenoid glycoside | 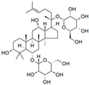 | Inhibits the IL-1β-induced effect, decreasing the expression of MMP-1, MMP-13, iNOS, and COX-2; the level of PGE2; promoting the expression of ACAN and Col II. | Anti-inflammatory, anti-apoptotic, antioxidant, and anti-degradative effects. | [244,245,246,247,248,249,250,251,252,253,254] |
| Promotes osteoblast differentiation and suppresses osteoclastogenesis by inhibiting MAPK and NF-κB signaling. | Bone protection | [255,256,257,258,259,260] | |||
| Harpagophytum procumbens | Iridoid glycosides | 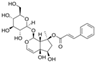 | Inhibits IL-1β-induced anti-inflammatory effects, decreasing the expression of IL-6 and MMP-13 via the suppression of c-Fos/AP-1 activity. | Anti-inflammatory effect. | [263] |
| Stimulates osteoblast dif-ferentiation and inhibits osteoclast differentiation. | Bone protection. | [264] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Lozano, M.-L.; Cesaro, A.; Mazor, M.; Esteve, E.; Berteina-Raboin, S.; Best, T.M.; Lespessailles, E.; Toumi, H. Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants 2021, 10, 265. https://doi.org/10.3390/antiox10020265
Pérez-Lozano M-L, Cesaro A, Mazor M, Esteve E, Berteina-Raboin S, Best TM, Lespessailles E, Toumi H. Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants. 2021; 10(2):265. https://doi.org/10.3390/antiox10020265
Chicago/Turabian StylePérez-Lozano, Maria-Luisa, Annabelle Cesaro, Marija Mazor, Eric Esteve, Sabine Berteina-Raboin, Thomas M. Best, Eric Lespessailles, and Hechmi Toumi. 2021. "Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis" Antioxidants 10, no. 2: 265. https://doi.org/10.3390/antiox10020265
APA StylePérez-Lozano, M.-L., Cesaro, A., Mazor, M., Esteve, E., Berteina-Raboin, S., Best, T. M., Lespessailles, E., & Toumi, H. (2021). Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants, 10(2), 265. https://doi.org/10.3390/antiox10020265









