Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Availability
2.2. NOX4 Expression by Cancer Stages
2.3. Correlation of NOXs with Genetic Programs of Cancer Progression and Macrophage Markers
2.4. Survival Analysis
3. Results
3.1. Data Retrieval and Dataset Composition
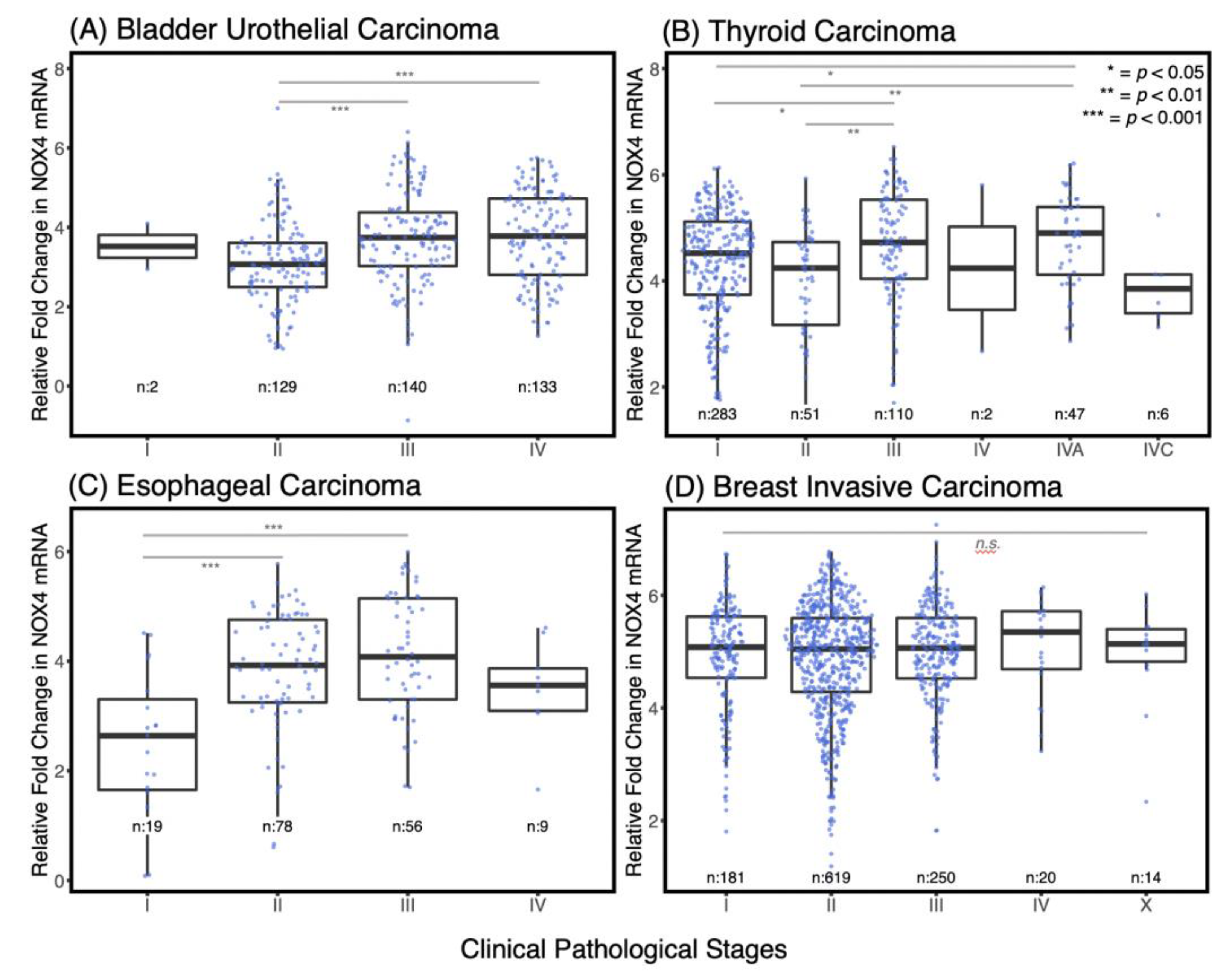
3.2. NOX4 Expression Is Increased in the Advanced Stages of Several Cancers
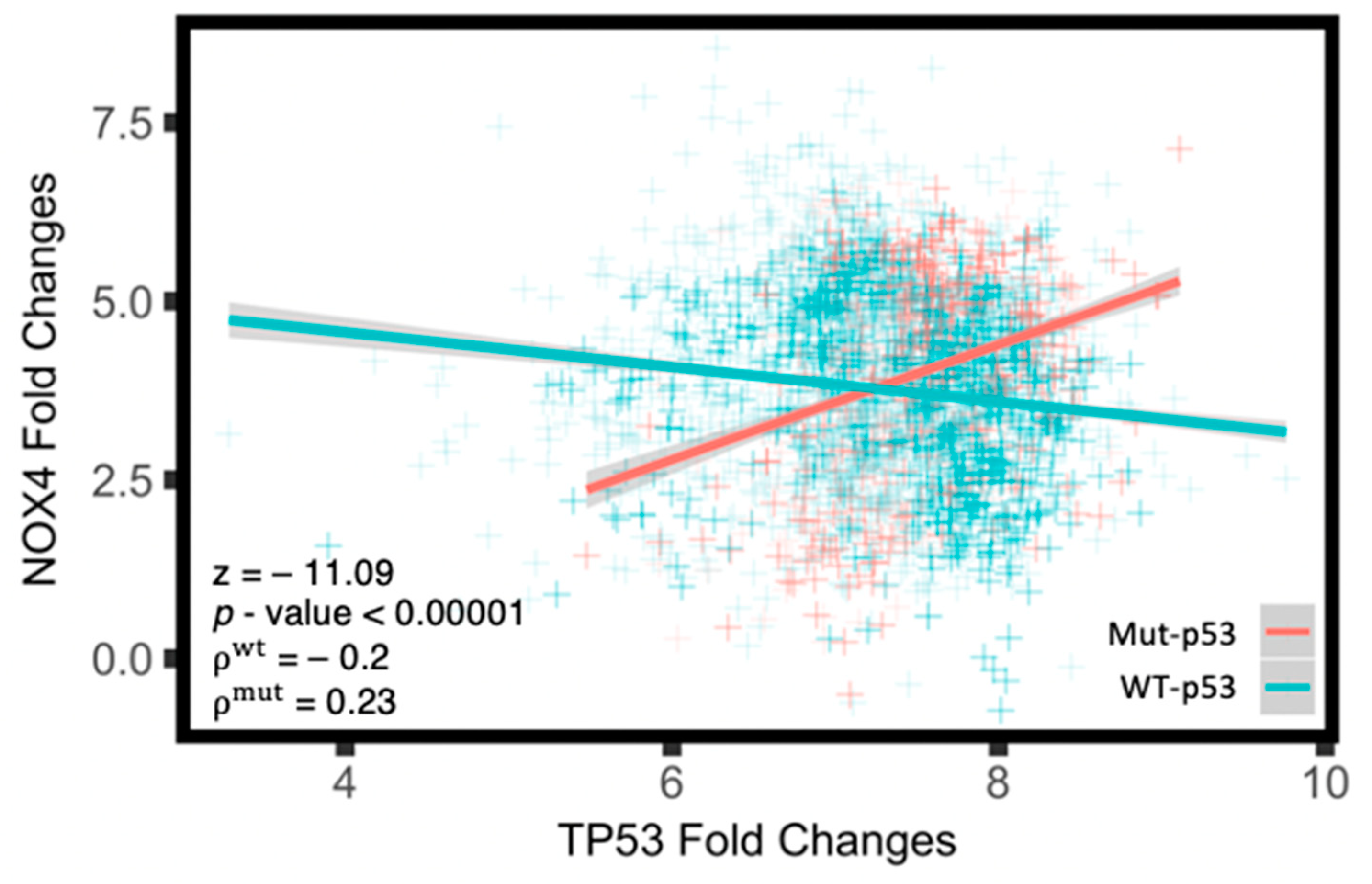
3.3. Mut-p53 mRNA Positively Correlates with NOX4 mRNA
3.4. NOX4 Shows Strong Positive Correlations with Transcriptional Programs of Cancer Progression Including EMT, Cell Migration, Cell Adhesion, and Angiogenesis
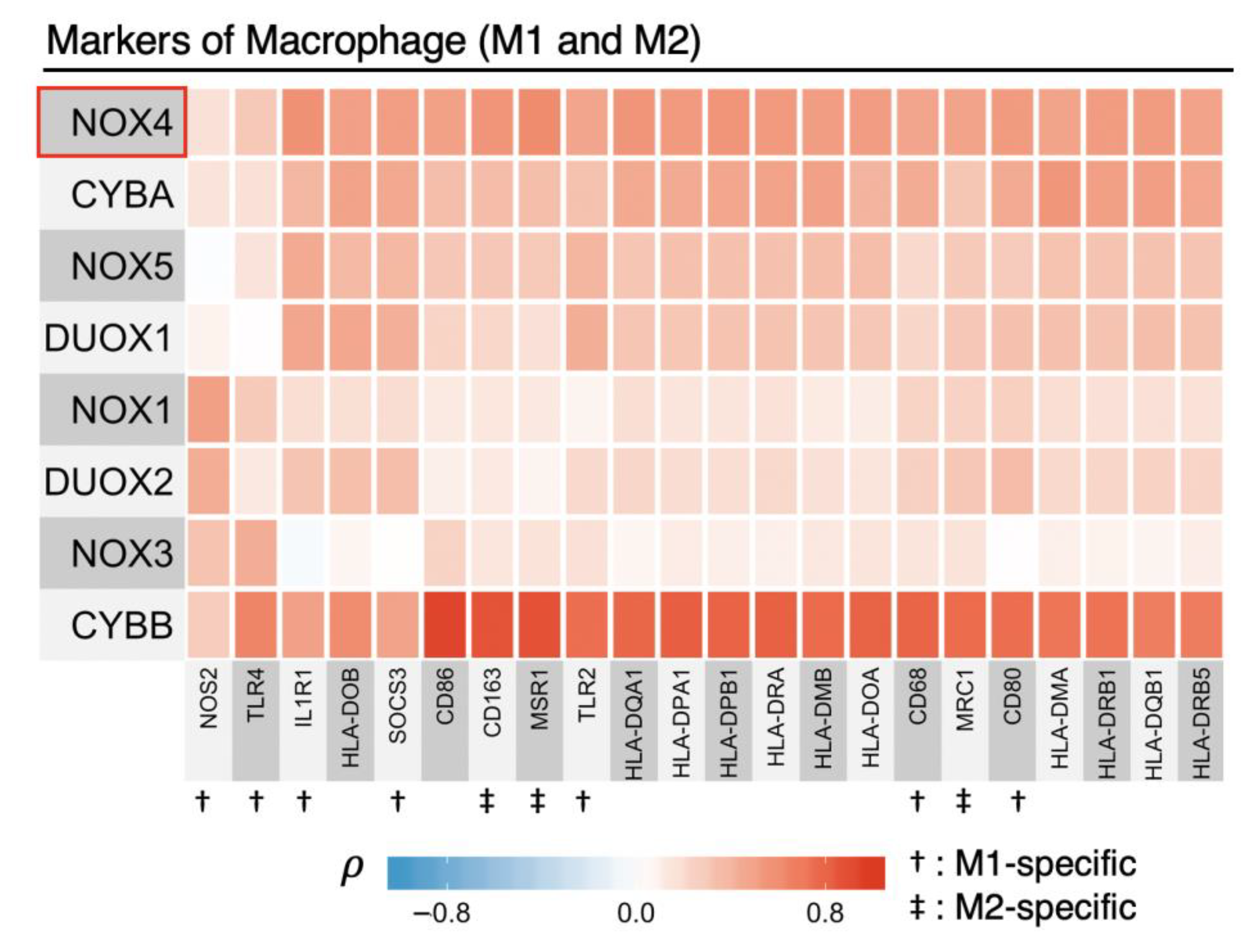
3.5. NOX2 (CYBB) Is Tightly Linked to Other Macrophage Markers
3.6. TP53 Mutations Alter NOX4 mRNA Correlation with EMT Gene Expression
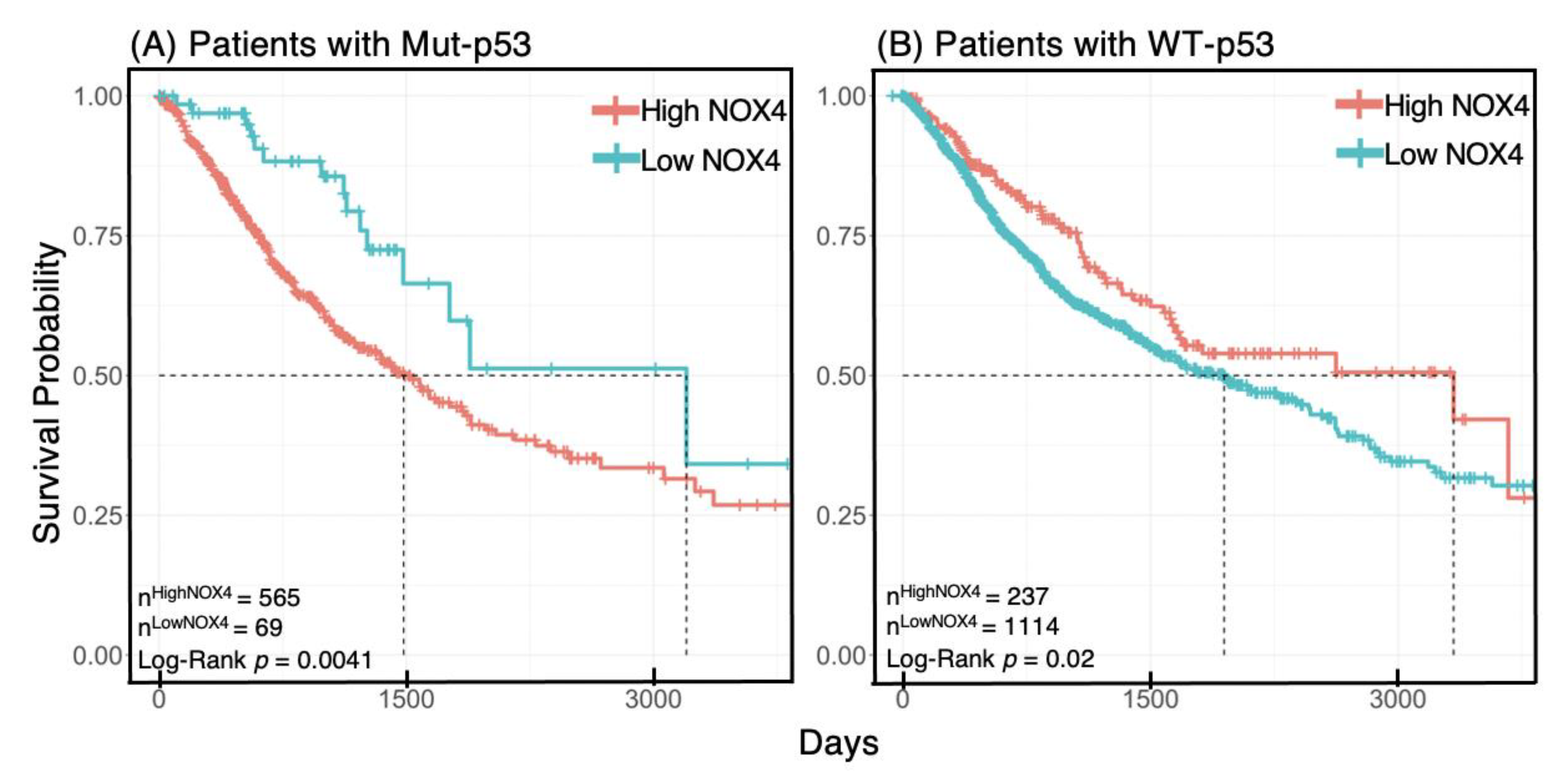
3.7. NOX4 Expression Affects Survival Outcome Depending on p53 Status
3.8. Mut-p53 and WT-p53 Divergently Affect NOX4 Correlations with Cell Proliferation and Apoptosis
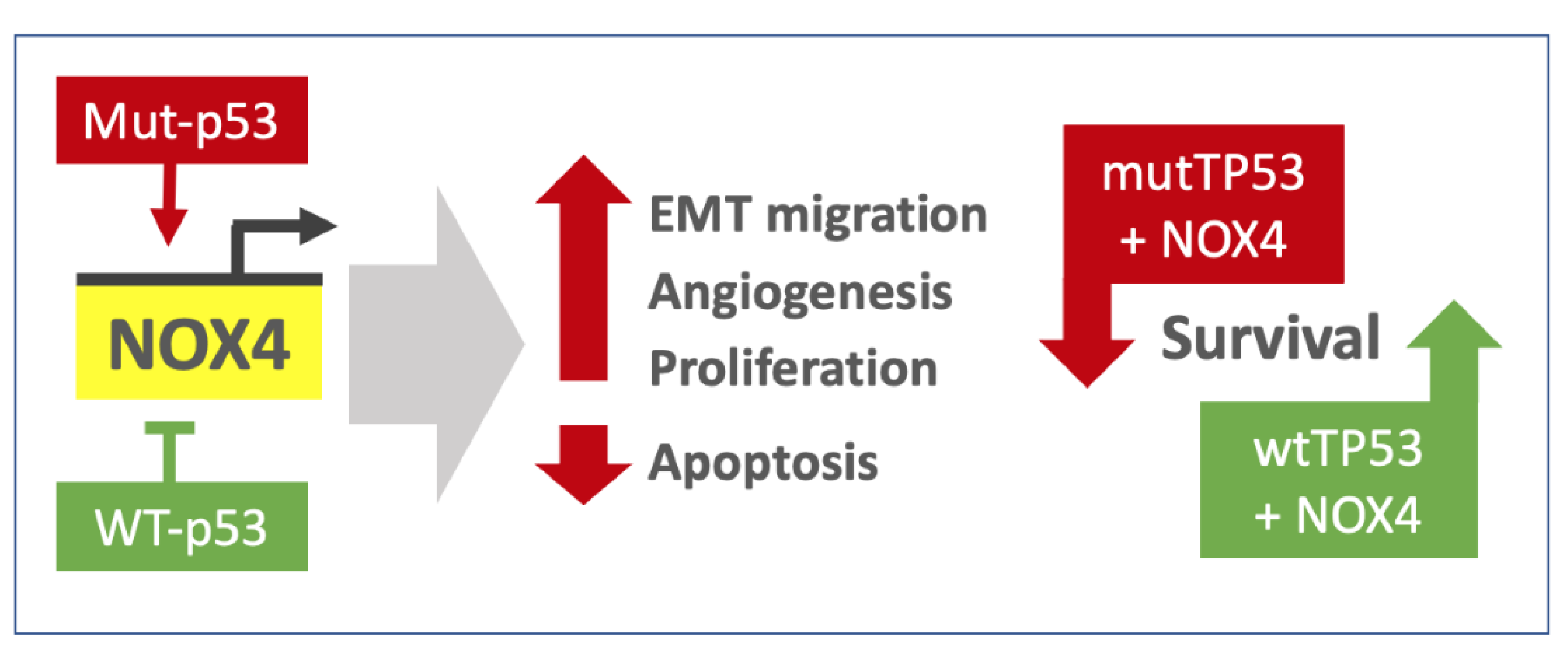
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, K.; O’Neill, A.; Prencipe, M.; Bugler, J.; Murphy, L.; Fabre, A.; Puhr, M.; Culig, Z.; Murphy, K.; Watson, R.W. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel- resistant prostate cancer. Mol. Oncol. 2017, 11, 251–265. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Leto, T.L. The NOX Family of NADPH Oxidases: Host Defense and Beyond. J. Biol. Chem. 2004, 279, 51715–51718. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of NADPH oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 24–313. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Kumar, P.; Griendling, K.K.; Schmidt, H.H.H.W.; Busse, R.; Brandes, R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004, 279, 45935–45941. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Longo-Guess, C.M.; Bergstrom, D.E.; Nauseef, W.M.; Jones, S.M.; Banfi, B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J. Clin. Investig. 2008, 118, 1176–1185. [Google Scholar] [CrossRef]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Sorescu, D. NADPH Oxidase 4 Mediates Transforming Growth Factor-beta1-induced differentiation of cardiac fibroblasts in myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Casterline, B.W.; Rada, B.; Korzeniowska, A.; Leto, T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012, 53, 1489–1499. [Google Scholar] [CrossRef]
- Hecker, L.; Vittal, R.; Jones, T.; Jagidar, R.; Luckhardt, T.R.; Horowitz, J.C.; Subramaniam, P.; Martinez, F.J.; Thannickal, V.J. NADPH Oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef]
- Hiraga, R.; Kato, M.; Miyagawa, S.; Kamata, T. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013, 33, 4431–4438. [Google Scholar]
- Jafari, N.; Kim, H.; Park, R.; Li, L.; Jang, M.; Morris, A.J.; Park, J.; Huang, C. CRISPR-Cas9 Mediated NOX4 Knockout Inhibits Cell Proliferation and Invasion in HeLa Cells. PLoS ONE 2017, 12, e0170327. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.; Hu, X. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem. Pharmacol. 2017, 86, 253–266. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Casterline, B.W.; Burke, D.J.; Leto, T.L. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Br. J. Cancer 2014, 110, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, H.E.; Ma, W.F.; Korzeniowska, A.; Park, J.J.; Bhagwat, M.A.; Leto, T.L. Histone modifications affect differential regulation of TGFβ- induced NADPH oxidase 4 (NOX4) by wild-type and mutant p53. Oncotarget 2017, 8, 44379–44397. [Google Scholar] [CrossRef] [PubMed]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Dürr, P. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, L.; Fu, S.; Lin, W.; Gao, Y. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget 2017, 5, 33586–33600. [Google Scholar] [CrossRef]
- Helfinger, V.; Henke, N.; Harenkamp, S.; Walter, S.; Epah, J.; Penski, C.; Mittelbronn, M.; Schröder, K. The NADPH Oxidase Nox4 mediates tumour angiogenesis. Acta Physiol. 2016, 216, 435–446. [Google Scholar] [CrossRef]
- Tang, C.-T.; Lin, X.-L.; Wu, S.; Liang, Q.; Yang, L.; Gao, Y.-J.; Ge, Z.Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell. Signal. 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Furuta, F.; Mitsushita, J.; Shang, W.H.; Ito, M.; Yokoo, Y.; Yamaura, M.; Ishizone, J.; Nakayama, J.; Konagai, A.; et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 2006, 25, 3699–3707. [Google Scholar] [CrossRef]
- Tanaka, M.; Miura, Y.; Numanami, H.; Sivasundaram, K.; Ota, A.; Konishi, H.; Hosokawa, Y.; Hanyuda, M. Inhibition of NADPH oxidase 4 induces apoptosis in malignant mesothelioma: Role of reactive oxygen species. Oncol. Rep. 2015, 34, 1726–1732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorrson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 17, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Creighton, C.J.; Davis, C.; Donehower, L.; Drummond, J.; Wheeler, D.; Ally, A.; Balasundaram, M.; Briol, I.; Butterfield, S.N.; et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Hu, Z.; Yau, C.; Ahmed, A.A. A pan-cancer genome-wide analysis reveals tumour dependencies by induction of nonsense-mediated decay. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samur, M.K. RTCGAToolbox: A New Tool for Exporting TCGA Firehose Data. PLoS ONE 2014, 9, e106397. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.; Dogrusoz, U.; Dresdner, G. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, 1–20. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.-S.; Zhou, B.-H.; Li, C.-L.; Zhang, F.; Wang, X.-F.; Zhang, G.; Bu, X.-Z.; Cai, S.-H.; Du, J. Epithelial–Mesenchymal Transition (EMT) Induced by TNF-α Requires AKT/GSK-3β-Mediated Stabilization of Snail in Colorectal Cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef] [PubMed]
- Röszer, T.; Corporation, H.P. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2017, 25, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M.; Przemyslaw, B. Survminer: Drawing Survival Curves using “ggplot2”. Available online: http://www.sthda.com/english/rpkgs/survminer/ (accessed on 7 January 2021).
- Chen, C.; Li, L.; Zhou, H.; Min, W. The Role of NOX4 and TRX2 in Angiogenesis and Their Potential Cross Talk. Antioxidants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-Q.Q.; Ying, H.; Tian, T.; Ling, J.; Fu, J.; Lu, Y.; Wu, M.; Yang, H.; Achreja, A.; Chen, G.; et al. Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma. Nat. Commun. 2017, 8, 14437. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by Nox/Duox Family NADPH oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, H.; Song, G.; Liao, X.; Xian, Y.; Li, W. Regulation of epithelial-mesenchymal transition by tumor-associated macrophages in cancer. Am. J. Transl. Res. 2015, 7, 1699–1711. [Google Scholar]
- Meziani, L.; de Thore, M.G.; Hamon, P.; Bockel, S.; Louzada, R.A.; Clemenson, C.; Corre, R.; Liu, W.; Dupuy, C.; Mondini, M.; et al. Dual oxidase 1 limits the IFNγ-associated antitumor effect of macrophages. J. Immunother. Cancer 2020, 8, e000622. [Google Scholar] [CrossRef]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef]
- Helfinger, V.; Palfi, K.; Weigert, A.; Schröder, K. The NADPH Oxidase Nox4 Controls Macrophage Polarization in an NFκB-Dependent Manner. Ox. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Helfinger, V.; von Gall, F.F.; Henke, N.; Kunze, M.M.; Schmid, T.; Heidler, J.; Ilka, W.; Radeka, H.H.; Marschall, V.; Anderson, K.; et al. Hydrogen Peroxide Formation by NOX4 Limits Malignant Transformation. bioRxiv 2017. [Google Scholar] [CrossRef]
- Hanley, C.J.; Mellone, M.; Ford, K.; Thirdborough, S.M.; Mellows, T.; Smith, D.M.; Harden, E.; Szyndralewiez, C.; Bullock, M. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J. Natl. Cancer Inst. 2018, 110, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Brunner, E.; Weber, A.; Puhr, M.; Schäfer, G.; Szyndralewiez, C.; Klocker, H. Inhibition of Nox4-dependent ROS signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int. J. Cancer 2018, 143, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.; Hanley, C.J.; Mellone, M.; Szyndralewiez, C.; Heitz, F.; Wiesel, P.; Wood, O.; Machado, M.; Lopez, M.A.; Ganesan, A.P.; et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020, 80, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
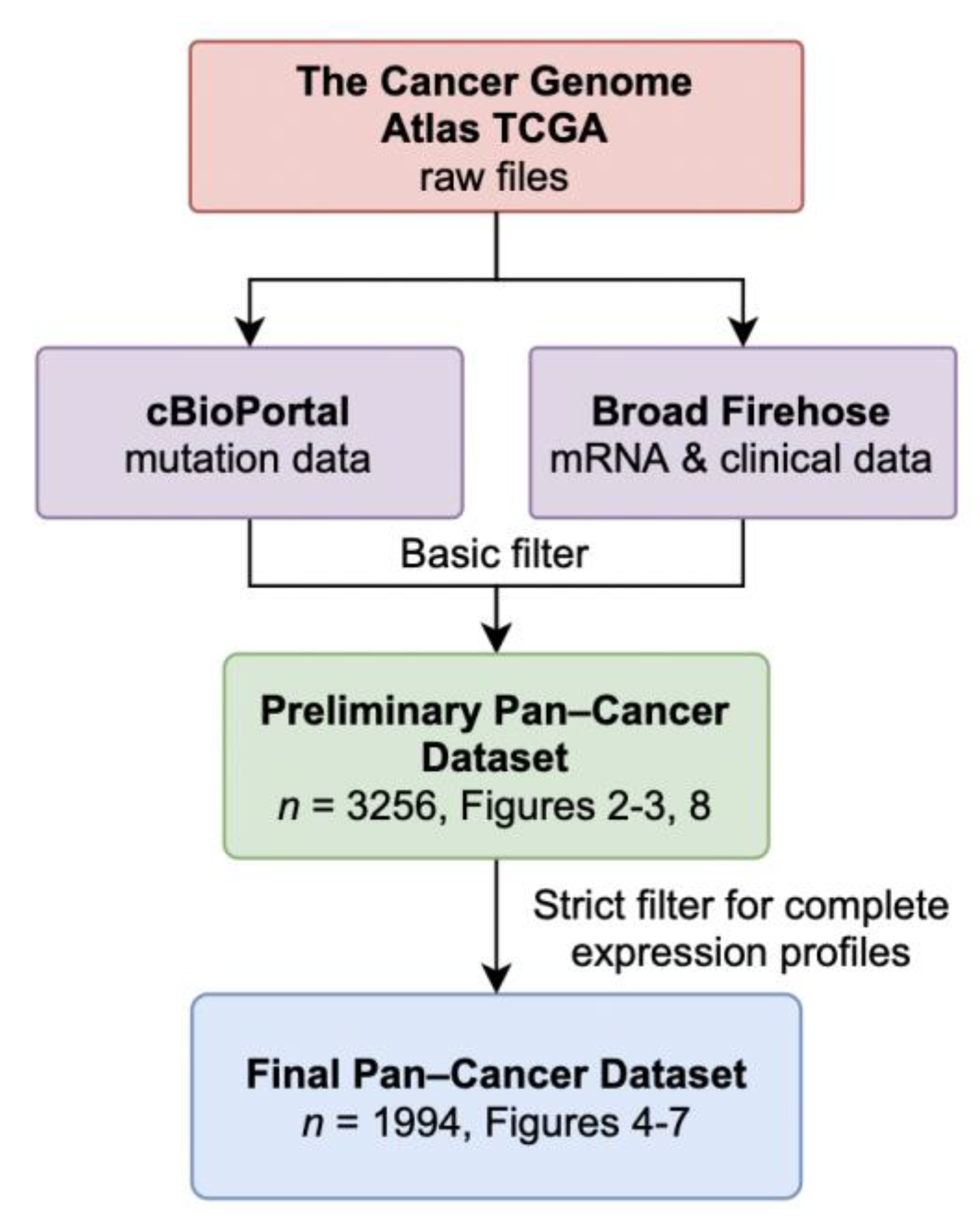



| Study | Abbrv. | Prelim. n | Final n |
|---|---|---|---|
| Bladder Urothelial Carcinoma | BLC A | 309 | 294 |
| Brain Lower Grade Glioma | LGG | 299 | 247 |
| Breast invasive carcinoma | BRCA | 246 | 172 |
| Cervical squamous cell carcinoma and endocervical adenocarcinoma | CESC | 113 | 113 |
| Colon adenocarcinoma | COAD | 562 | 39 |
| Esophageal carcinoma | ESAD | 48 | 50 |
| Glioblastoma multiforme | GBM | 43 | 27 |
| Head and Neck squamous cell carcinoma | HNSC | 102 | 69 |
| Kidney renal clear cell carcinoma | KIRC | 86 | 86 |
| Kidney renal papillary cell carcinoma | KIRP | 10 | 10 |
| Liver hepatocellular carcinoma | LIHC | 29 | 29 |
| Lung adenocarcinoma | LUAD | 171 | 153 |
| Lung squamous cell carcinoma | LUSC | 173 | 137 |
| Mesothelioma | MESO | 87 | 87 |
| Ovarian serous cystadenocarcinoma | OV | 194 | 158 |
| Pancreatic adenocarcinoma | PAAD | 59 | 59 |
| Prostate adenocarcinoma | PRAD | 21 | 18 |
| Rectum adenocarcinoma | READ | 192 | 17 |
| Sarcoma | SARC | 27 | 27 |
| Stomach adenocarcinoma | STAD | 86 | 54 |
| Thyroid carcinoma | THCA | 212 | 106 |
| Uterine Carcinosarcoma | UCS | 17 | 17 |
| Uterine Corpus Endometrial Carcinoma | UCEC | 170 | 25 |
| Total | 3256 | 1994 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.F.; Boudreau, H.E.; Leto, T.L. Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants 2021, 10, 235. https://doi.org/10.3390/antiox10020235
Ma WF, Boudreau HE, Leto TL. Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants. 2021; 10(2):235. https://doi.org/10.3390/antiox10020235
Chicago/Turabian StyleMa, Wei Feng, Howard E. Boudreau, and Thomas L. Leto. 2021. "Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome" Antioxidants 10, no. 2: 235. https://doi.org/10.3390/antiox10020235
APA StyleMa, W. F., Boudreau, H. E., & Leto, T. L. (2021). Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants, 10(2), 235. https://doi.org/10.3390/antiox10020235






