Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
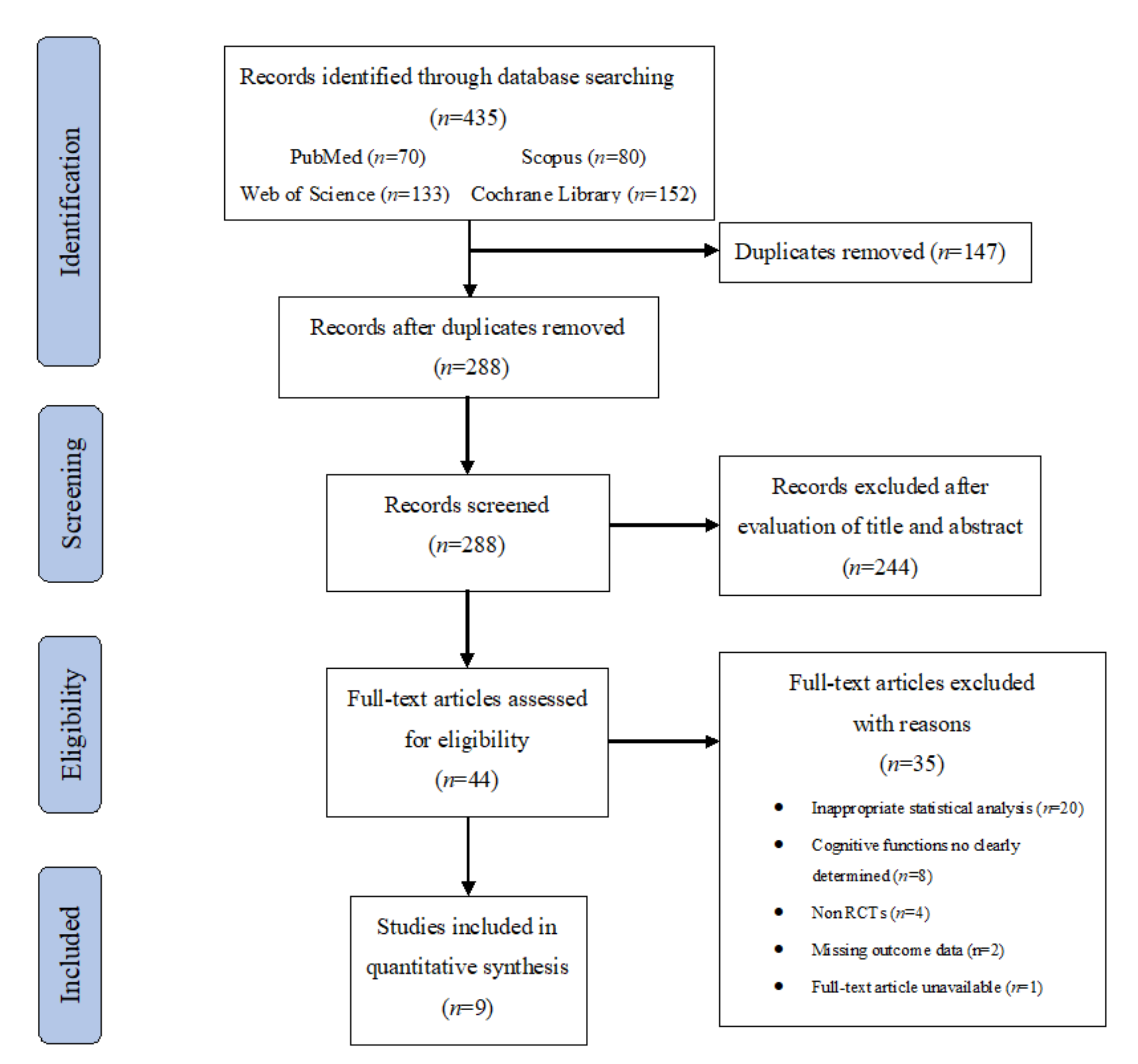
2.3. Selection Process and Data Extraction
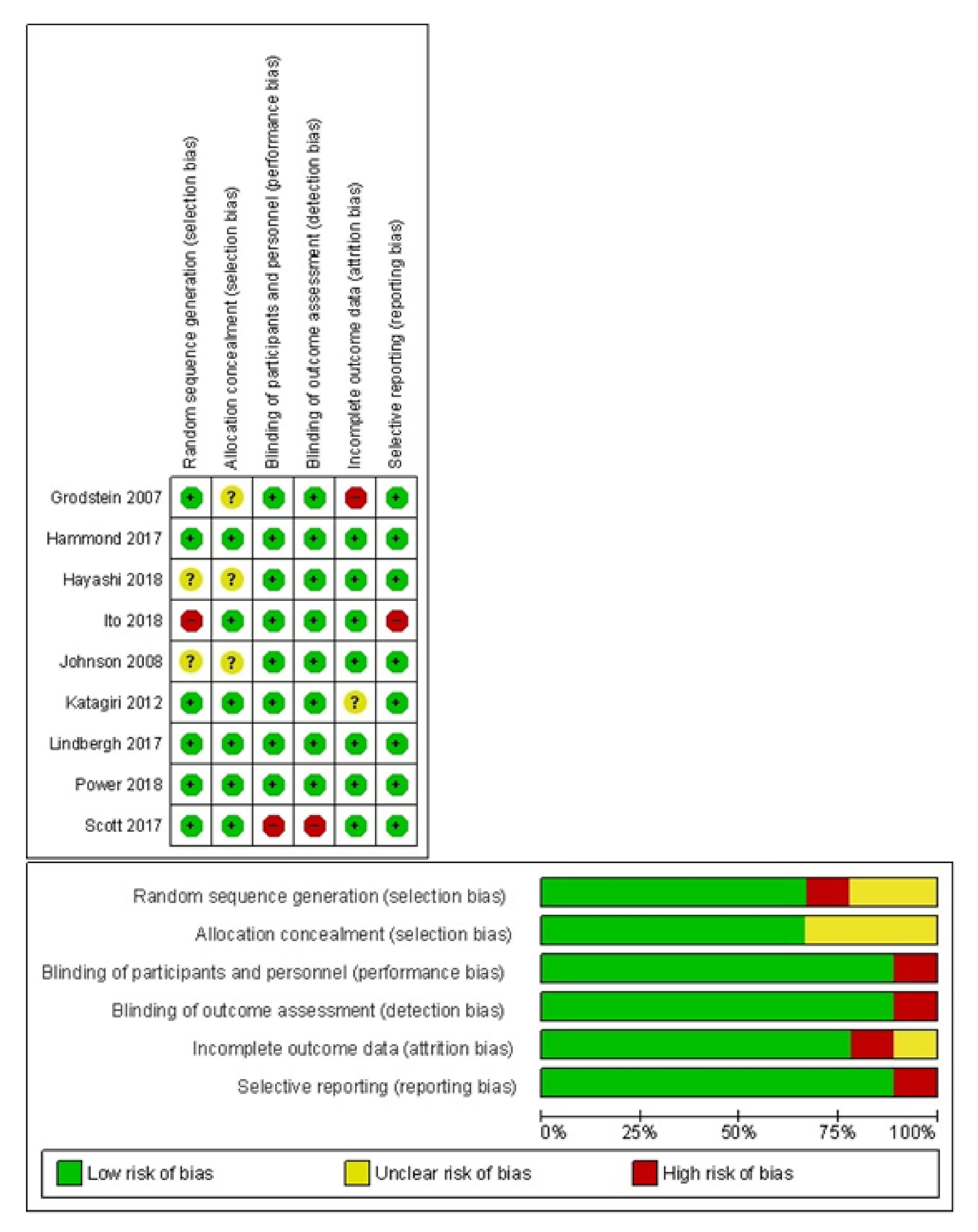
2.4. Quality Assessment
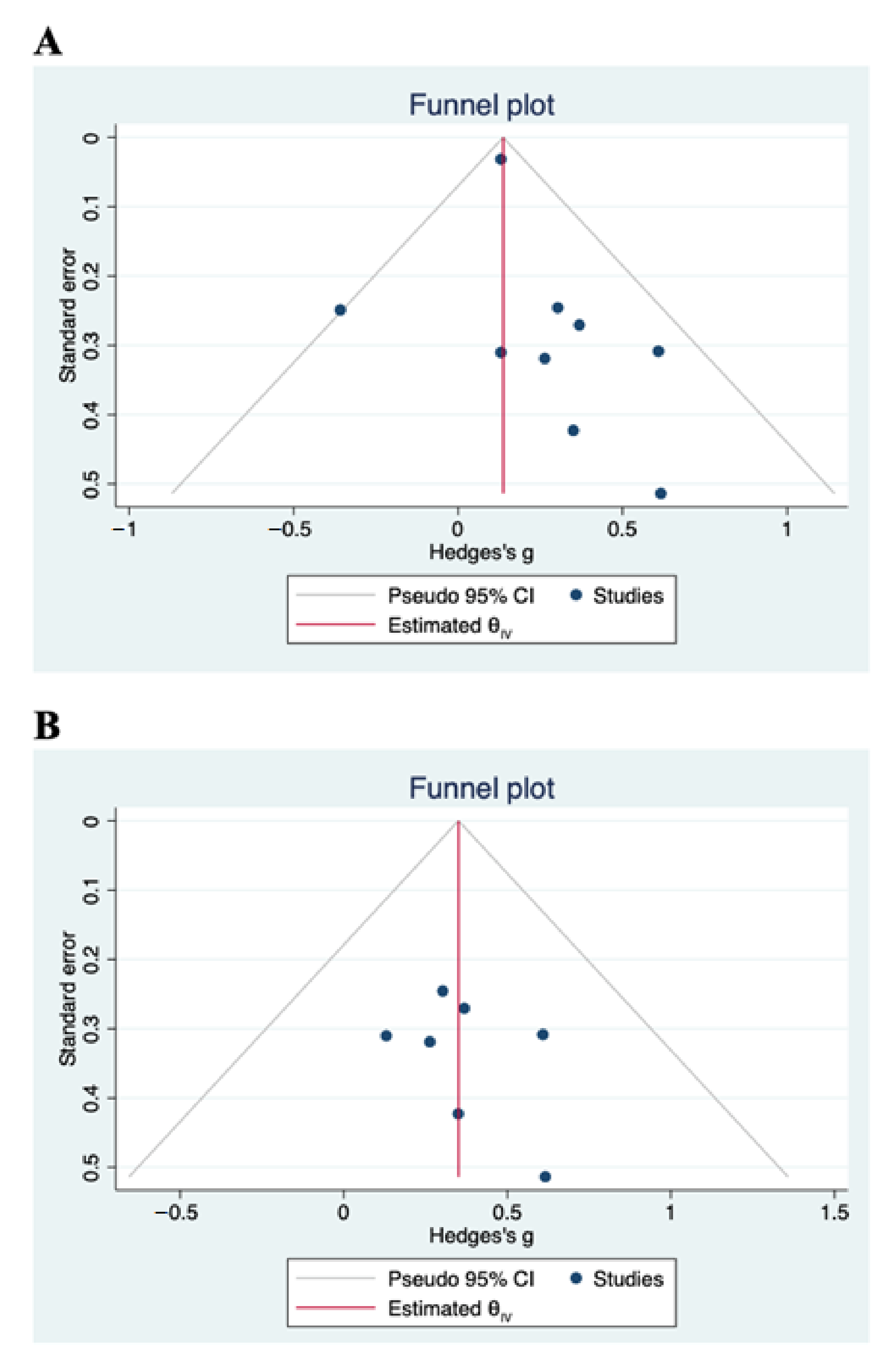
2.5. Statistical Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment
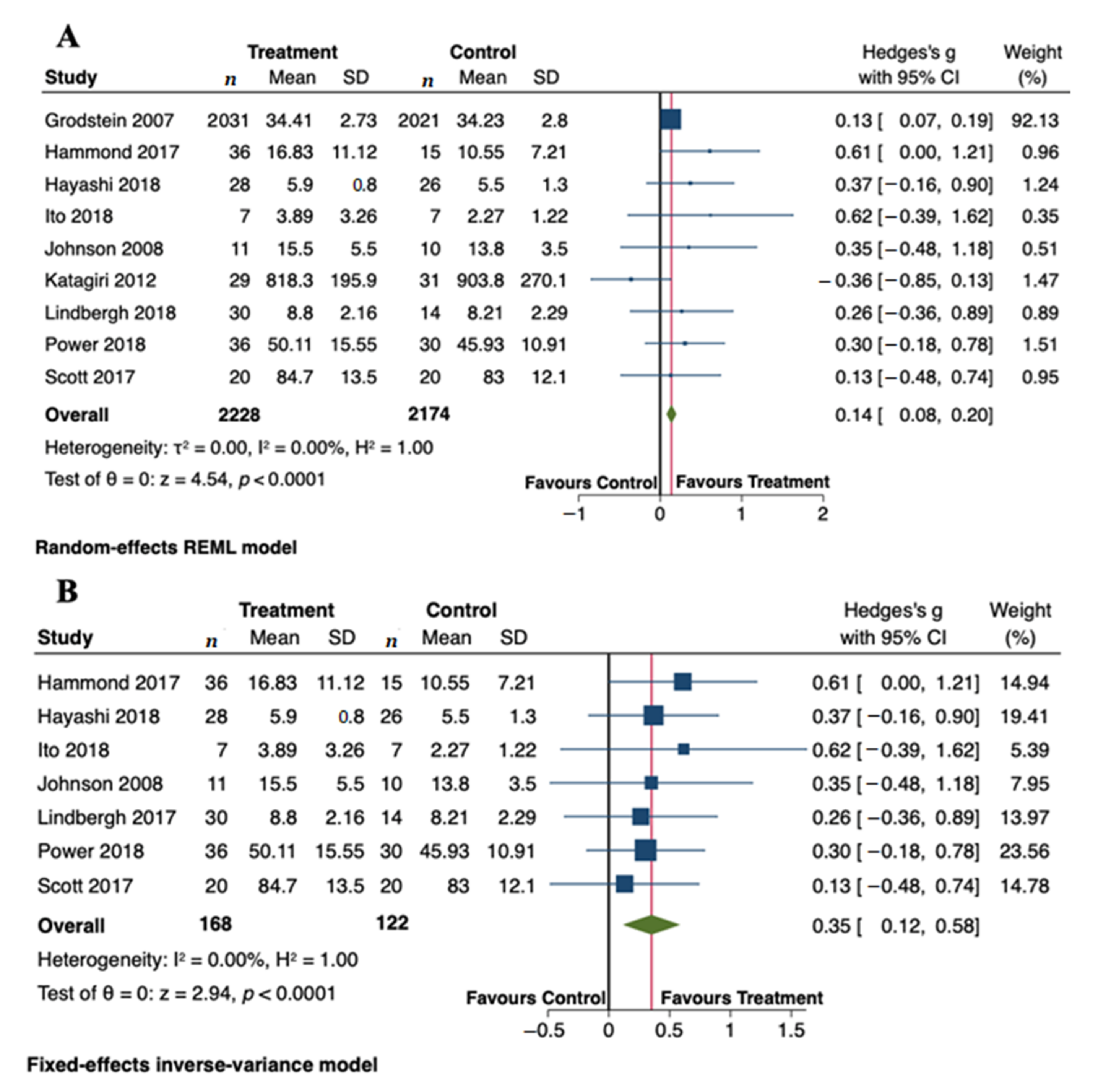
3.4. Meta-Analysis of the Effect of Carotenoids on Cognitive Outcomes
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.; Van Boxtel, M.; Bosma, H.; Bosmans, E.; Delanghe, J.; De Bruijn, C.; Wauters, A.; Maes, M.; Jolles, J.; Steinbusch, H.; et al. Inflammation markers in relation to cognition in a healthy aging population. J. Neuroimmunol. 2003, 134, 142–150. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.C.; Stout, J.C.; Bonner, J.; Hesselink, J.R. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 2001, 22, 581–594. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Ismail, Z.; Livingston, G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against.” Alzheimer’s Res. Ther. 2020, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chalfont, G.; Milligan, C.; Simpson, J. A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia. Dement. 2018, 19, 1086–1130. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Panza, F.; Torres, F.; Mastroianni, F.; Del Parigi, A.; Venezia, A.; Capurso, A. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology 1999, 52, 1563. [Google Scholar] [CrossRef]
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Barberger-Gateau, P.; Ritchie, K. Olive oil and cognition: Results from the three-city study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Ashley, S.; Bradburn, S.; Murgatroyd, C. A meta-analysis of peripheral tocopherol levels in age-related cognitive decline and Alzheimer’s disease. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mangels, A.R.; Holden, J.M.; Beecher, G.R.; Forman, M.R.; Lanza, E. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J. Am. Diet. Assoc. 1993, 93, 284–296. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2016, 174, 1290–1324. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Scott, T.M.; Schalch, W.; Wittwer, J.; Hausman, D.B.; Davey, A.; Johnson, M.A.; Green, R.C.; Gearing, M.; et al. Serum carotenoids as a biomarker for carotenoid concentrations in the brain. FASEB J. 2011, 25, 344.2. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Joffe, S.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef]
- Tanprasertsuk, M.J.; Mohn, P.E.S.; Matthan, P.N.R.; Lichtenstein, D.A.H.; Barger, P.K.; Vishwanathan, P.R.; Johnson, M.A.; Poon, P.L.W.; Johnson, E.J. Serum carotenoids, tocopherols, total n-3 polyunsaturated fatty acids, and n-6/n-3 polyunsaturated fatty acid ratio reflect brain concentrations in a cohort of centenarians. J. Gerontol. Ser. A 2019, 74, 306–314. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, N.M.; Saedisomeolia, A.; Abdolahi, M.; Shayeganrad, A.; Sangsari, G.T.; Rad, B.H.; Muench, G. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer’s disease: A review of current evidence. J. Mol. Neurosci. 2017, 61, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a putative geroprotector: Molecular basis and focus on brain aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W. Carotenoid orientation: Role in membrane stabilization. In Carotenoids in Health and Disease, 1st ed.; Norman, I.K., Susan, T.M., Helmut, S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 170–183. [Google Scholar]
- Stahl, W.; Sies, H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors 2001, 15, 95–98. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions, version 6.1; [Updated September 2020]; The Cochrane Collaboration: 2020. Available online: www.training.cochrane.org/handbook (accessed on 13 June 2020).
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Kang, J.H.; Glynn, R.J.; Cook, N.R.; Gaziano, J.M. A randomized trial of beta carotene supplementation and cognitive function in men: The physicians’ health study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R.J.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: A randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J. Alzheimer’s Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.-Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.; Terry, U.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and zeaxanthin influence brain function in older adults: A randomized controlled trial. J. Int. Neuropsychol. Soc. 2017, 24, 77–90. [Google Scholar] [CrossRef]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental retinal carotenoids enhance memory in healthy individuals with low levels of macular pigment in a randomized, double-blind, placebo-controlled clinical trial. J. Alzheimer’s Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef]
- Scott, T.; Rasmussen, H.M.; Chen, C.-Y.O.; Johnson, E.J. Avocado consumption increases macular pigment density in older adults: A randomized, controlled trial. Nutrients 2017, 9, 919. [Google Scholar] [CrossRef]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. J. Gerontol. Ser. A 2007, 62, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2020, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Kim, S.H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef]
- Zuniga, K.E.; Moran, N.E. Low serum carotenoids are associated with self-reported cognitive dysfunction and inflammatory markers in breast cancer survivors. Nutrients 2018, 10, 1111. [Google Scholar] [CrossRef]
- Feeney, J.; Finucane, C.; Savva, G.M.; Cronin, H.; Beatty, S.; Nolan, J.M.; Kenny, R.A. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol. Aging 2013, 34, 2449–2456. [Google Scholar] [CrossRef]
- Crichton, G.E.; Bryan, J.; Murphy, K.J. Dietary antioxidants, cognitive function and dementia—A systematic review. Plant Foods Hum. Nutr. 2013, 68, 279–292. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Walk, A.M.; Khan, N.A.; Barnett, S.; Raine, L.B.; Kramer, A.F.; Cohen, N.J.; Moulton, C.J.; Renzi-Hammond, L.M.; Hammond, B.R.; Hillman, C.H. From neuro-pigments to neural efficiency: The relationship between retinal carotenoids and behavioral and neuroelectric indices of cognitive control in childhood. Int. J. Psychophysiol. 2017, 118, 1–8. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2013, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Koh, H.-K.; Kim, D.-S. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
| Reference | Location | Population Characteristics and Study Design | Intervention | Outcome of Interest | Cognitive Test Used | Results |
|---|---|---|---|---|---|---|
| Grodstein et al. (2007) [34] | USA | n = 4052 M (mean age 55.9 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 18 years | β-carotene (50 mg on alternate days) | Global cognition Verbal memory Category fluency | TICS (MMSE) EBMT CFT | Improvement in global cognitive score (p = 0.03), verbal memory (p = 0.007), and TICS score (p = 0.04) |
| Hammond et al. (2017) [35] | USA | n = 51 (21 M and 30 F; mean age 73.7 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 12 months | Lutein + zeaxanthin (12 mg/d; 10 mg of lutein and 2 mg of zeaxanthin) | Verbal memory Visual memory Reasoning ability Executive function Psychomotor speed Complex attention Cognitive flexibility Global cognition | CNS Vital Signs | Improvement in complex attention (p < 0.02), cognitive flexibility (p < 0.04) ,and composite memory in male (p = 0.04) |
| Hayashi et al. (2018) [36] | Japan | n = 54 (25 M and 29 F, age range 45–64 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 2 months | Astaxanthin (8 mg/d) | Immediate and short-term memory Memory-recall ability Cognitive interference inhibition | Word memory test Verbal fluency test Stroop test | Improvement in immediate recall (p < 0.05), recall after 5 min + cued recall (p < 0.05), verbal fluency (p < 0.01), and Stroop test score (p < 0.01) |
| Ito et al. (2018) [37] | Japan | n = 14 (9 M and 5 F; age range 57–78 years) Condition: MCI Design: Randomized, double-blind, placebo-controlled trial Duration: 3 months | Astaxanthin + sesamin (16 mg/d; 6 mg of astaxanthin) | Composite memory Verbal memory Visual memory Processing speed Psychomotor speed Executive function Reaction attention Complex attention Simple attention Cognitive flexibility Motor speed | CNS Vital Signs ADAS-Cog | Improvement in psychomotor speed (p < 0.05) and processing speed (p < 0.05) |
| Johnson et al. (2008) [38] | USA | n = 21 F (mean age 67.3 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 4 months | Lutein (12 mg/d) | Memory Processing speed Attention | Verbal fluency test Digit span forward Digit span backward MIR apartment test Shopping list test Pattern recognition test | Improvement in verbal fluency scores (p < 0.03) |
| Katagiri et al. (2012) [39] | Japan | n = 60 (M & F; mean age 51.5 years) Condition: Healthy subjects with age-related forgetfulness Design: Randomized, double-blind, placebo-controlled trial Duration: 3 months | Astaxanthin (high dosage group 12 mg/d) | Response time Accuracy Spatial working memory | CogHealth battery GMLT | Improvement in working memory (p = 0.044), delayed recall score (p = 0.028), and improvement of GMLT total errors (p < 0.01) |
| Lindbergh et al. (2018) [40] | USA | n = 44 (M 18 and F 26; mean age 72 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 12 months | Lutein + zeaxanthin (12 mg/d; 10 mg of lutein and 2 mg of zeaxanthin) | Verbal learning | fMRI-adapted learning Word recall tests | No effect in word recall score (p > 0.05). Improvement in verbal learning (p < 0.05) |
| Power et al. (2018) [41] | Ireland | n = 66 (M & F; mean age 46.4 years) Condition: Healthy subjects Design: Randomized, double-blind, placebo-controlled trial Duration: 12 months | Carotenoids (22 mg/d; 10 mg of lutein, 10 mg of meso-zeaxanthin, and 2 mg of zeaxanthin) | Phonemic fluency | FAS test | Improvement in phonemic fluency (p < 0.001) |
| Scott et al. (2017) [42] | USA | n = 40 (M 15 and F 25, mean age 63.3 years) Condition: Healthy subjects Design: Randomized, placebo-controlled trial Duration: 6 months | Lutein (0.5 mg/d) | Attention Visual memory Executive function Working memory Planning | CANTAB | Improvement in sustained attention (p = 0.033), memory (p = 0.001), and spatial working memory (p = 0.032) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. https://doi.org/10.3390/antiox10020223
Davinelli S, Ali S, Solfrizzi V, Scapagnini G, Corbi G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants. 2021; 10(2):223. https://doi.org/10.3390/antiox10020223
Chicago/Turabian StyleDavinelli, Sergio, Sawan Ali, Vincenzo Solfrizzi, Giovanni Scapagnini, and Graziamaria Corbi. 2021. "Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials" Antioxidants 10, no. 2: 223. https://doi.org/10.3390/antiox10020223
APA StyleDavinelli, S., Ali, S., Solfrizzi, V., Scapagnini, G., & Corbi, G. (2021). Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants, 10(2), 223. https://doi.org/10.3390/antiox10020223







