The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts Preparation
2.2. Reference Compounds
2.3. Isolation of Human Blood Plasma and Fibrinogen
2.4. Effects on Oxidative/Nitrative Modifications of Fibrinogen
2.5. Effects on Blood Clotting Times and Enzymatic Properties of Thrombin
2.6. Effects on Hyaluronidase Activity
2.7. Statistical Analysis
3. Results and Discussion
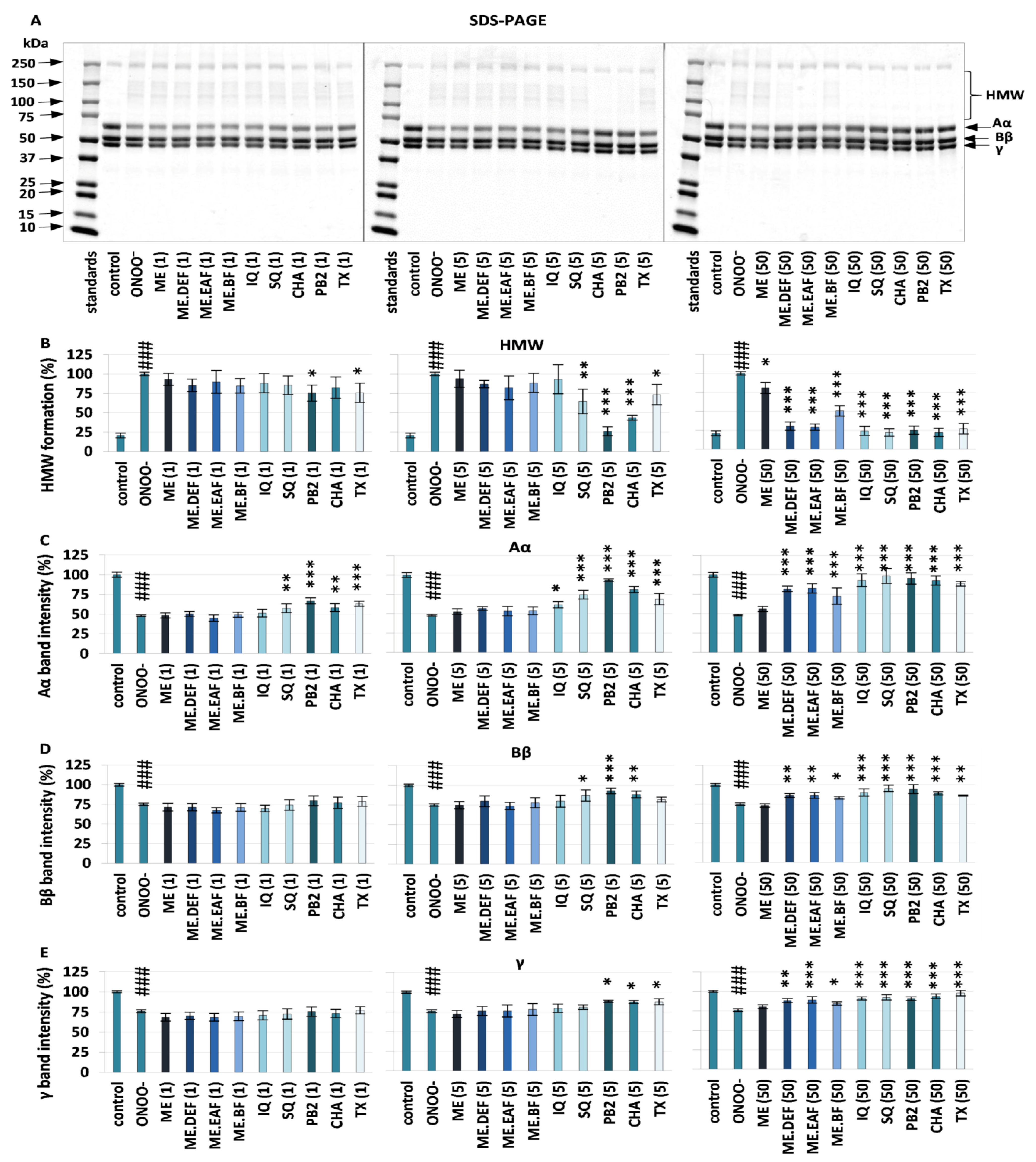
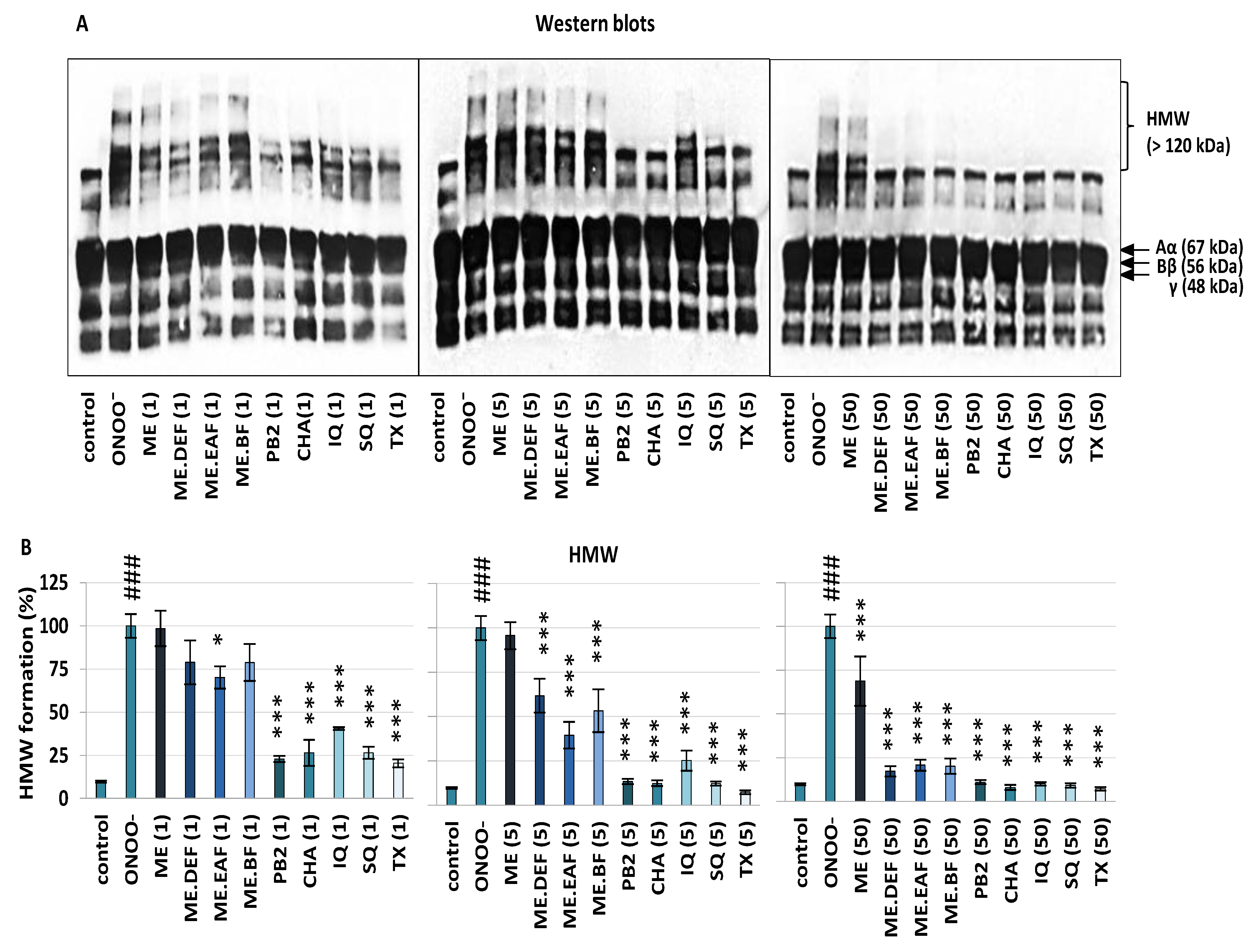
3.1. Effects on Oxidative/Nitrative Modifications of Human Fibrinogen
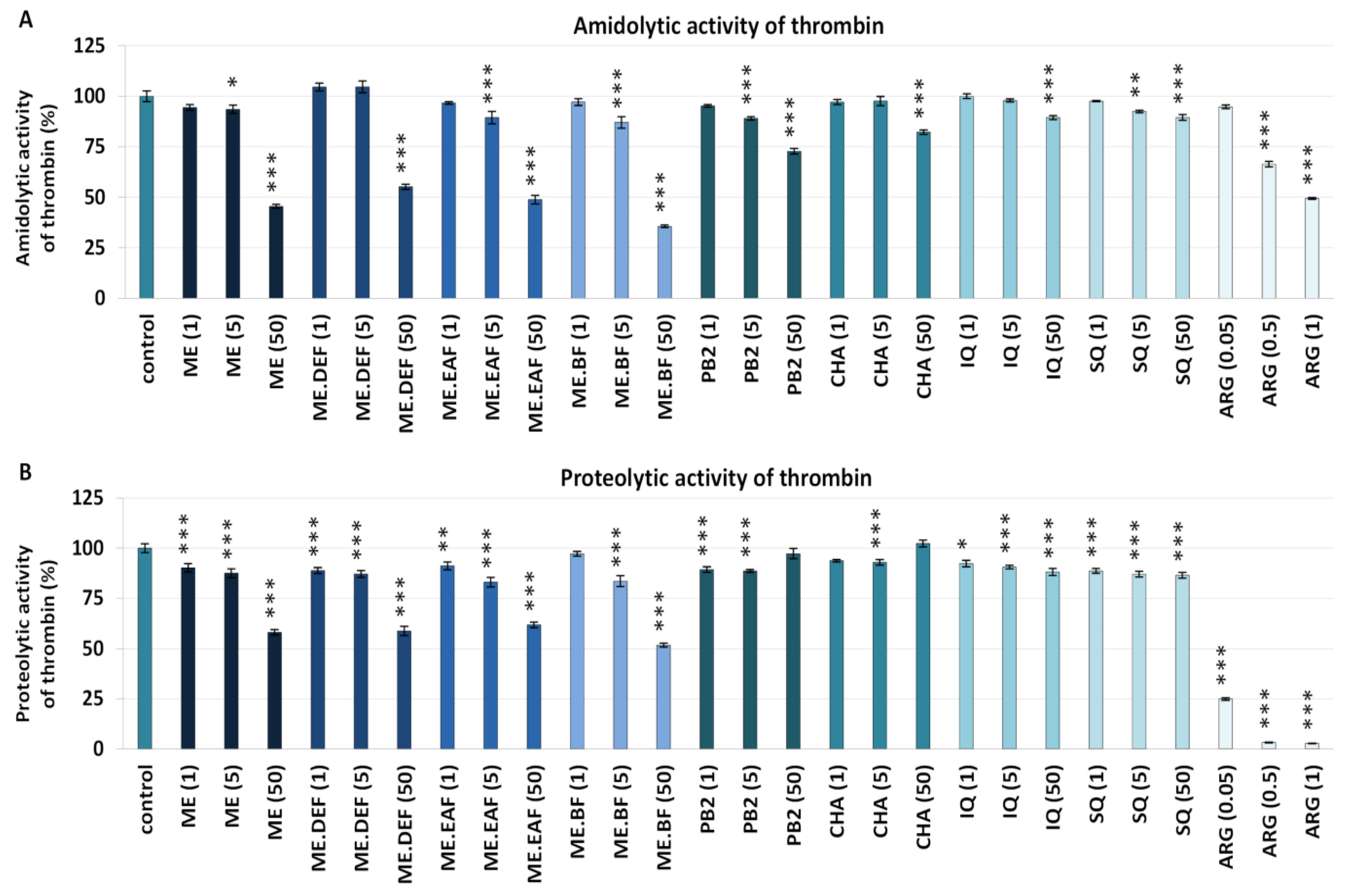
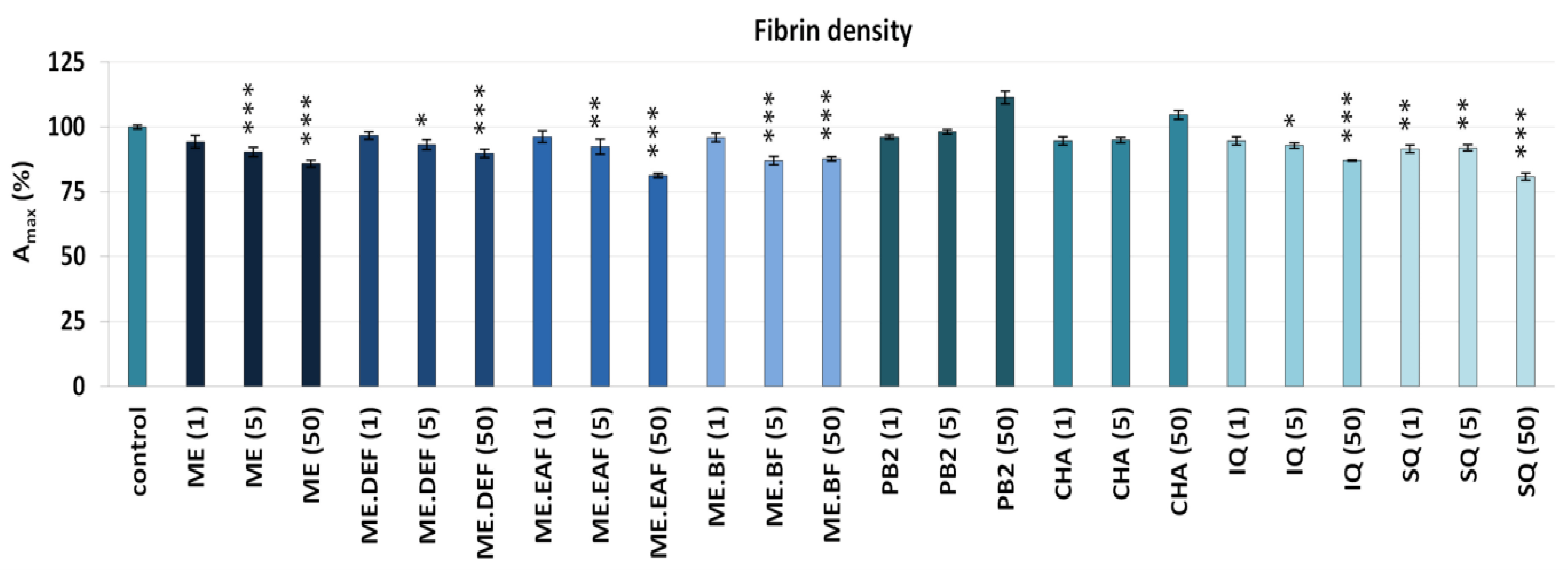
3.2. Effects on Blood Clotting Times, Enzymatic Properties of Thrombin, and Fibrin Density
3.3. Effects on Hyaluronidase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batsatsashvili, K.; Mehdiyeva, N.P.; Fayvush, G.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Aleksanyan, A.; Alizade, V.M.; et al. Sorbus aucuparia L. Sorbus torminalis (L.) Crantz Rosaceae. In Ethnobotany of the Caucasus; Bussmann, R.W., Ed.; Springer: Cham, Switzerland, 2017; pp. 665–672. ISBN 978-3-319-49411-1. [Google Scholar]
- Facciola, S. Cornucopia II: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1998; ISBN 9780962808722. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; Their History and Applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [Green Version]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry Components Inhibit α-Glucosidase in Vitro: Synergies between Acarbose and Polyphenols from Black Currant and Rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, G.; Zhang, X.; Gao, J.; Zhou, Z.; Fan, J. Polyphenols from Sorbus Aucuparia Ameliorate Insulin Resistance and Metabolic Disorders in Diabetic Mice. Curr. Top. Nutraceutical Res. 2016, 14, 227–233. [Google Scholar]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel Insight into Biological Activity and Phytochemical Composition of Sorbus Aucuparia L. Fruits: Fractionated Extracts as Inhibitors of Protein Glycation and Oxidative/Nitrative Damage of Human Plasma Components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, A.J. Current Understanding of Hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Weber, N.C.; Cohn, D.M.; Hollmann, M.W.; DeVries, J.H.; Hermanides, J.; Preckel, B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J. Clin. Med. 2021, 10, 2419. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary Polyphenols: Structures, Bioavailability and Protective Effects against Atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, Food and Pharma. Current Knowledge and Directions for Future Research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(Ogen) in Human Disease: Both Friend and Foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [Green Version]
- De Vries, J.J.; Snoek, C.J.M.; Rijken, D.C.; De Maat, M.P.M. Effects of Post-Translational Modifications of Fibrinogen on Clot Formation, Clot Structure, and Fibrinolysis: A Systematic Review. Arterioscler. Thromb. Vasc. Biol. 2019, 40, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Ponczek, M.; Sady-Janczak, M.; Pilarski, R.; Bukowska, B. Extracts from Uncaria Tomentosa as Antiplatelet Agents and Thrombin Inhibitors—The in Vitro and in Silico Study. J. Ethnopharmacol. 2021, 267, 113494. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial Cell Control of Thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant Efficacy of Kalanchoe Daigremontiana Bufadienolide-Rich Fraction in Blood Plasma in Vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different Vulnerability of Fibrinogen Subunits to Oxidative/Nitrative Modifications Induced by Peroxynitrite: Functional Consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The Effects of Prunus Spinosa l. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen in Vitro. Antioxidants 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Ngu-Schwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A Practical Method for Preparing Peroxynitrite Solutions of Low Ionic Strength and Free of Hydrogen Peroxide. Free Radic. Biol. Med. 1995, 18, 75–83. [Google Scholar] [CrossRef]
- Khan, J.; Brennan, M.D.; Bradley, N.; Gao, B.; Bruckdorfer, R.; Jackobs, M. 3-Nitrotyrosine in the Proteins of Human Plasma Determined by an ELISA Method. Biochem. J. 1998, 330, 795–801. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Wachowicz, B.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A. Antioxidative Effects of Extracts from Trifolium Species on Blood Platelets Exposed to Oxidative Stress. J. Physiol. Biochem. 2013, 69, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Owczarek, A.; Matczak, M.; Kosno, M.; Szymański, P.; Mikiciuk-Olasik, E.; Kilanowicz, A.; Wesołowski, W.; Olszewska, M.A. Metabolite Profiling of Eastern Teaberry (Gaultheria procumbens L.) Lipophilic Leaf Extracts with Hyaluronidase and Lipoxygenase Inhibitory Activity. Molecules 2017, 22, 412. [Google Scholar] [CrossRef] [Green Version]
- Verkleij, C.J.N.; De Bruijn, R.E.; Meesters, E.W.; Gerdes, V.E.A.; Meijers, J.C.M.; Marx, P.F. The Hemostatic System in Patients with Type 2 Diabetes with and without Cardiovascular Disease. Clin. Appl. Thromb. 2011, 17, E57–E63. [Google Scholar] [CrossRef] [Green Version]
- Sovová, Ž.; Štikarová, J.; Kaufmanová, J.; Májek, P.; Suttnar, J.; Šácha, P.; Malý, M.; Dyr, J.E. Impact of Posttranslational Modifications on Atomistic Structure of Fibrinogen. PLoS ONE 2020, 15, e0227543. [Google Scholar] [CrossRef]
- Štikarová, J.; Kotlín, R.; Riedel, T.; Suttnar, J.; Pimková, K.; Chrastinová, L.; Dyr, J.E. The Effect of Reagents Mimicking Oxidative Stress on Fibrinogen Function. Sci. World J. 2013, 2013, 359621. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Szabo, C. Role of Peroxynitrite in the Pathogenesis of Cardiovascular Complications of Diabetes. Curr. Opin. Pharmacol. 2006, 6, 136–141. [Google Scholar] [CrossRef]
- Vadseth, C.; Souza, J.M.; Thomson, L.; Seagraves, A.; Nagaswami, C.; Scheiner, T.; Torbet, J.; Vilaire, G.; Bennett, J.S.; Murciano, J.-C.; et al. Pro-Thrombotic State Induced by Post-Translational Modification of Fibrinogen by Reactive Nitrogen Species. J. Biol. Chem. 2004, 279, 8820–8826. [Google Scholar] [CrossRef] [Green Version]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Herkert, O.; Djordjevic, T.; Belaiba, R.S.; Görlach, A. Insights into the Redox Control of Blood Coagulation: Role of Vascular NADPH Oxidase-Derived Reactive Oxygen Species in the Thrombogenic Cycle. Antioxid. Redox Signal. 2004, 6, 765–776. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract Are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Manach, C. Bioavailability and Bioefficacy of Polyphenols in Humans. II. Review of 93 Intervention Studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijak, M.; Saluk, J.; Antosik, A.; Ponczek, M.B.; Zbikowska, H.M.; Borowiecka, M.; Nowak, P. Aronia Melanocarpa as a Protector against Nitration of Fibrinogen. Int. J. Biol. Macromol. 2013, 55, 264–268. [Google Scholar] [CrossRef]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Zbikowska, H.M.; Wachowicz, B. Protective Effects of (-)-Epicatechin against Nitrative Modifications of Fibrinogen. Thromb. Res. 2012, 130, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Sohn, H.-Y. Anti-Coagulation and Anti-Platelet Aggregation Activity of the Mature Fruit of Sorbus Commixta. Microbiol. Biotechnol. Lett. 2015, 43, 373–377. [Google Scholar] [CrossRef]
- Marchi, R.; López, M. Thrombin Structural and Functional Determinants as Therapeutic Targets. Int. Blood Res. Rev. 2016, 6, 1–22. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk, J.; Ponczek, M.B.; Nowak, P. Antithrombin Effect of Polyphenol-Rich Extracts from Black Chokeberry and Grape Seeds. Phyther. Res. 2013, 27, 71–76. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Pasiński, B.; Ponczek, M.B.; Moniuszko-Szajwaj, B.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Bufadienolides from Kalanchoe Daigremontiana Modulate the Enzymatic Activity of Plasmin—In Vitro and In Silico Analyses. Int. J. Biol. Macromol. 2018, 120, 1591–1600. [Google Scholar] [CrossRef]
- Hahn, D.; Bae, J.S. Recent Progress in the Discovery of Bioactive Components from Edible Natural Sources with Antithrombotic Activity. J. Med. Food 2019, 22, 109–120. [Google Scholar] [CrossRef]
- Li, Q.Q.; Yang, Y.X.; Qv, J.W.; Hu, G.; Hu, Y.J.; Xia, Z.N.; Yang, F.Q. Investigation of Interactions between Thrombin and Ten Phenolic Compounds by Affinity Capillary Electrophoresis and Molecular Docking. J. Anal. Methods Chem. 2018, 2018, 4707609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yang, Z.; Su, F.; Li, J.; Boadi, E.O.; Chang, Y.-X.; Wang, H. Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation in Vitro. Molecules 2020, 25, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Kim, K.J.; Kim, S. Comparative Effect of Quercetin and Quercetin-3-O-β-D-Glucoside on Fibrin Polymers, Blood Clots, and in Rodent Models. J. Biochem. Mol. Toxicol. 2016, 30, 548–558. [Google Scholar] [CrossRef]
- Sinegre, T.; Milenkovic, D.; Teissandier, D.; Fully, P.; Bourdin, J.; Morand, C.; Lebreton, A. Impact of Epicatechin on Fibrin Clot Structure. Eur. J. Pharmacol. 2021, 893, 173830. [Google Scholar] [CrossRef]
- Wang, G.; Tiemeier, G.L.; van den Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790. [Google Scholar] [CrossRef]
- Molinari, A.C.; Banov, L.; Bertamino, M.; Barabino, P.; Lassandro, G.; Giordano, P. A Practical Approach to the Use of Low Molecular Weight Heparins in VTE Treatment and Prophylaxis in Children and Newborns. Pediatr. Hematol. Oncol. 2015, 32, 1–10. [Google Scholar] [CrossRef]
- Girish, K.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B. Hyaluronidase Inhibitors: A Biological and Therapeutic Perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Hetta, M.H.; Rateb, M.E.; Selim, M.A.; AboulMagd, A.M.; Badria, F.A.; Abdelmohsen, U.R.; Alhadrami, H.A.; Hassan, H.M. Bioassay-Guided Isolation, Metabolic Profiling, and Docking Studies of Hyaluronidase Inhibitors from Ravenala Madagascariensis. Molecules 2020, 25, 1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Yin, H.; Dong, J.; Xiao, L.; Liu, G.; Qian, Z.; Miao, J. Inhibition and Interaction with Hyaluronidase by Compounds from Hop (Humulus Lupulus L.) Flowers. Asian J. Chem. 2013, 25, 10262–10266. [Google Scholar] [CrossRef]
- Tomohara, K.; Ito, T.; Onikata, S.; Kato, A.; Adachi, I. Discovery of Hyaluronidase Inhibitors from Natural Products and Their Mechanistic Characterization under DMSO-Perturbed Assay Conditions. Bioorganic Med. Chem. Lett. 2017, 27, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus Domestica L. Leaf Extracts as Functional Products: Phytochemical Profiling, Cellular Safety, pro-Inflammatory Enzymes Inhibition and Protective Effects against Oxidative Stress In Vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]

| ME | ME.DEF | ME.EAF | ME.BF | |
|---|---|---|---|---|
| Total phenolic content | 26.03 ± 0.33 | 155.96 ± 2.99 | 198.18 ± 8.53 | 76.93 ± 1.78 |
| Total content of mono- and dicaffeoylqunic acids | 8.70 ± 0.11 | 103.09 ± 1.12 | 187.99 ± 5.50 | 41.78 ± 0.61 |
| 3-O-caffeoylquinic acid | 2.93 ± 0.04 | 11.25 ± 0.08 | 21.64 ± 0.54 | 14.51 ± 0.22 |
| 5-O-caffeoylquinic acid (CHA) | 4.88 ± 0.04 | 35.12 ± 0.15 | 140.18 ± 3.40 | 23.84 ± 0.35 |
| 4-O-caffeoylquinic acid | 0.36 ± 0.01 | 3.65 ± 0.02 | 8.10 ± 0.23 | 1.88 ± 0.03 |
| 1-O-caffeoylquinic acid | 0.20 ± 0.00 | 6.01 ± 0.22 | 6.81 ± 0.12 | 1.55 ± 0.02 |
| 3,5-O-dicaffeoylquinic acid | 0.33 ± 0.01 | 32.05 ± 0.17 | 10.38 ± 0.31 | n.d. |
| 1,3-O-dicaffeoylquinic acid | n.d. | 10.93 ± 0.45 | n.d. | n.d. |
| 4,5-O-dicaffeoylquinic acid | n.d. | 4.07 ± 0.03 | 0.89 ± 0.02 | n.d. |
| Total content of other hydroxycinnamic acid derivatives | 1.05 ± 0.02 | 33.52 ± 1.05 | 51.75 ± 0.66 | 21.10 ± 0.30 |
| Total content of hydroxybenzoic acid derivatives | n.d. | 2.64 ± 0.04 | n.d. | n.d. |
| Total content of flavonoids | 0.62 ± 0.00 | 1.43 ± 0.03 | 5.79 ± 0.14 | 5.76 ± 0.06 |
| quercetin 3-O-β-sophoroside (SQ) | 0.39 ± 0.00 | n.d. | n.d | 3.57 ± 0.04 |
| quercetin 3-O-β-D-galactoside | 0.03 ± 0.00 | n.d. | 1.36 ± 0.03 | 0.38 ± 0.01 |
| quercetin 3-O-β-D-glucoside (IQ) | 0.07 ± 0.00 | 0.37 ± 0.01 | 2.25 ± 0.05 | 0.28 ± 0.00 |
| quercetin | n.d. | 1.06 ± 0.01 | 0.34 ± 0.02 | n.d. |
| Total content of oligomeric and polymeric proanthocyanidins | 14.18 ± 0.37 | n.d. | n.d. | 13.34 ± 0.46 |
| Analyte | IC50 (µg/mL) a | IC50 (mg HP/ mg dw) b |
|---|---|---|
| ME | 151.54 ± 8.99 E | 0.32 ± 0.02 |
| ME.DEF | 87.05 ± 4.55 B | 0.56 ± 0.03 |
| ME.EAF | 108.53 ± 3.95 C | 0.45 ± 0.02 |
| ME.BF | 118.79 ± 2.45 D | 0.41 ± 0.01 |
| PB2 | >120 | - |
| CHA | >120 | - |
| IQ | >200 | - |
| SQ | >200 | - |
| HP | 48.98 ± 3.71 A | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, M.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro. Antioxidants 2021, 10, 2009. https://doi.org/10.3390/antiox10122009
Rutkowska M, Kolodziejczyk-Czepas J, Olszewska MA. The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro. Antioxidants. 2021; 10(12):2009. https://doi.org/10.3390/antiox10122009
Chicago/Turabian StyleRutkowska, Magdalena, Joanna Kolodziejczyk-Czepas, and Monika Anna Olszewska. 2021. "The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro" Antioxidants 10, no. 12: 2009. https://doi.org/10.3390/antiox10122009
APA StyleRutkowska, M., Kolodziejczyk-Czepas, J., & Olszewska, M. A. (2021). The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro. Antioxidants, 10(12), 2009. https://doi.org/10.3390/antiox10122009






