Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Sampling Procedure
2.2. Assessment
2.3. Nutritional Management
2.4. Statistical Analysis
2.4.1. Factors Associated with Ascorbic Acid Deficiency and Insufficiency
2.4.2. Factors Associated with MoCA Score
2.5. Ethics
3. Results
3.1. Characteristics of Subjects
3.2. Prevalence of Ascorbic Acid Deficiency and Insufficiency
3.3. Factors Associated with Ascorbic Acid Deficiency and Insufficiency
3.4. Factors Associated with MoCA Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gautron, M.-A.; Questel, F.; Lejoyeux, M.; Bellivier, F.; Vorspan, F. Nutritional Status during Inpatient Alcohol Detoxification. Alcohol Alcohol. 2017, 53, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Coulbault, L.; Ritz, L.; Vabret, F.; Lannuzel, C.; Boudehent, C.; Nowoczyn, M.; Beaunieux, H.; Pitel, A.L. Thiamine and phosphate esters concentrations in whole blood and serum of patients with alcohol use disorder: A relation with cognitive deficits. Nutr. Neurosci. 2021, 24, 530–541. [Google Scholar] [CrossRef]
- Rao, R.; Topiwala, A. Alcohol use disorders and the brain. Addiction 2020, 115, 1580–1589. [Google Scholar] [CrossRef]
- Luquiens, A.; Rolland, B.; Pelletier, S.; Alarcon, R.; Donnadieu-Rigole, H.; Benyamina, A.; Nalpas, B.; Perney, P. Role of Patient Sex in Early Recovery from Alcohol-Related Cognitive Impairment: Women Penalized. J. Clin. Med. 2019, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Maillard, A.; Poussier, H.; Boudehent, C.; Lannuzel, C.; Vicente, A.; Vabret, F.; Cabe, N.; Pitel, A.-L. Short-term neuropsychological recovery in alcohol use disorder: A retrospective clinical study. Addict. Behav. 2020, 105, 106350. [Google Scholar] [CrossRef]
- McLean, C.; Tapsell, L.; Grafenauer, S.; McMahon, A.T. Systematic review of nutritional interventions for people admitted to hospital for alcohol withdrawal. Nutr. Diet. 2020, 77, 76–89. [Google Scholar] [CrossRef]
- Addolorato, G.; Capristo, E.; Stefanini, G.F.; Gasbarrini, G. Metabolic Features and Nutritional Status in Chronic Alcoholics. Am. J. Gastroenterol. 1998, 93, 665–666. [Google Scholar] [CrossRef]
- Ross, L.J.; Wilson, M.; Banks, M.; Rezannah, F.; Daglish, M. Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition 2012, 28, 738–743. [Google Scholar] [CrossRef]
- LeComte, E.; Herbeth, B.; Pirollet, P.; Chancerelle, Y.; Arnaud, J.; Musse, N.; Paille, F.; Siest, G.; Artur, Y. Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am. J. Clin. Nutr. 1994, 60, 255–261. [Google Scholar] [CrossRef]
- Lux-Battistelli, C.; Battistelli, D. Alcohol Withdrawal: Possible Risk of Latent Scurvy Appearing as Tiredness: A STROBE-Compliant Study. J. Clin. Med. Res. 2019, 11, 26–34. [Google Scholar] [CrossRef][Green Version]
- Marik, P.E.; Liggett, A. Adding an orange to the banana bag: Vitamin C deficiency is common in alcohol use disorders. Crit. Care 2019, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; A Lacher, D. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- Harrison, F.E.; Bowman, G.L.; Polidori, M.C. Ascorbic Acid and the Brain: Rationale for the Use against Cognitive Decline. Nutrients 2014, 6, 1752–1781. [Google Scholar] [CrossRef]
- Ballaz, S.; Morales, I.; Rodríguez, M.; Obeso, J.A. Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons: Ascorbate Prevents Cell Death from Glutamate. J. Neurosci. Res. 2013, 91, 1609–1617. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Fain, O.; Pariés, J.; Jacquart, B.; Le Moël, G.; Kettaneh, A.; Stirnemann, J.; Héron, C.; Sitbon, M.; Taleb, C.; Letellier, E.; et al. Hypovitaminosis C in hospitalized patients. Eur. J. Intern. Med. 2003, 14, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Miller, M.; Shahi, R.; Doyle, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Vitamin C deficiency in Australian hospitalised patients: An observational study: Vitamin C deficiency. Intern. Med. J. 2019, 49, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Mosdøl, A.; Erens, B.; Brunner, E. Estimated prevalence and predictors of vitamin C deficiency within UK’s low-income population. J. Public Health 2008, 30, 456–460. [Google Scholar] [CrossRef]
- Malmauret, L.; Leblanc, J.; Cuvelier, I.; Verger, P. Dietary intakes and vitamin status of a sample of homeless people in Paris. Eur. J. Clin. Nutr. 2002, 56, 313–320. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Cholongitas, E.; Papatheodoridis, G.; Vangeli, M.; Terreni, N.; Patch, D.; Burroughs, A.K. Systematic review: The model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment. Pharmacol. Ther. 2005, 22, 1079–1089. [Google Scholar] [CrossRef]
- Johnston, C.S.; Thompson, L.L. Vitamin C status of an outpatient population. J. Am. Coll. Nutr. 1998, 17, 366–370. [Google Scholar] [CrossRef]
- Heirene, R.; John, B.; Roderique-Davies, G. Identification and Evaluation of Neuropsychological Tools Used in the Assessment of Alcohol-Related Cognitive Impairment: A Systematic Review. Front. Psychol. 2018, 9, 2618. [Google Scholar] [CrossRef]
- Alarcon, R.; Nalpas, B.; Pelletier, S.; Perney, P. MoCA as a Screening Tool of Neuropsychological Deficits in Alcohol-Dependent Patients. Alcohol. Clin. Exp. Res. 2015, 39, 1042–1048. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, J.; Hong, K.J.; Jung, J.H. Vitamin C Deficiency of Korean Homeless Patients Visiting to Emergency Department with Acute Alcohol Intoxication. J. Korean Med. Sci. 2015, 30, 1874–1880. [Google Scholar] [CrossRef]
- Beattie, A.D.; Sherlock, S. Ascorbic acid deficiency in liver disease. Gut 1976, 17, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Clot, P.; Tabone, M.; Arico, S.; Albano, E. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut 1994, 35, 1637–1643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernandez-Guerra, M.; Garcia-Pagan, J.C.; Turnes, J.; Bellot, P.; Deulofeu, R.; Abraldes, J.G.; Bosch, J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology 2006, 43, 485–491. [Google Scholar] [CrossRef]
- Guo, Y.-E.; Suo, N.; Cui, X.; Yuan, Q.; Xie, X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia 2018, 66, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Xu, Z.-P.; Wang, W.; Cao, J.-B.; Fu, Q.; Zhao, W.-X.; Li, Y.; Huo, X.-L.; Zhang, L.-M.; Li, Y.-F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef]
- Michels, A.; Frei, B. Myths, Artifacts, and Fatal Flaws: Identifying Limitations and Opportunities in Vitamin C Research. Nutrients 2013, 5, 5161–5192. [Google Scholar] [CrossRef] [PubMed]
- Valcour, V.; Paul, R.; Chiao, S.; Wendelken, L.A.; Miller, B. Screening for Cognitive Impairment in Human Immunodeficiency Virus. Clin. Infect. Dis. 2011, 53, 836–842. [Google Scholar] [CrossRef]
- Hercberg, S.; Preziosi, P.; Galan, P.; Devanlay, M.; Keller, H.; Bourgeois, C.; De Courcy, G.P.; Cherouvrier, F. Vitamin status of a healthy French population: Dietary intakes and biochemical markers. Int. J. Vitam. Nutr. Res. 1994, 64, 220–232. [Google Scholar]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Smith, A.; Di Primio, G.; Humphrey-Murto, S. Scurvy in the developed world. Can. Med. Assoc. J. 2011, 183, E752–E755. [Google Scholar] [CrossRef]
- Fain, O. Carences en vitamine C. La Rev. de Médecine Interne 2004, 25, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. Available online: https://www.nap.edu/catalog/9810 (accessed on 25 October 2021).
| All Patients | Subsample with Available MoCA (n = 53) | ||
|---|---|---|---|
| (n = 96) | |||
| Sex | Women | 22 (22.9%) | 12 (22.6%) |
| Men | 74 (77.1%) | 41 (77.4%) | |
| Age (years) | Mean (SD) | 49.1 (±11.5) | 50.2 (±11.2) |
| Min–Max | 24–79 | 29–79 | |
| Body mass index (kg/m2) (n = 91) | <21.0 | 19 (20.7%) | 10 (19.2%) |
| 21.0–24.99 | 43 (46.7%) | 27 (51.9%) | |
| ≥25.0 | 30 (32.6%) | 15 (28.8%) | |
| Housing | Private home | 63 (65.6%) | 39 (73.6%) |
| Homeless in a shelter | 16 (16.7%) | 9 (17.0%) | |
| Street homelessness | 17 (17.7%) | 5 (9.4%) | |
| Educational attainment (n = 94) | ≥Bachelor’s degree | 28 (29.8%) | 25 (47.2%) |
| High school degree | 23 (24.5%) | 11 (20.8%) | |
| <High school degree | 43 (45.7%) | 17 (32.1%) | |
| Socio-professional category * | Higher | 24 (25.0%) | 18 (34.0%) |
| Intermediate | 12 (12.5%) | 9 (17.0%) | |
| Lower | 60 (62.5%) | 26 (49.0%) | |
| Type of admission | Planned | 77 (80.2%) | 46 (86.8%) |
| Via the Emergency Department | 19 (19.8%) | 7 (13.2%) | |
| Alcohol intake per day (grams) (n = 95) | Mean (SD) | 225 (±130) | 212 (±108) |
| Min–Max | 20–600 | 64–550 | |
| Tobacco use | Current smoker | 76 (79.2%) | 42 (79.2%) |
| Former smoker | 12 (12.5%) | 7 (13.2%) | |
| Non-smoker | 8 (8.3%) | 4 (7.5%) | |
| Cigarettes per day (n = 93) | Mean (SD) | 15.0 (±12.7) | 16.1 (±13.8) |
| Number of tobacco pack years (n = 84) | Mean (SD) | 31.4 (±17.9) | 32.1 (±15.9) |
| Number of years of tobacco smoking (n = 88) | Mean (SD) | 28.6 (±13.5) | 30.4 (±13.1) |
| Cirrhosis | Compensated | 13 (13.5%) | 9 (17.0%) |
| No cirrhosis | 83 (86.5%) | 44 (83.0%) | |
| HIV status | Yes | 8 (8.3%) | 5 (9.4%) |
| No | 88 (91.7%) | 48 (90.6%) | |
| Hypertension | Yes | 11 (11.5%) | 8 (15.1%) |
| No | 85 (88.5%) | 45 (85.9%) | |
| Cannabis use disorder ** | Yes | 20 (20.8%) | 12 (22.6%) |
| No | 76 (79.2%) | 41 (77.4%) | |
| Sedative use disorder ** | Yes | 12 (12.5%) | 6 (11.3%) |
| No | 84 (87.5%) | 47 (88.7%) | |
| Cocaine use disorder ** | Yes | 13 (13.5%) | 4 (7.5%) |
| No | 83 (86.5%) | 49 (92.5%) | |
| Opiate use disorder ** | Active | 2 (2.1%) | 0 (0%) |
| Opioid maintenance treatment | 10 (10.4%) | 3 (5.7%) | |
| No | 84 (87.5%) | 50 (94.7%) | |
| Psychiatric comorbidity | Bipolar disorder | 4 (4.2%) | 2 (3.8%) |
| Schizophrenia | 2 (2.1%) | 1 (1.9%) | |
| Selective serotonin reuptake inhibitor current use | Yes | 33 (34.4%) | 24 (45.3%) |
| No | 63 (65.6%) | 29 (54.7%) | |
| Ascorbic Acid Level (mg/L) | |||||
|---|---|---|---|---|---|
| Deficiency (<2.0) | Insufficiency (2.0–4.99) | Normal Level (≥5.0) | p | ||
| Sex | Women | 1 (4.5%) | 5 (22.7%) | 16 (72.7%) | 0.011 †† |
| Men | 26 (35.1%) | 17 (23.0%) | 31 (41.9%) | ||
| Age (years) | Mean (SD) | 46.7 (±10.1) | 49.4 (±10.8) | 50.3 (±12.5) | 0.43 ††† |
| Body mass index (kg/m2) | <21.0 | 5 (26.3%) | 3 (15.8%) | 11 (57.9%) | 0.93 † |
| 21.0–24.99 | 11 (25.6%) | 11 (25.6%) | 21 (48.8%) | ||
| ≥25.0 | 8 (26.7%) | 8 (26.7%) | 14 (46.7%) | ||
| Prealbumin (g/L) | Mean (SD) | 0.24 (±0.11) | 0.29 (±0.11) | 0.28 (±0.10) | 0.23 ††† |
| Albumin (g/L) | Mean (SD) | 40.2 (±5.3) | 42.5 (±3.8) | 42.5 (±3.9) | 0.074 ††† |
| Vitamin D | <10 ng/L | 19 (36.5%) | 13 (25.0%) | 20 (38.5%) | 0.28 † |
| 10–20 ng/L | 4 (19.0%) | 4 (19.0%) | 13 (61.9%) | ||
| ≥20 ng/L | 4 (18.2%) | 5 (22.7%) | 13 (59.1%) | ||
| Vitamin B9 | <3.9 µg/L | 7 (33.3%) | 6 (28.6%) | 8 (38.1%) | 0.50 † |
| ≥3.9 µg/L | 20 (26.7%) | 16 (21.3%) | 39 (52.0%) | ||
| Vitamin B12 | <197 ng/L | 0 (0%) | 1 (50.0%) | 1 (50.0%) | 0.47 † |
| ≥197 ng/L | 27 (21.5%) | 20 (29.0%) | 46 (49.5%) | ||
| Housing | Private home | 13 (20.6%) | 12 (19.0%) | 38 (60.3%) | 0.014 † |
| Homeless in a shelter | 5 (31.2%) | 5 (31.2%) | 6 (37.5%) | ||
| Street homelessness | 9 (52.9%) | 5 (29.4%) | 3 (17.6%) | ||
| Academic attainment | ≥Bachelor’s degree | 6 (21.4%) | 6 (21.4%) | 16 (57.1%) | 0.24 † |
| High school degree | 3 (13.0%) | 6 (29.1%) | 14 (60.9%) | ||
| <High school degree | 16 (37.2%) | 10 (23.3%) | 17 (39.5%) | ||
| Socioprofessional category * | Higher | 5 (20.8%) | 3 (12.5%) | 16 (66.7%) | 0.23 † |
| Intermediate | 2 (16.7%) | 3 (25%) | 7 (58.3%) | ||
| Lower | 20 (33.3%) | 16 (26.7%) | 24 (40.0%) | ||
| Type of admission | Planned | 18 (23.4%) | 18 (23.4%) | 41 (53.2%) | 0.13 † |
| Urgently | 9 (47.4%) | 4 (21.1%) | 6 (31.6%) | ||
| Alcohol intake per day (grams) | Mean (SD) | 248 (±136) | 251 (±139) | 198 (±119) | 0.20 *** |
| Min–Max | 60–600 | 80–600 | 20–600 | ||
| Tobacco use | Current smoker | 23 (30.3%) | 17 (22.4%) | 36 (47.4%) | 0.72 † |
| Former or non-smoker | 4 (20.0%) | 5 (25.0%) | 11 (55.0%) | ||
| Cigarettes per day | Mean (SD) | 15.2 (±12.3) | 17.4 (±13.7) | 13.8 (±12.6) | 0.51 *** |
| Number of tobacco pack years | Mean (SD) | 27.8 (±15.2) | 33.5 (±16.0) | 32.5 (±20.3) | 0.55 *** |
| Number of years of tobacco smoking | Mean (SD) | 27.0 (±13.1) | 29.5 (±11.1) | 29.1 (±14.9) | 0.73 *** |
| Cirrhosis | Compensated | 8 (61.5%) | 1 (7.7%) | 4 (30.8%) | 0.028 † |
| No cirrhosis | 19 (22.9%) | 21 (25.3%) | 43 (51.8%) | ||
| HIV status | Yes | 4 (50.0%) | 2 (25.0%) | 2 (25.0%) | 0.27 † |
| No | 23 (26.1%) | 20 (22.7%) | 45 (51.1%) | ||
| Cannabis use disorder ** | Yes | 2 (10.0%) | 5 (25.0%) | 13 (65.0%) | 0.099 † |
| No | 25 (22.4%) | 17 (32.9%) | 34 (44.7%) | ||
| Sedative use disorder ** | Yes | 2 (16.7%) | 3 (25.0%) | 7 (58.3%) | 0.71 † |
| No | 25 (29.8%) | 19 (22.6%) | 40 (47.6%) | ||
| Cocaine use disorder ** | Yes | 2 (15.4%) | 3 (23.1%) | 8 (61.5%) | 0.54 † |
| No | 25 (30.1%) | 19 (22.9%) | 39 (47.0%) | ||
| Opiate use disorder ** | Active | 0 | 0 | 2 (100%) | 0.21 † |
| Opioid maintenance treatment | 5 (55.6%) | 2 (22.2%) | 2 (22.2%) | ||
| No | 22 (25.9%) | 20 (23.5%) | 43 (50.6%) | ||
| Univariate Analysis | Multivariate Analysis *** (n = 47) | ||||
|---|---|---|---|---|---|
| MoCA Score | p-Value | β | p-Value | ||
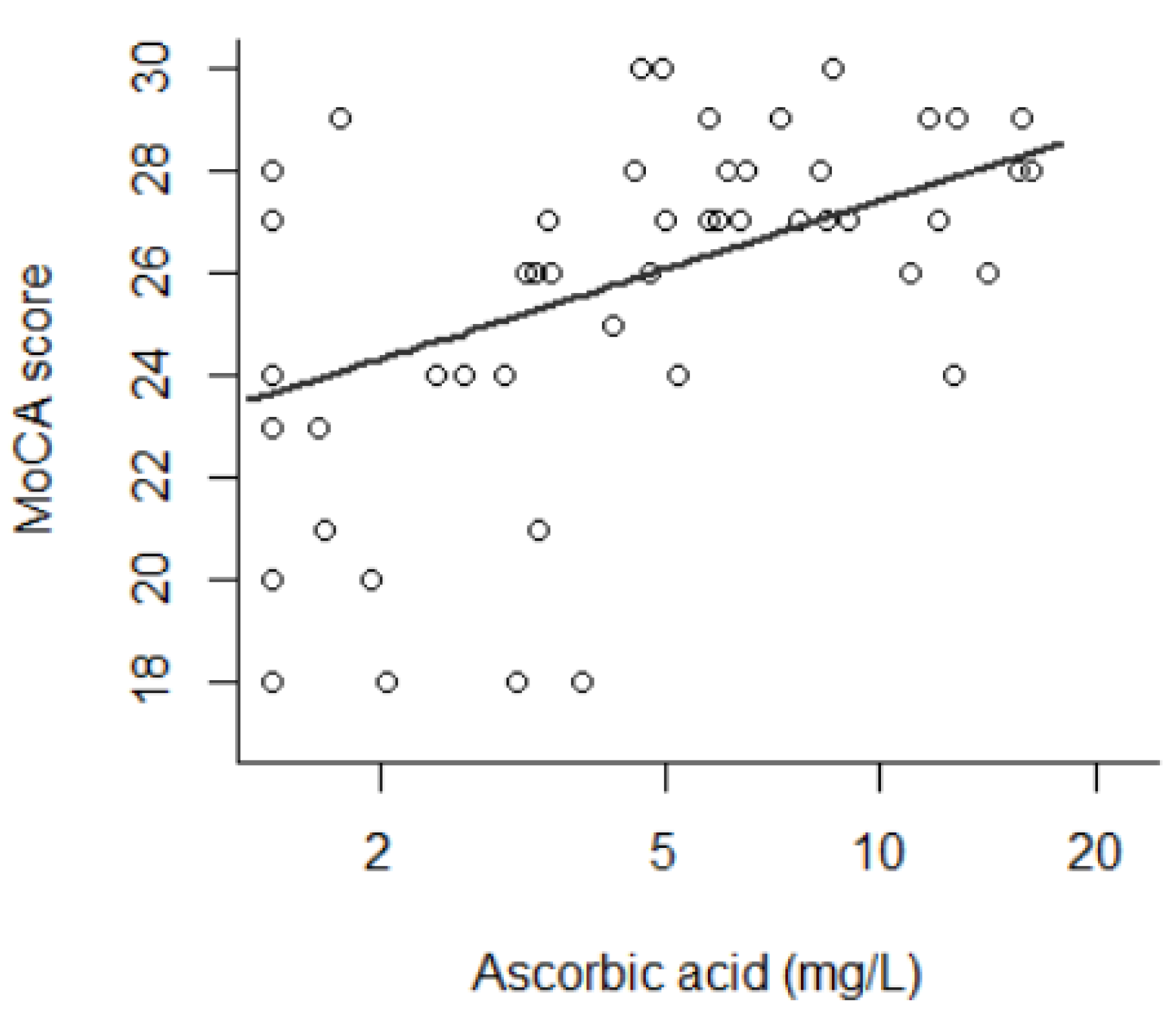
| Ascorbic acid | natural logarithm (mg/L) | β = 1.91 | 5.3 × 10−4 ††† | 1.18 | 0.037 |
| Sex | Women | 27.2 (±1.8) | 0.15 †† | ref | 0.54 |
| Men | 25.3 (±3.5) | −0.53 | |||
| Age (years) | /decade | β = −6.9 | 0.013 *** | −4.1 | 0.099 |
| /decade2 | β’ = 0.64 | 0.016 *** | 0.31 | 0.19 | |
| Prealbumin (g/L) (n = 47) | β = 8.3 | 0.046 ††† | 2.50 | 0.51 | |
| Albumin (g/L) | β = 0.10 | 0.37 ††† | - | - | |
| Vitamin D (ng/L) (n = 52) | β = −1.7 × 10−3 | 0.96 ††† | - | - | |
| Vitamin B9 (µg/L) | β = −0.014 | 0.92 ††† | - | - | |
| Vitamin B12 (ng/L) (n = 52) | β = 5.6 × 10−4 | 0.84 ††† | - | - | |
| Body mass index (kg/m2) (n = 52) | <21.0 | 25.7 (±2.9) | 0.95 †† | - | - |
| 21.0–24.99 | 25.7 (±3.5) | ||||
| ≥25.0 | 25.8 (±3.4) | ||||
| Housing | Private home | 26.5 (±2.7) | 4.4 × 10−3 †† | ref | ref |
| Homeless in a shelter | 25.2 (±3.4) | 1.29 | 0.19 | ||
| Street homelessness | 20.1 (±2.5) | −2.01 | 0.17 | ||
| Socioprofessional category * | Higher | 27.0 (±2.4) | 6.1 × 10−3 †† | ref | ref |
| Intermediate | 26.0 (±3.3) | 1.23 | 0.29 | ||
| Lower | 24.1 (±3.6) | −1.69 | 0.053 | ||
| Alcohol intake | by 10 g per day | β = −0.052 | 0.21 ††† | - | - |
| Tobacco use | Current smoker | 25.4 (±3.4) | 0.98 † | - | - |
| Former or non-smoker | 25.5 (±3.7) | ||||
| Cigarettes (n = 52) | Number use per day | β = 0.014 | 0.69 ††† | - | - |
| Cirrhosis | Compensated | 24.9 (±4.3) | 0.78 † | - | - |
| No cirrhosis | 25.9 (±3.1) | ||||
| HIV status | Yes | 22.0 (±3.5) | 0.021 † | −2.67 | 0.075 |
| No | 26.1 (±3.0) | ||||
| Cannabis use disorder ** | Yes | 26.6 (±3.3) | 0.35 † | 0.72 | 0.50 |
| No | 25.5 (±3.3) | ref | |||
| Sedative use disorder ** | Yes | 24.8 (±3.6) | 0.35 † | −2.77 | 0.046 |
| No | 25.8 (±3.3) | ref | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clergue-Duval, V.; Azuar, J.; Fonsart, J.; Delage, C.; Rollet, D.; Amami, J.; Frapsauce, A.; Gautron, M.-A.; Hispard, E.; Bellivier, F.; et al. Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study. Antioxidants 2021, 10, 1892. https://doi.org/10.3390/antiox10121892
Clergue-Duval V, Azuar J, Fonsart J, Delage C, Rollet D, Amami J, Frapsauce A, Gautron M-A, Hispard E, Bellivier F, et al. Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study. Antioxidants. 2021; 10(12):1892. https://doi.org/10.3390/antiox10121892
Chicago/Turabian StyleClergue-Duval, Virgile, Julien Azuar, Julien Fonsart, Clément Delage, Dorian Rollet, Jihed Amami, Alexia Frapsauce, Marie-Astrid Gautron, Eric Hispard, Frank Bellivier, and et al. 2021. "Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study" Antioxidants 10, no. 12: 1892. https://doi.org/10.3390/antiox10121892
APA StyleClergue-Duval, V., Azuar, J., Fonsart, J., Delage, C., Rollet, D., Amami, J., Frapsauce, A., Gautron, M.-A., Hispard, E., Bellivier, F., Bloch, V., Laplanche, J.-L., Questel, F., & Vorspan, F. (2021). Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study. Antioxidants, 10(12), 1892. https://doi.org/10.3390/antiox10121892






