Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ovulation Induction Protocol
2.3. Isolation of hGL Cells
2.4. Cell Culture and Treatments
2.4.1. Glucose Treatment
2.4.2. Peroxynitrite Treatment
2.4.3. Antioxidant Treatment
2.5. Gene Expression Analysis by qRT-PCR
2.6. DNA Damage Assay
2.7. Oxidative Stress Assay
2.8. Statistical Analysis
3. Results
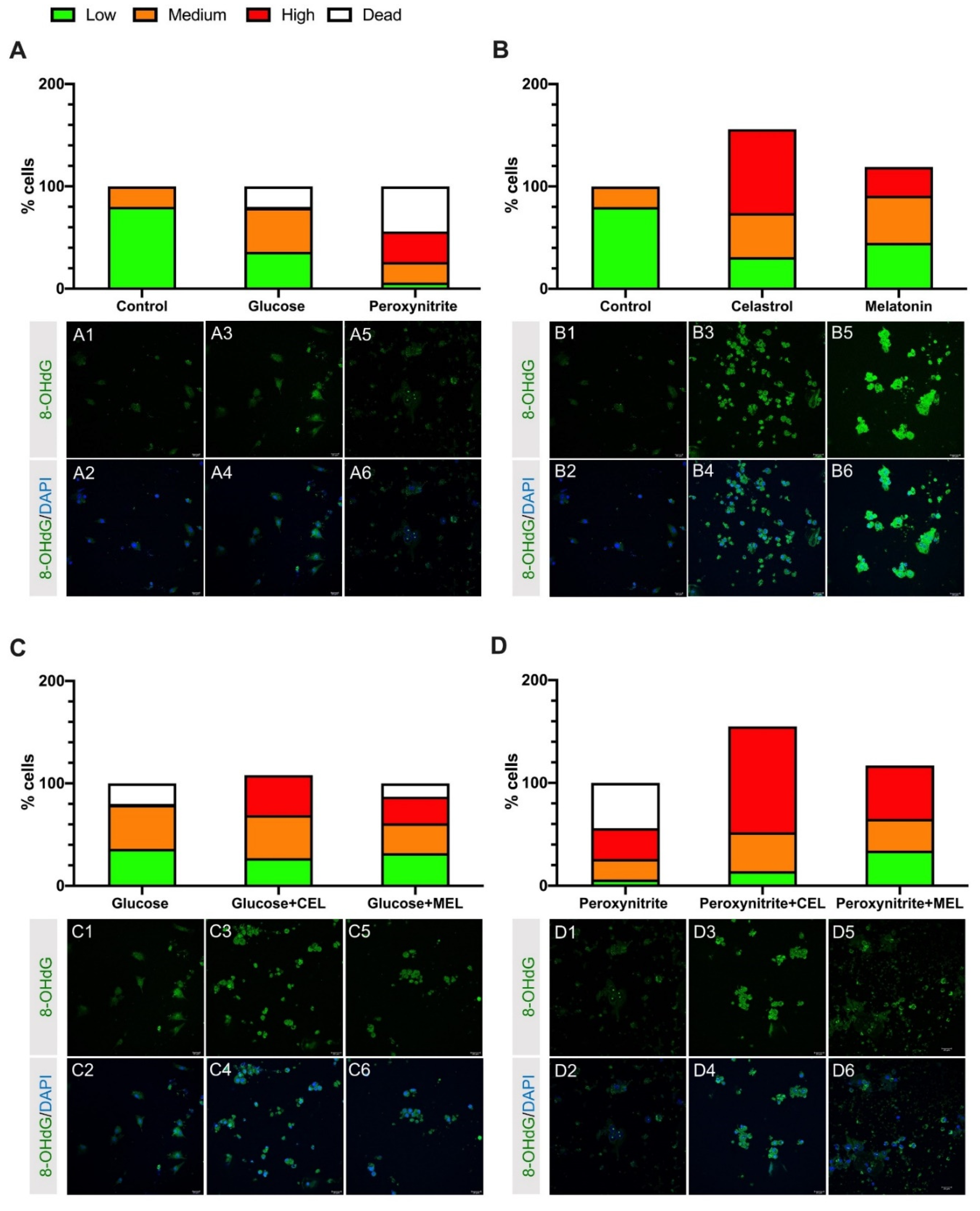
3.1. DNA Damage in Control and Treated Cells
3.2. Effect of Melatonin on hGL Cultured Cells
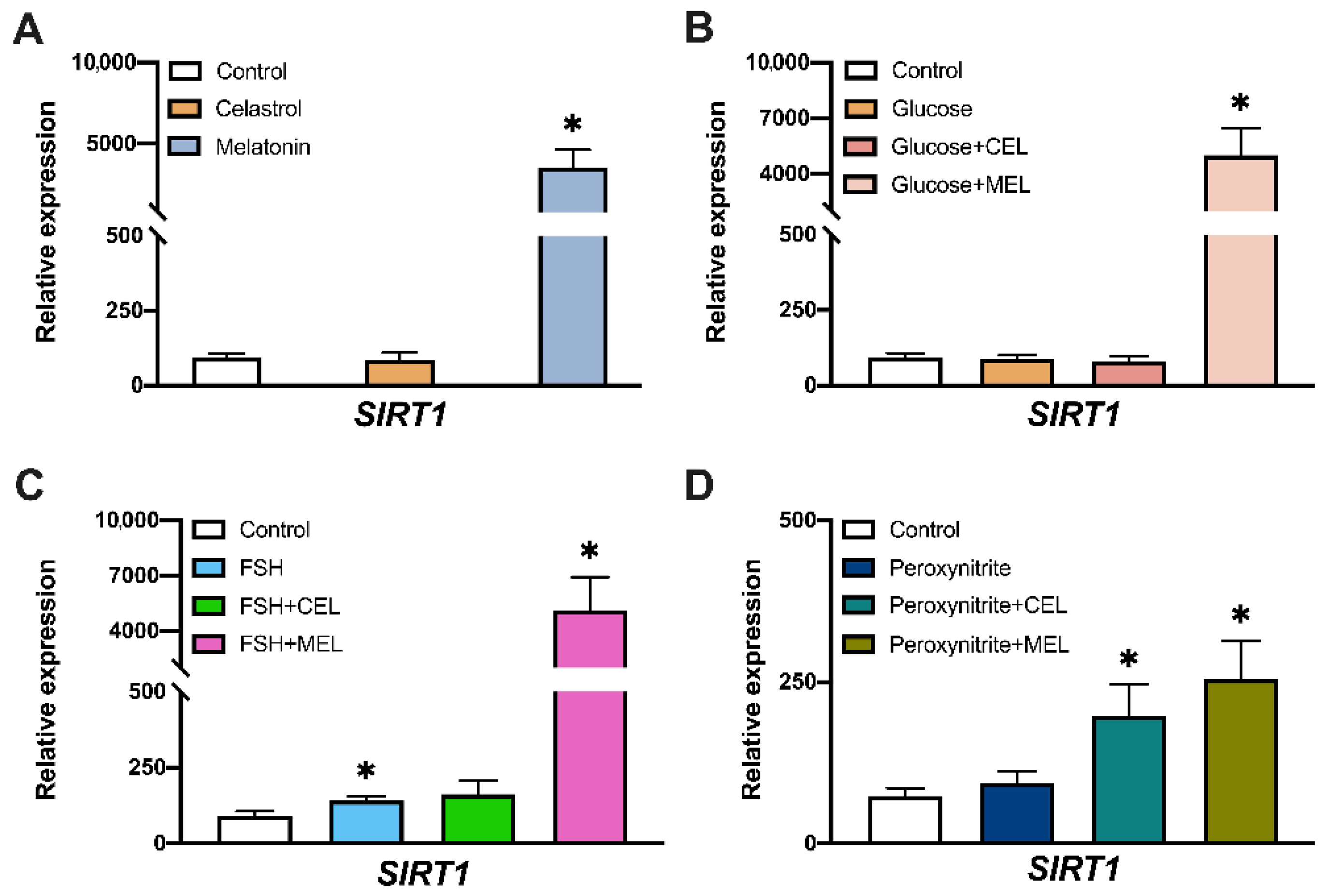
3.3. SIRT1 Expression in Control and Treated Cells
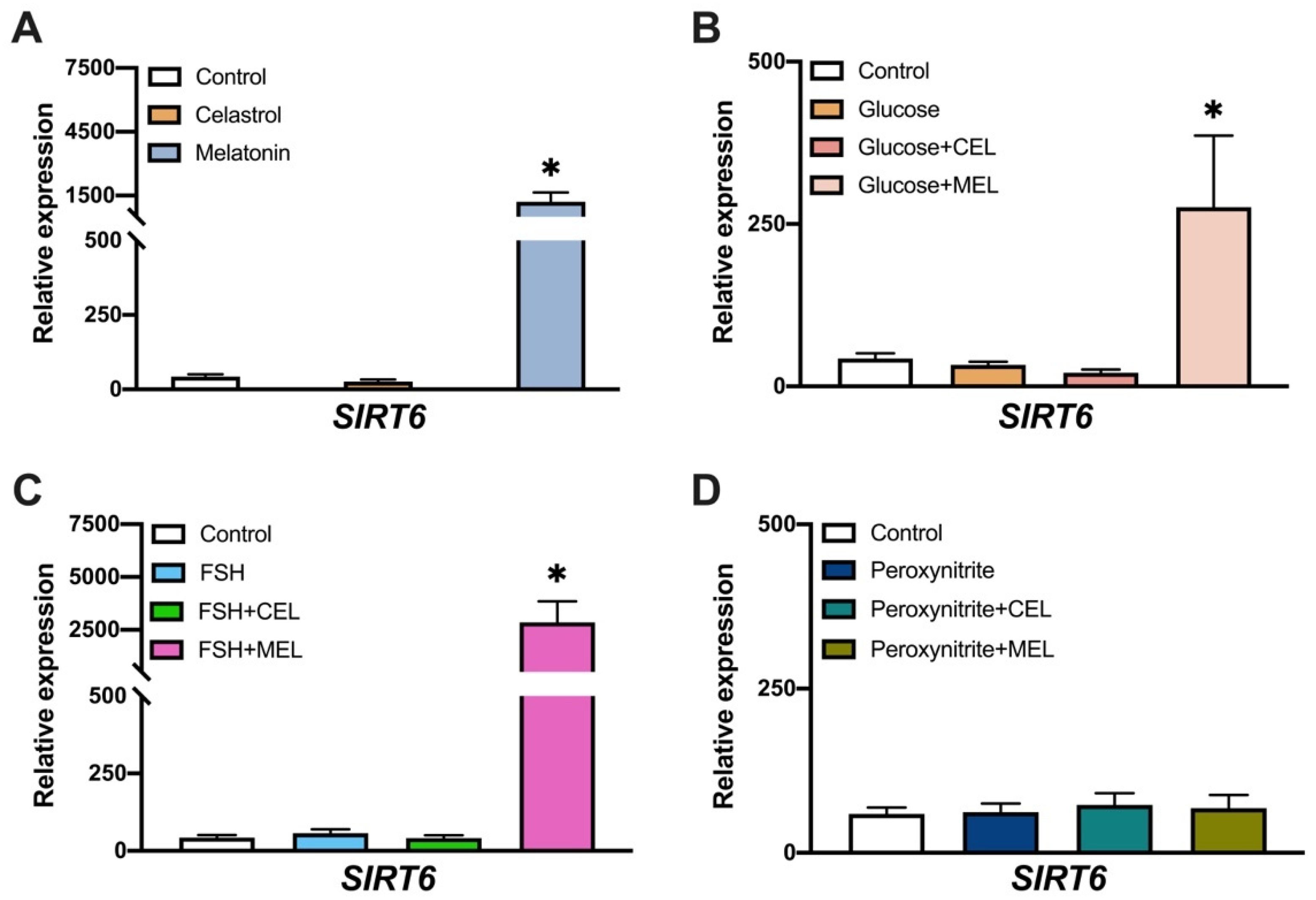
3.4. SIRT6 Expression in Control and Treated Cells
3.5. SIRT7 Expression in Control and Treated Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary Evidence on the Physiological Role of Reactive Oxygen Species in Human Sperm Function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, W. The Controversial World of Sirtuins. Drug Discov. Today Technol. 2014, 12, e9–e17. [Google Scholar] [CrossRef] [Green Version]
- Morris, B.J. Seven Sirtuins for Seven Deadly Diseases Ofaging. Free Radic. Biol. Med. 2013, 56, 133–171. [Google Scholar] [CrossRef]
- Cantó, C.; Sauve, A.A.; Bai, P. Crosstalk between Poly(ADP-Ribose) Polymerase and Sirtuin Enzymes. Mol. Asp. Med. 2013, 34, 1168–1201. [Google Scholar] [CrossRef] [Green Version]
- Ford, E. Mammalian Sir2 Homolog SIRT7 Is an Activator of RNA Polymerase I Transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic Shuttling of the NAD+-Dependent Histone Deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental Defects and P53 Hyperacetylation in Sir2 Homolog (SIRT1)-Deficient Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT 7 Promotes Genome Integrity and Modulates Non-homologous End Joining DNA Repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, I.H.; Kim, D.H.; Park, S.W. Knockout of Longevity Gene Sirt1 in Zebrafish Leads to Oxidative Injury, Chronic Inflammation, and Reduced Life Span. PLoS ONE 2019, 14, e0220581. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.-S.; Chang, Y.S.; Park, C.W.; et al. Age-Associated Molecular Changes in the Kidney in Aged Mice. Oxidative Med. Cell. Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Washida, N.; Tokuyama, H.; Hayashi, K.; Itoh, H. Sirt1 Protects against Oxidative Stress-Induced Renal Tubular Cell Apoptosis by the Bidirectional Regulation of Catalase Expression. Biochem. Biophys. Res. Commun. 2008, 372, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Toiber, D.; Erdel, F.; Bouazoune, K.; Silberman, D.M.; Zhong, L.; Mulligan, P.; Sebastian, C.; Cosentino, C.; Martinez-Pastor, B.; Giacosa, S.; et al. SIRT6 Recruits SNF2H to DNA Break Sites, Preventing Genomic Instability through Chromatin Remodeling. Mol. Cell 2013, 51, 454–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Hine, C.; Tian, X.; van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 Promotes DNA Repair under Stress by Activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.-J.; Jin, J.; Gao, Y.; Shi, G.; Madabushi, A.; Yan, A.; Guan, X.; Zalzman, M.; Nakajima, S.; Lan, L.; et al. SIRT6 Protein Deacetylase Interacts with MYH DNA Glycosylase, APE1 Endonuclease, and Rad9–Rad1–Hus1 Checkpoint Clamp. BMC Mol. Biol. 2015, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grummt, I.; Pikaard, C.S. Epigenetic Silencing of RNA Polymerase I Transcription. Nat. Rev. Mol. Cell Biol. 2003, 4, 641–649. [Google Scholar] [CrossRef]
- Chen, S.; Blank, M.F.; Iyer, A.; Huang, B.; Wang, L.; Grummt, I.; Voit, R. SIRT7-Dependent Deacetylation of the U3-55k Protein Controls Pre-RRNA Processing. Nat. Commun. 2016, 7, 10734. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; He, M.; Liu, Y.; Paredes, S.; Villanova, L.; Brown, K.; Qiu, X.; Nabavi, N.; Mohrin, M.; Wojnoonski, K.; et al. SIRT7 Represses Myc Activity to Suppress ER Stress and Prevent Fatty Liver Disease. Cell Rep. 2013, 5, 654–656. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Jaswal, V.; Sharma, A.; Kashyap, D.; Tuli, H.S.; Garg, V.K.; Das, S.K.; Srinivas, R. Celastrol as a Pentacyclic Triterpenoid with Chemopreventive Properties. Pharm. Pat. Anal. 2018, 7, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Sarmidi, M.R.; Tan, J.S.; Mohamad Rosdi, M.N. Celastrol Attenuates Mitochondrial Dysfunction and Inflammation in Palmitate-Mediated Insulin Resistance in C3A Hepatocytes. Eur. J. Pharmacol. 2017, 799, 73–83. [Google Scholar] [CrossRef]
- Ng, S.W.; Chan, Y.; Chellappan, D.K.; Madheswaran, T.; Zeeshan, F.; Chan, Y.L.; Collet, T.; Gupta, G.; Oliver, B.G.; Wark, P.; et al. Molecular Modulators of Celastrol as the Keystones for Its Diverse Pharmacological Activities. Biomed. Pharmacother. 2019, 109, 1785–1792. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Zhou, K.; Zhang, J.; Shang, F.; Zhang, X. Celastrol Protects RPE Cells from Oxidative Stress-Induced Cell Death via Activation of Nrf2 Signaling Pathway. Curr. Mol. Med. 2019, 19, 172–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, C.; Liu, X.; Li, M.; Gao, M.; Liu, X.; Fang, F.; Chang, Y. Celastrol Ameliorates Liver Metabolic Damage Caused by a High-Fat Diet through Sirt1. Mol. Metab. 2017, 6, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Rosales-Corral, S.; Manchester, L.; Tan, D.-X. Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time. Int. J. Mol. Sci. 2013, 14, 7231–7272. [Google Scholar] [CrossRef] [Green Version]
- Lan, M.; Han, J.; Pan, M.-H.; Wan, X.; Pan, Z.-N.; Sun, S.-C. Melatonin Protects against Defects Induced by Deoxynivalenol during Mouse Oocyte Maturation. J. Pineal Res. 2018, 65, e12477. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, K.; Sritawan, N.; Prajit, R.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Protects against the Side-Effects of 5-Fluorouracil on Hippocampal Neurogenesis and Ameliorates Antioxidant Activity in an Adult Rat Hippocampus and Prefrontal Cortex. Antioxidants 2021, 10, 615. [Google Scholar] [CrossRef]
- Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, W.; Ao, H.; Zhang, Y.; Zhou, R.; Li, K. Effects of Melatonin on the Synthesis of Estradiol and Gene Expression in Pig Granulosa Cells. J. Pineal Res. 2019, 66, e12546. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-Related Genes Expressed in the Mouse Uterus during Early Gestation Promote Embryo Implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.-Y. Melatonin and the Circadian System: Contributions to Successful Female Reproduction. Fertil. Steril. 2014, 102, 293–307. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Inflammation-Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Dong, X.; Xue, X.; Xu, S.; Zhang, X.; Xu, Y.; Wang, Z.; Wang, Y.; Gao, H.; Liang, Y.; et al. Melatonin Attenuates Diabetic Cardiomyopathy and Reduces Myocardial Vulnerability to Ischemia-reperfusion Injury by Improving Mitochondrial Quality Control: Role of SIRT6. J. Pineal Res. 2021, 70, e12698. [Google Scholar] [CrossRef] [PubMed]
- Nargund, G.; Datta, A.K.; Fauser, B.C.J.M. Mild Stimulation for in Vitro Fertilization. Fertil. Steril. 2017, 108, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 309–315. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring Reactive Oxygen and Nitrogen Species with Fluorescent Probes: Challenges and Limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Martín-Ramírez, R.; González-Fernández, R.; Rotoli, D.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Celastrol Prevents Oxidative Stress Effects on FSHR, PAPP, and CYP19A1 Gene Expression in Cultured Human Granulosa-Lutein Cells. Int. J. Mol. Sci. 2021, 22, 3596. [Google Scholar] [CrossRef]
- Sirotkin, A.; Dekanová, P.; Harrath, A. FSH, Oxytocin and IGF-I Regulate the Expression of Sirtuin 1 in Porcine Ovarian Granulosa Cells. Physiol. Res. 2020, 69, 461–466. [Google Scholar] [CrossRef]
- Kim, J.K.; Noh, J.H.; Jung, K.H.; Eun, J.W.; Bae, H.J.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Park, W.S.; Lee, J.Y.; et al. Sirtuin7 Oncogenic Potential in Human Hepatocellular Carcinoma and Its Regulation by the Tumor Suppressors MiR-125a-5p and MiR-125b. Hepatology 2013, 57, 1055–1067. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Ha, Y.; Luo, X.; Motamedi, M.; Gupta, M.P.; Ma, J.-X.; Tilton, R.G.; Zhang, W. Posttranslational Modification of Sirt6 Activity by Peroxynitrite. Free Radic. Biol. Med. 2015, 79, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin and the Pathologies of Weakened or Dysregulated Circadian Oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef]
- Hardeland, R. Extended Signaling by Melatonin. Cell Cell. Life Sci. J. 2018, 3, 000123. [Google Scholar] [CrossRef]
- Hardeland, R. Recent Findings in Melatonin Research and Their Relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, X.-Y.; Li, B.; Li, X.-T. Melatonin Protects Premature Ovarian Insufficiency Induced by Tripterygium Glycosides: Role of SIRT1. Am. J. Transl. Res. 2017, 9, 1580. [Google Scholar] [PubMed]
- Zhang, M.; Zhang, Q.; Hu, Y.; Xu, L.; Jiang, Y.; Zhang, C.; Ding, L.; Jiang, R.; Sun, J.; Sun, H.; et al. MiR-181a Increases FoxO1 Acetylation and Promotes Granulosa Cell Apoptosis via SIRT1 Downregulation. Cell Death Dis. 2017, 8, e3088. [Google Scholar] [CrossRef]
- Lee, F.-Y.; Sun, C.-K.; Sung, P.-H.; Chen, K.-H.; Chua, S.; Sheu, J.-J.; Chung, S.-Y.; Chai, H.-T.; Chen, Y.-L.; Huang, T.-H.; et al. Daily Melatonin Protects the Endothelial Lineage and Functional Integrity against the Aging Process, Oxidative Stress, and Toxic Environment and Restores Blood Flow in Critical Limb Ischemia Area in Mice. J. Pineal Res. 2018, 65, e12489. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qin, B.; Wu, F.; Qin, S.; Nowsheen, S.; Shan, S.; Zayas, J.; Pei, H.; Lou, Z.; Wang, L. Regulation of Serine-Threonine Kinase Akt Activation by NAD+-Dependent Deacetylase SIRT7. Cell Rep. 2017, 18, 1229–1240. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Blengini, C.S.; Hernandez, Y.; Serrano, L.; Schindler, K. SIRT7 Promotes Chromosome Synapsis during Prophase I of Female Meiosis. Chromosoma 2019, 128, 369–383. [Google Scholar] [CrossRef]
- Si, H.; Wang, H.; Xiao, H.; Fang, Y.; Wu, Z. Anti-Tumor Effect of Celastrol on Hepatocellular Carcinoma by the Circ_SLIT3/MiR-223-3p/CXCR4 Axis. Cancer Manag. Res. 2021, 13, 1099. [Google Scholar] [CrossRef]
- Liu, P.; Wang, M.; Tang, W.; Li, G.; Gong, N. Circ_SATB2 Attenuates the Anti-Tumor Role of Celastrol in Non-Small-Cell Lung Carcinoma Through Targeting MiR-33a-5p/E2F7 Axis. OncoTargets Ther. 2020, 13, 11899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol Exerts Anti-Inflammatory Effect in Liver Fibrosis via Activation of AMPK-SIRT3 Signalling. J. Cell. Mol. Med. 2020, 24, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Wang, X.; Teng, H.; Li, C.; Wang, B.; Wang, J. Celastrol Inhibits Microglial Pyroptosis and Attenuates Inflammatory Reaction in Acute Spinal Cord Injury Rats. Int. Immunopharmacol. 2019, 66, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol Induces ROS-Mediated Apoptosis via Directly Targeting Peroxiredoxin-2 in Gastric Cancer Cells. Theranostics 2020, 10, 10290. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.M.; Álvarez, X.A.; Vigo, C. Celastrol, a Potent Antioxidant and Anti-Inflammatory Drug, as a Possible Treatment for Alzheimer’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 108–114. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, G.; Song, Y.; Wang, L.; Tu, L.; Zhang, L.; Zhang, C. Celastrol-Induced Suppression of the MiR-21/ERK Signalling Pathway Attenuates Cardiac Fibrosis and Dysfunction. Cell. Physiol. Biochem. 2016, 38, 1928–1938. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Lee, H.; Han, S.; Lee, Y.-S.; Choe, J.; Kim, Y.-M.; Hahn, J.-H.; Ro, J.Y.; Jeoung, D. Celastrol Binds to ERK and Inhibits FcεRI Signaling to Exert an Anti-Allergic Effect. Eur. J. Pharmacol. 2009, 612, 131–142. [Google Scholar] [CrossRef]
- Huang, X.-P.; Chen, J.-K.; Wei, X.; Dong, Y.-F.; Lang, Y.; Zhang, X.-F.; Pan, Y.-M.; Chang, W.-J.; Zhu, J.-B. Systematic Identification of Celastrol-Binding Proteins Reveals That Shoc2 Is Inhibited by Celastrol. Biosci. Rep. 2018, 38, BSR20181233. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ye, X.; Chen, R.; Gao, Q.; Zhao, D.; Ling, C.; Qian, Y.; Xu, C.; Tao, M.; Xie, Y. Sirtuin 7 Promotes Non-small Cell Lung Cancer Progression by Facilitating G1/S Phase and Epithelial-mesenchymal Transition and Activating AKT and ERK1/2 Signaling. Oncol. Rep. 2020, 44, 959–972. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Ramírez, R.; González-Fernández, R.; Hernández, J.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress. Antioxidants 2021, 10, 1871. https://doi.org/10.3390/antiox10121871
Martín-Ramírez R, González-Fernández R, Hernández J, Martín-Vasallo P, Palumbo A, Ávila J. Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress. Antioxidants. 2021; 10(12):1871. https://doi.org/10.3390/antiox10121871
Chicago/Turabian StyleMartín-Ramírez, Rita, Rebeca González-Fernández, Jairo Hernández, Pablo Martín-Vasallo, Angela Palumbo, and Julio Ávila. 2021. "Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress" Antioxidants 10, no. 12: 1871. https://doi.org/10.3390/antiox10121871
APA StyleMartín-Ramírez, R., González-Fernández, R., Hernández, J., Martín-Vasallo, P., Palumbo, A., & Ávila, J. (2021). Celastrol and Melatonin Modify SIRT1, SIRT6 and SIRT7 Gene Expression and Improve the Response of Human Granulosa-Lutein Cells to Oxidative Stress. Antioxidants, 10(12), 1871. https://doi.org/10.3390/antiox10121871






