The Role of NRF2 in Mycobacterial Infection
Abstract
1. Introduction
2. Epidemiology of M. tuberculosis and Pulmonary NTM Disease
3. The Effects of Reactive Oxygen Species in General Bacteria and Mycobacteria
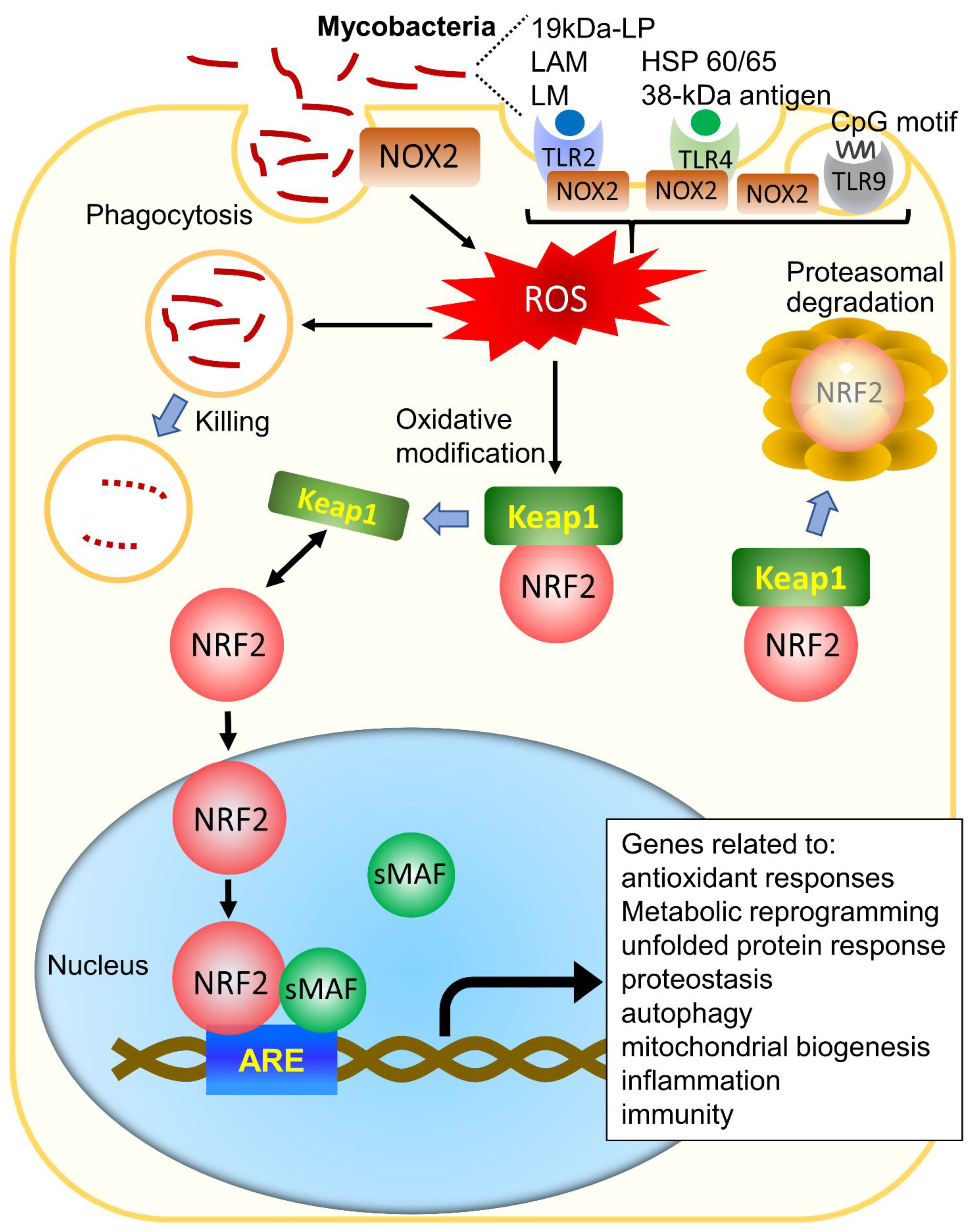
4. The Role of NRF2 in the Regulation of Oxidative Stress and Its Role Other than the Antioxidant Effect
5. The Role of NRF2 in Tuberculous Infection
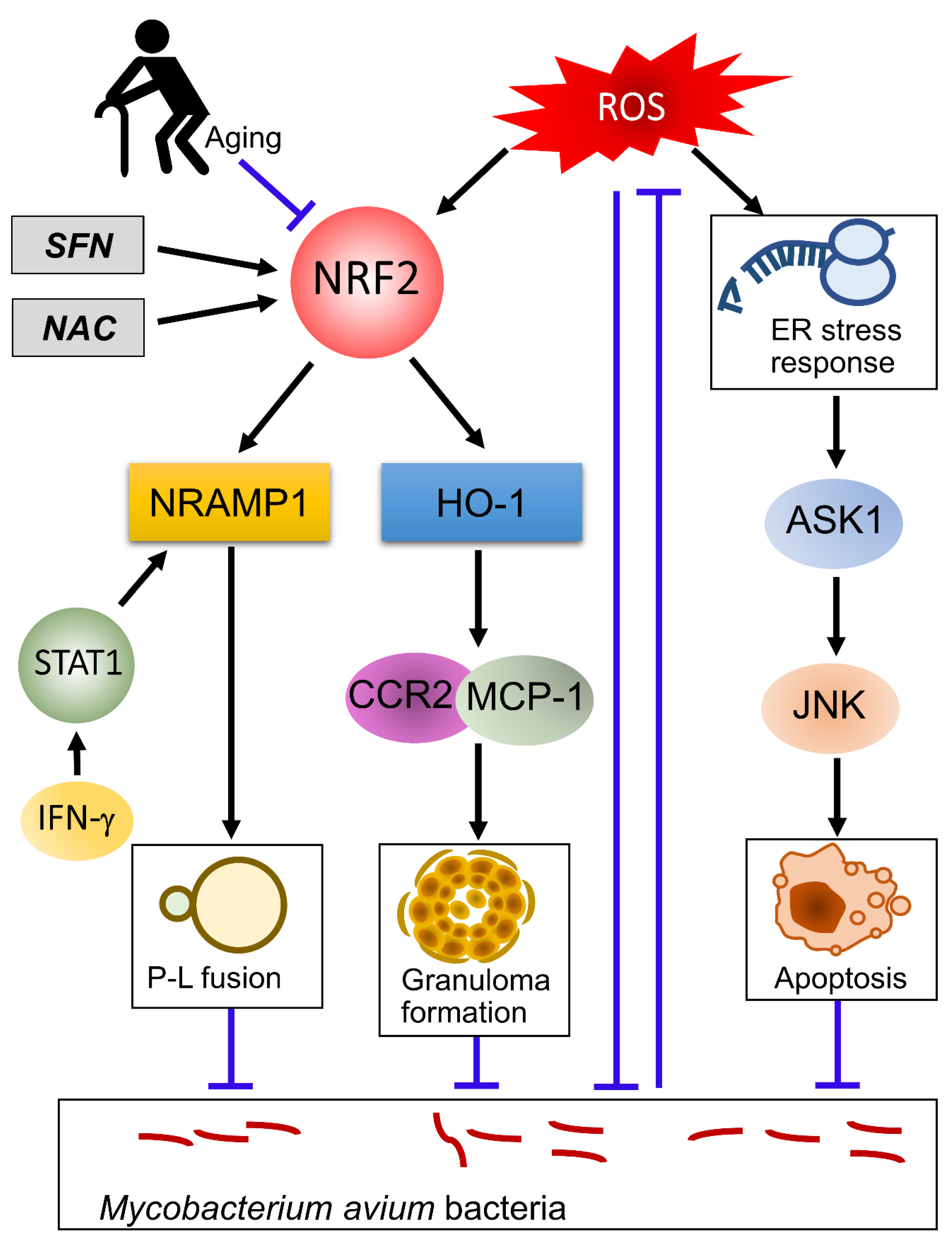
6. The Role of NRF2 in NTM Infection
7. Aging and NRF2 in Mycobacterial Infection
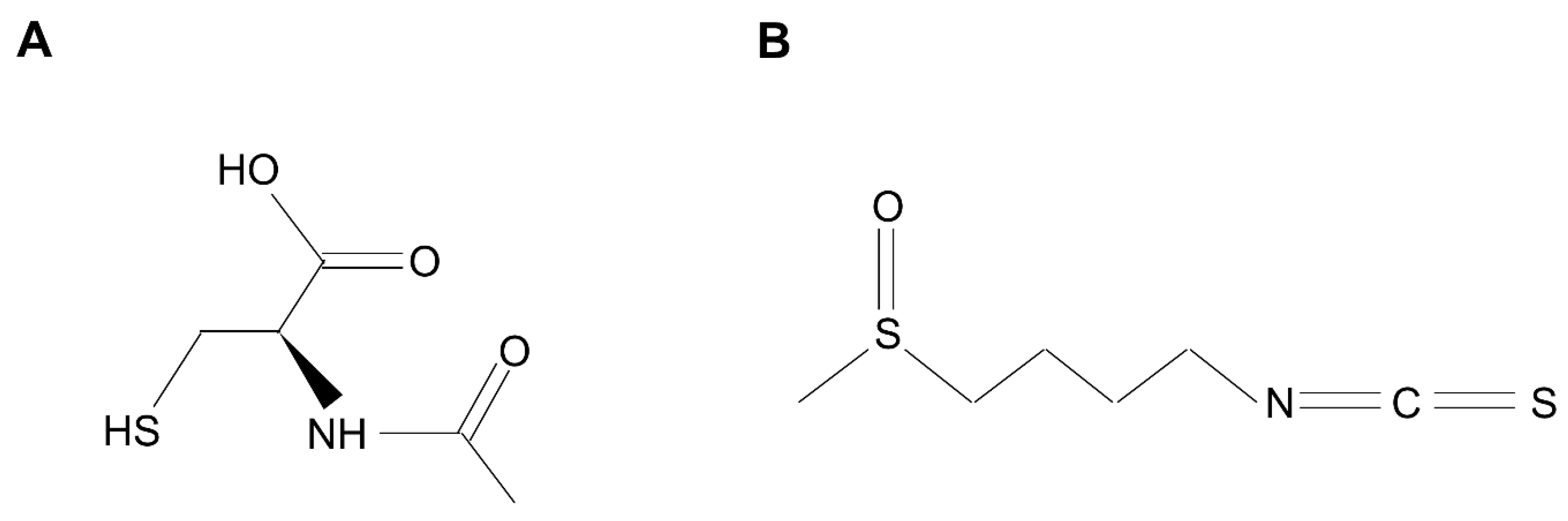
8. Developing New Therapeutic Options Targeting NRF2 and Oxidative Stress for NTM and TB Infection
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.C.; Kang, M.J.; Han, C.H.; Lee, S.M.; Kim, C.J.; Lee, J.M.; Kang, Y.A. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: A nationwide population-based study. BMC Pulm. Med. 2019, 19, 140. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef]
- Loewenberg, S. India reports cases of totally drug-resistant tuberculosis. Lancet 2012, 379, 205. [Google Scholar] [CrossRef]
- Martial, N.T.; Mubarik, S.; Yu, C. Long-term trends of tuberculosis incidence and mortality in four central African countries. Sci. Rep. 2021, 11, 16624. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med. Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 14 July 2021).
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- Zignol, M.; van Gemert, W.; Falzon, D.; Sismanidis, C.; Glaziou, P.; Floyd, K.; Raviglione, M. Surveillance of anti-tuberculosis drug resistance in the world: An updated analysis, 2007–2010. Bull. World Health Organ. 2012, 90, 111–119. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, S.; Wang, L.; Chin, D.P.; Wang, S.; Jiang, G.; Xia, H.; Zhou, Y.; Li, Q.; Ou, X.; et al. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med. 2012, 366, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Beer, J.; Emmrich, F.; Sack, U.; Rodloff, A.C. Analysis of gene mutations associated with isoniazid, rifampicin and ethambutol resistance among Mycobacterium tuberculosis isolates from Ethiopia. BMC Infect. Dis. 2012, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef]
- Silva, M.T. When two is better than one: Macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010, 87, 93–106. [Google Scholar] [CrossRef]
- Prasla, Z.; Sutliff, R.L.; Sadikot, R.T. Macrophage Signaling Pathways in Pulmonary Nontuberculous Mycobacteria Infections. Am. J. Respir. Cell Mol. Biol. 2020, 63, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Brown, G.D. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 2011, 14, 392–399. [Google Scholar] [CrossRef]
- Basu, J.; Shin, D.M.; Jo, E.K. Mycobacterial signaling through toll-like receptors. Front. Cell. Infect. Microbiol. 2012, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Senaratne, R.H.; Hoppstadter, J.; Diesel, B.; Riley, L.W.; Tabeta, K.; Bauer, S.; Beutler, B.; Zuraw, B.L. Attenuated activation of macrophage TLR9 by DNA from virulent mycobacteria. J. Innate Immun. 2009, 1, 29–45. [Google Scholar] [CrossRef]
- Hossain, M.M.; Norazmi, M.N. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection—The double-edged sword? BioMed Res. Int. 2013, 2013, 179174. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Ogier-Denis, E.; Mkaddem, S.B.; Vandewalle, A. NOX enzymes and Toll-like receptor signaling. Semin. Immunopathol. 2008, 30, 291–300. [Google Scholar] [CrossRef]
- Li, P.; Chang, M. Roles of PRR-Mediated Signaling Pathways in the Regulation of Oxidative Stress and Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7688. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, H.; Tomioka, H.; Jidoi, J. Susceptibility of micro-organisms to active oxygen species: Sensitivity to the xanthine-oxidase-mediated antimicrobial system. J. Gen. Microbiol. 1987, 133, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Saito, H.; Tomioka, H.; Jidoi, J. Relationship between the susceptibility of various bacteria to active oxygen species and to intracellular killing by macrophages. J. Gen. Microbiol. 1987, 133, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Farhana, A.; Guidry, L.; Saini, V.; Hondalus, M.; Steyn, A.J. Redox homeostasis in mycobacteria: The key to tuberculosis control? Expert Rev. Mol. Med. 2011, 13, e39. [Google Scholar] [CrossRef]
- Hinchey, J.; Lee, S.; Jeon, B.Y.; Basaraba, R.J.; Venkataswamy, M.M.; Chen, B.; Chan, J.; Braunstein, M.; Orme, I.M.; Derrick, S.C.; et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 2007, 117, 2279–2288. [Google Scholar] [CrossRef]
- Oberley-Deegan, R.E.; Rebits, B.W.; Weaver, M.R.; Tollefson, A.K.; Bai, X.; McGibney, M.; Ovrutsky, A.R.; Chan, E.D.; Crapo, J.D. An oxidative environment promotes growth of Mycobacterium abscessus. Free Radic. Biol. Med. 2010, 49, 1666–1673. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, B.J.; Kook, Y.H.; Kim, B.J. Phagosome Escape of Rough Mycobacterium abscessus Strains in Murine Macrophage via Phagosomal Rupture Can Lead to Type I Interferon Production and Their Cell-To-Cell Spread. Front. Immunol. 2019, 10, 125. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, B.J.; Kook, Y.H.; Kim, B.J. Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLoS Pathog. 2020, 16, e1008294. [Google Scholar] [CrossRef]
- McNamara, M.; Tzeng, S.C.; Maier, C.; Wu, M.; Bermudez, L.E. Surface-exposed proteins of pathogenic mycobacteria and the role of cu-zn superoxide dismutase in macrophages and neutrophil survival. Proteome Sci. 2013, 11, 45. [Google Scholar] [CrossRef]
- McNamara, M.; Tzeng, S.C.; Maier, C.; Zhang, L.; Bermudez, L.E. Surface proteome of “Mycobacterium avium subsp. hominissuis” during the early stages of macrophage infection. Infect. Immun. 2012, 80, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Danelishvili, L.; Wagner, D.; Petrofsky, M.; Bermudez, L.E. Identification of virulence determinants of Mycobacterium avium that impact on the ability to resist host killing mechanisms. J. Med. Microbiol. 2010, 59, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Yang, C.S.; Shin, D.M.; Lee, H.M.; Son, J.W.; Lee, S.J.; Akira, S.; Gougerot-Pocidalo, M.A.; El-Benna, J.; Ichijo, H.; Jo, E.K. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell. Microbiol. 2008, 10, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Noguchi, T.; Naguro, I.; Ichijo, H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 199–225. [Google Scholar] [CrossRef]
- Miller, J.L.; Velmurugan, K.; Cowan, M.J.; Briken, V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog. 2010, 6, e1000864. [Google Scholar] [CrossRef]
- Go, D.; Lee, J.; Choi, J.A.; Cho, S.N.; Kim, S.H.; Son, S.H.; Song, C.H. Reactive oxygen species-mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium-infected macrophages by activating regulated IRE1-dependent decay pathway. Cell. Microbiol. 2019, 21, e13094. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Choi, H.G.; Son, Y.J.; Whang, J.; Kim, K.; Jeon, H.S.; Park, H.S.; Back, Y.W.; Choi, S.; Kim, S.W.; et al. Mycobacterium avium MAV2052 protein induces apoptosis in murine macrophage cells through Toll-like receptor 4. Apoptosis 2016, 21, 459–472. [Google Scholar] [CrossRef]
- Lee, K.I.; Whang, J.; Choi, H.G.; Son, Y.J.; Jeon, H.S.; Back, Y.W.; Park, H.S.; Paik, S.; Park, J.K.; Choi, C.H.; et al. Mycobacterium avium MAV2054 protein induces macrophage apoptosis by targeting mitochondria and reduces intracellular bacterial growth. Sci. Rep. 2016, 6, 37804. [Google Scholar] [CrossRef]
- Bruns, H.; Stenger, S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Future Microbiol. 2014, 9, 327–341. [Google Scholar] [CrossRef]
- Whang, J.; Back, Y.W.; Lee, K.I.; Fujiwara, N.; Paik, S.; Choi, C.H.; Park, J.K.; Kim, H.J. Mycobacterium abscessus glycopeptidolipids inhibit macrophage apoptosis and bacterial spreading by targeting mitochondrial cyclophilin D. Cell Death Dis. 2017, 8, e3012. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Shin, S.J. Genetic Involvement of Mycobacterium avium Complex in the Regulation and Manipulation of Innate Immune Functions of Host Cells. Int. J. Mol. Sci. 2021, 22, 3011. [Google Scholar] [CrossRef] [PubMed]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Alejo, N.; Santos-Argumedo, L. Innate defects of the IL-12/IFN-γ axis in susceptibility to infections by mycobacteria and salmonella. J. Interferon Cytokine Res. 2014, 34, 307–317. [Google Scholar] [CrossRef]
- Holland, S.M.; Pierce, V.M.; Shailam, R.; Glomski, K.; Farmer, J.R. Case 28-2017. A 13-Month-Old Girl with Pneumonia and a 33-Year-Old Woman with Hip Pain. N. Engl. J. Med. 2017, 377, 1077–1091. [Google Scholar] [CrossRef]
- Wu, U.I.; Holland, S.M. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect. Dis. 2015, 15, 968–980. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef]
- Türei, D.; Papp, D.; Fazekas, D.; Földvári-Nagy, L.; Módos, D.; Lenti, K.; Csermely, P.; Korcsmáros, T. NRF2-ome: An integrated web resource to discover protein interaction and regulatory networks of NRF2. Oxid. Med. Cell. Longev. 2013, 2013, 737591. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Ohl, K.; Fragoulis, A.; Klemm, P.; Baumeister, J.; Klock, W.; Verjans, E.; Böll, S.; Möllmann, J.; Lehrke, M.; Costa, I.; et al. Nrf2 Is a Central Regulator of Metabolic Reprogramming of Myeloid-Derived Suppressor Cells in Steady State and Sepsis. Front. Immunol. 2018, 9, 1552. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Kikuchi, N.; Ishii, Y.; Morishima, Y.; Yageta, Y.; Haraguchi, N.; Itoh, K.; Yamamoto, M.; Hizawa, N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010, 11, 31. [Google Scholar] [CrossRef]
- Lv, H.; Yang, H.; Wang, Z.; Feng, H.; Deng, X.; Cheng, G.; Ci, X. Nrf2 signaling and autophagy are complementary in protecting lipopolysaccharide/d-galactosamine-induced acute liver injury by licochalcone A. Cell Death Dis. 2019, 10, 313. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, Z.H.; Talukder, M.; Lin, J.; Li, X.N.; Zhang, C.; Li, J.L. Crosstalk between unfolded protein response and Nrf2-mediated antioxidant defense in Di-(2-ethylhexyl) phthalate-induced renal injury in quail (Coturnix japonica). Environ. Pollut. 2018, 242, 1871–1879. [Google Scholar] [CrossRef]
- Vijayamalini, M.; Manoharan, S. Lipid peroxidation, vitamins C, E and reduced glutathione levels in patients with pulmonary tuberculosis. Cell Biochem. Funct. 2004, 22, 19–22. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Kirk, N.M.; Ackart, D.F.; Shanley, C.A.; Orme, I.M.; Basaraba, R.J. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS ONE 2011, 6, e26254. [Google Scholar] [CrossRef]
- Rockwood, N.; Costa, D.L.; Amaral, E.P.; Du Bruyn, E.; Kubler, A.; Gil-Santana, L.; Fukutani, K.F.; Scanga, C.A.; Flynn, J.L.; Jackson, S.H.; et al. Mycobacterium tuberculosis Induction of Heme Oxygenase-1 Expression Is Dependent on Oxidative Stress and Reflects Treatment Outcomes. Front. Immunol. 2017, 8, 542. [Google Scholar] [CrossRef]
- Brodin, P.; Rosenkrands, I.; Andersen, P.; Cole, S.T.; Brosch, R. ESAT-6 proteins: Protective antigens and virulence factors? Trends Microbiol. 2004, 12, 500–508. [Google Scholar] [CrossRef] [PubMed]
- MacGurn, J.A.; Cox, J.S. A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect. Immun. 2007, 75, 2668–2678. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Bottai, D.; Brosch, R. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr. Opin. Microbiol. 2009, 12, 4–10. [Google Scholar] [CrossRef]
- Van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Aggerbeck, H.; Madsen, S.M. Safety of ESAT-6. Tuberculosis 2006, 86, 363–373. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Chinta, K.C.; Rahman, M.A.; Saini, V.; Glasgow, J.N.; Reddy, V.P.; Lever, J.M.; Nhamoyebonde, S.; Leslie, A.; Wells, R.M.; Traylor, A.; et al. Microanatomic Distribution of Myeloid Heme Oxygenase-1 Protects against Free Radical-Mediated Immunopathology in Human Tuberculosis. Cell Rep. 2018, 25, 1938–1952.e1935. [Google Scholar] [CrossRef]
- Costa, D.L.; Namasivayam, S.; Amaral, E.P.; Arora, K.; Chao, A.; Mittereder, L.R.; Maiga, M.; Boshoff, H.I.; Barry, C.E., 3rd; Goulding, C.W.; et al. Pharmacological Inhibition of Host Heme Oxygenase-1 Suppresses Mycobacterium tuberculosis Infection In Vivo by a Mechanism Dependent on T Lymphocytes. mBio 2016, 7, e01675-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, S.; Liu, Q.; Wang, Y.; Ji, G.; Sandford, A.J.; He, J.Q. Association of heme oxygenase-1 single nucleotide polymorphisms with susceptibility to tuberculosis in Chinese Han population. J. Clin. Lab. Anal. 2020, 34, e23276. [Google Scholar] [CrossRef]
- Qian, Z.; Lv, J.; Kelly, G.T.; Wang, H.; Zhang, X.; Gu, W.; Yin, X.; Wang, T.; Zhou, T. Expression of nuclear factor, erythroid 2-like 2-mediated genes differentiates tuberculosis. Tuberculosis 2016, 99, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Yamamoto, M.; Sugawara, I. Significant reduction of granulomas in Nrf2-deficient mice infected with Mycobacterium tuberculosis. Indian J. Tuberc. 2010, 57, 108–113. [Google Scholar]
- Rothchild, A.C.; Olson, G.S.; Nemeth, J.; Amon, L.M.; Mai, D.; Gold, E.S.; Diercks, A.H.; Aderem, A. Alveolar macrophages generate a noncanonical NRF2-driven transcriptional response to Mycobacterium tuberculosis in vivo. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Bonay, M.; Roux, A.L.; Floquet, J.; Retory, Y.; Herrmann, J.L.; Lofaso, F.; Deramaudt, T.B. Caspase-independent apoptosis in infected macrophages triggered by sulforaphane via Nrf2/p38 signaling pathways. Cell Death Discov. 2015, 1, 15022. [Google Scholar] [CrossRef]
- Bonay, M.; Deramaudt, T.B. Nrf2: New insight in cell apoptosis. Cell Death Dis. 2015, 6, e1897. [Google Scholar] [CrossRef]
- Awuh, J.A.; Haug, M.; Mildenberger, J.; Marstad, A.; Do, C.P.; Louet, C.; Stenvik, J.; Steigedal, M.; Damås, J.K.; Halaas, Ø.; et al. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E4272–E4280. [Google Scholar] [CrossRef]
- Regev, D.; Surolia, R.; Karki, S.; Zolak, J.; Montes-Worboys, A.; Oliva, O.; Guroji, P.; Saini, V.; Steyn, A.J.; Agarwal, A.; et al. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Lab. Investig. 2012, 92, 1541–1552. [Google Scholar] [CrossRef]
- Silva-Gomes, S.; Appelberg, R.; Larsen, R.; Soares, M.P.; Gomes, M.S. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect. Immun. 2013, 81, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Surolia, R.; Karki, S.; Wang, Z.; Kulkarni, T.; Li, F.J.; Vohra, S.; Batra, H.; Nick, J.A.; Duncan, S.R.; Thannickal, V.J.; et al. Attenuated heme oxygenase-1 responses predispose the elderly to pulmonary nontuberculous mycobacterial infections. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L928–L940. [Google Scholar] [CrossRef]
- Nakajima, M.; Matsuyama, M.; Kawaguchi, M.; Kiwamoto, T.; Matsuno, Y.; Morishima, Y.; Yoshida, K.; Sherpa, M.; Yazaki, K.; Osawa, H.; et al. Nrf2 Regulates Granuloma Formation and Macrophage Activation during Mycobacterium avium Infection via Mediating Nramp1 and HO-1 Expressions. mBio 2021, 12, e01947-20. [Google Scholar] [CrossRef]
- Koh, W.J.; Kwon, O.J.; Kim, E.J.; Lee, K.S.; Ki, C.S.; Kim, J.W. NRAMP1 gene polymorphism and susceptibility to nontuberculous mycobacterial lung diseases. Chest 2005, 128, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, N.S.; Horsburgh, C.R., Jr. Prevention of tuberculosis in older adults in the United States: Obstacles and opportunities. Clin. Infect. Dis. 2013, 56, 1240–1247. [Google Scholar] [CrossRef]
- Jhun, B.W.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Yoo, H.; Carriere, K.C.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: A 15-year follow-up study. Eur. Respir. J. 2020, 55, 1900798. [Google Scholar] [CrossRef]
- Han, S.N.; Adolfsson, O.; Lee, C.K.; Prolla, T.A.; Ordovas, J.; Meydani, S.N. Age and vitamin E-induced changes in gene expression profiles of T cells. J. Immunol. 2006, 177, 6052–6061. [Google Scholar] [CrossRef]
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef]
- Vilchèze, C.; Kim, J.; Jacobs, W.R., Jr. Vitamin C Potentiates the Killing of Mycobacterium tuberculosis by the First-Line Tuberculosis Drugs Isoniazid and Rifampin in Mice. Antimicrob. Agents Chemother. 2018, 62, e02165-17. [Google Scholar] [CrossRef] [PubMed]
- Andosca, J.B.; Foley, J.A. Calcium ribonate and vitamin C (Nu 240-10) in the treatment of tuberculosis. Dis. Chest 1948, 14, 107–114. [Google Scholar] [CrossRef][Green Version]
- Volchegorskiĭ, I.A.; Novoselov, P.N.; Astakhova, T.V. The effectiveness of ascorbic acid and emoxipin in treatment of infiltrative pulmonary tuberculosis. Klin. Med. 2007, 85, 55–58. [Google Scholar]
- Sadowska, A.M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4628–4633. [Google Scholar] [CrossRef]
- Venketaraman, V.; Rodgers, T.; Linares, R.; Reilly, N.; Swaminathan, S.; Hom, D.; Millman, A.C.; Wallis, R.; Connell, N.D. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Res. Ther. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Mahakalkar, S.M.; Nagrale, D.; Gaur, S.; Urade, C.; Murhar, B.; Turankar, A. N-acetylcysteine as an add-on to Directly Observed Therapy Short-I therapy in fresh pulmonary tuberculosis patients: A randomized, placebo-controlled, double-blinded study. Perspect. Clin. Res. 2017, 8, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Hayati Roodbari, N.; Nasr-Esfahani, M.H. The Effect of N-Acetyl-Cysteine on NRF2 Antioxidant Gene Expression in Asthenoteratozoospermia Men: A Clinical Trial Study. Int. J. Fertil. Steril. 2020, 14, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef]
- Shiozawa, A.; Kajiwara, C.; Ishii, Y.; Tateda, K. N-acetyl-cysteine mediates protection against Mycobacterium avium through induction of human β-defensin-2 in a mouse lung infection model. Microbes Infect. 2020, 22, 567–575. [Google Scholar] [CrossRef]
- Gao, X.P.; Standiford, T.J.; Rahman, A.; Newstead, M.; Holland, S.M.; Dinauer, M.C.; Liu, Q.H.; Malik, A.B. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: Studies in p47phox-/- and gp91phox-/- mice. J. Immunol. 2002, 168, 3974–3982. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Thimmulappa, R.K.; Sethi, S.; Kong, X.; Yarmus, L.; Brown, R.H.; Feller-Kopman, D.; Wise, R.; Biswal, S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011, 3, 78ra32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuyama, M.; Nonaka, M.; Nakajima, M.; Morishima, Y.; Ishii, Y.; Hizawa, N. The Role of NRF2 in Mycobacterial Infection. Antioxidants 2021, 10, 1861. https://doi.org/10.3390/antiox10121861
Matsuyama M, Nonaka M, Nakajima M, Morishima Y, Ishii Y, Hizawa N. The Role of NRF2 in Mycobacterial Infection. Antioxidants. 2021; 10(12):1861. https://doi.org/10.3390/antiox10121861
Chicago/Turabian StyleMatsuyama, Masashi, Mizu Nonaka, Masayuki Nakajima, Yuko Morishima, Yukio Ishii, and Nobuyuki Hizawa. 2021. "The Role of NRF2 in Mycobacterial Infection" Antioxidants 10, no. 12: 1861. https://doi.org/10.3390/antiox10121861
APA StyleMatsuyama, M., Nonaka, M., Nakajima, M., Morishima, Y., Ishii, Y., & Hizawa, N. (2021). The Role of NRF2 in Mycobacterial Infection. Antioxidants, 10(12), 1861. https://doi.org/10.3390/antiox10121861





