Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile—A Cohort Study of a Caucasian Population of Polish Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants of the Study Cohort
2.2. Anthropometric Measurements and Body Composition Analysis
2.3. Oral Glucose Tolerance Test (OGTT) Performance
2.4. Blood Collection and Biochemical Analysis
2.5. Calculations
2.6. Daily Physical Activity and Dietary Intake Analyses
2.7. Genetic Analysis
2.8. Ethics Statement
2.9. Statistical Analysis
3. Results
| rs3751812 | rs8050136 | |||||||
|---|---|---|---|---|---|---|---|---|
| G/G | G/T | T/T | p Value | C/C | A/C | A/A | p Value | |
| N | 211 | 420 | 181 | 209 | 424 | 182 | ||
| Genotype frequency | 0.26 | 0.52 | 0.22 | >0.05 | 0.26 | 0.52 | 0.22 | >0.05 |
| Age (years) | 40.5 (14.2) | 41.2 (14.7) | 39.5 (14.3) | 0.33 | 40.2 (14.1) | 41.3 (14.8) | 39.4 (14.3) | 0.24 |
| BMI (kg/m2) | 27.6 (6.0) | 28.7 (6.8) | 28.9 (6.8) | 0.060 | 27.6 (6.1) | 28.7 (6.8) | 28.9 (6.8) | 0.063 |
| 81 (38.8%) | 136 (32.9%) | 53 (29.9%) | 0.410 | 80 (38.6%) | 138 (33.2%) | 54 (30.2%) | 0.422 |
| 69 (33.0%) | 141 (34.1%) | 66 (37.3%) | 69 (33.3%) | 140 (33.7%) | 67 (37.4%) | |||
| 59 (28.2%) | 136 (32.9%) | 58 (32.8%) | 58 (28.0%) | 138 (33.2%) | 58 (32.4%) | |||
| Total body fat content (kg) | 25.3 (12.4) | 27.6 (13.8) | 28.2 (15.2) | 0.080 | 25.3 (12.4) | 27.5 (13.8) | 28.2 (15.2) | 0.095 |
| Total body fat content (%) | 30.6 (9.1) | 31.8 (9.6) | 31.6 (10.3) | 0.377 | 30.6 (9.1) | 31.8 (9.6) | 31.6 (10.4) | 0.465 |
| Waist circumference (cm) | 94.3 (17.5) | 96.7 (17.2) | 97.5 (16.7) | 0.054 | 94.2 (17.6) | 96.7 (17.2) | 97.4 (16.6) | 0.053 |
| Hip circumference (cm) | 101.3 (12.4) | 104.2 (13.0) | 103.8 (12.5) | 0.008 | 101.2 (12.5) | 104.1 (13.0) | 103.8 (12.4) | 0.008 |
| WHR | 0.927 (0.091) | 0.925 (0.088) | 0.937 (0.085) | 0.327 | 0.927 (0.092) | 0.925 (0.088) | 0.936 (0.085) | 0.382 |
| Visceral fat (cm3) | 103.0 (81.0) | 110.0 (79.9) | 112.3 (83.0) | 0.379 | 103.3 (81.5) | 110.2 (80.0) | 111.8 (82.5) | 0.381 |
| Visceral fat (%) | 36.4 (11.8) | 37.5 (12.4) | 37.2 (11.7) | 0.587 | 36.4 (11.7) | 37.6 (12.5) | 37.1 (11.6) | 0.570 |
| Subcutaneous fat (cm3) | 163.5 (83.1) | 167.2 (80.5) | 175.0 (82.7) | 0.401 | 163.7 (83.5) | 166.9 (80.8) | 175.3 (83.1) | 0.405 |
| Subcutaneous fat (%) | 63.7 (11.7) | 62.3 (12.9) | 62.8 (11.7) | 0.557 | 63.7 (11.6) | 62.2 (13.0) | 62.9 (11.6) | 0.540 |
| Visceral/subcutaneous fat ratio | 0.642 (0.406) | 0.687 (0.475) | 0.665 (0.413) | 0.554 | 0.641 (0.404) | 0.690 (0.477) | 0.662 (0.410) | 0.536 |
| Total cholesterol (mg/dL) | 202.7 (56.0) | 191.7 (41.3) | 194.0 (43.2) | 0.070 | 201.9 (56.1) | 192.1 (41.4) | 193.7 (43.1) | 0.153 |
| HDL (mg/dL) | 60.7 (14.1) | 59.8 (15.6) | 59.5 (14.5) | 0.662 | 60.8 (14.0) | 59.6 (15.7) | 59.7 (14.4) | 0.422 |
| LDL (mg/dL) | 117.3 (43.3) | 109.4 (37.8) | 111.2 (41.8) | 0.095 | 116.3 (43.3) | 109.9 (37.9) | 111.1 (41.8) | 0.189 |
| TG (mg/dL) | 123.8 (143.9) | 111.9 (69.7) | 116.3 (61.9) | 0.289 | 124.1 (144.3) | 113.2 (71.1) | 115.1 (61.3) | 0.491 |
| Frequency of prediabetes or diabetes | ||||||||
| 103 (48.8%) | 209 (49.8%) | 95 (52.5%) | 0.751 | 100 (47.8%) | 213 (50.2%) | 96 (52.7%) | 0.628 |
| 108 (51.2%) | 211 (50.2%) | 86 (47.5%) | 109 (52.2%) | 211 (49.8%) | 86 (47.3%) | |||
| Corrected insulin response level during OGTT (10 × mU × mL × mg−2) | ||||||||
| 0.8 (0.7) | 0.8 (0.7) | 0.9 (1.0) | 0.875 | 0.8 (0.7) | 0.8 (0.7) | 0.9 (1.0) | 0.884 |
| 0.7 (1.1) | 0.6 (0.6) | 0.7 (0.8) | 0.325 | 0.7 (1.1) | 0.6 (0.6) | 0.7 (0.8) | 0.325 | |
| 1.0 (0.7) | 1.3 (1.0) | 1.5 (1.6) | 0.554 | 1.1 (0.9) | 1.2 (1.0) | 1.5 (1.6) | 0.764 | |
| Daily energy intake (kcal) | 1807.2 (732.3) | 1766.9 (676.0) | 1837.4 (713.4) | 0.849 | 1820.5 (734.9) | 1759.6 (673.3) | 1853.9 (716.3) | 0.645 |
| Dietary fiber intake (g) | 18.5 (7.2) | 18.5 (8.2) | 18.3 (7.6) | 0.901 | 18.6 (7.2) | 18.5 (8.2) | 18.4 (7.7) | 0.958 |
| Daily physical activity level | ||||||||
| 16 (7.6%) | 25 (6.0%) | 18 (9.9%) | 0.302 | 16 (7.7%) | 25 (5.9%) | 19 (10.4%) | 0.179 |
| 50 (23.7%) | 83 (19.8%) | 40 (22.1%) | 50 (23.9%) | 83 (19.6%) | 40 (22.0%) | |||
| 145 (68.7%) | 312 (74.3%) | 123 (68.0%) | 143 (68.4%) | 316 (74.5%) | 123 (67.6%) | |||
| rs6499640 | rs8044769 | |||||||
|---|---|---|---|---|---|---|---|---|
| A/A | A/G | G/G | p Value | C/C | C/T | T/T | p Value | |
| N | 307 | 377 | 134 | 270 | 406 | 138 | ||
| Genotype frequency | 0.37 | 0.46 | 0.16 | >0.05 | 0.33 | 0.50 | 0.17 | >0.05 |
| Age (years) | 41.0 (14.7) | 40.0 (14.2) | 41.4 (15.0) | 0.97 | 40.4 (14.8) | 41.2 (14.6) | 39.6 (13.7) | 0.54 |
| BMI (kg/m2) | 28.6 (6.8) | 28.6 (6.8) | 27.6 (5.8) | 0.358 | 28.5 (6.8) | 28.7 (6.7) | 27.8 (6.1) | 0.534 |
| 93 (30.8%) | 128 (34.5%) | 52 (39.4%) | 0.230 | 88 (33.2%) | 131 (32.8%) | 51 (37.5%) | 0.862 |
| 118 (39.1%) | 120 (32.3%) | 40 (30.3%) | 92 (34.7%) | 143 (35.8%) | 43 (31.6%) | |||
| 91 (30.1%) | 123 (33.2%) | 40 (30.3%) | 85 (32.1%) | 126 (31.5%) | 42 (30.9%) | |||
| Total body fat content (kg) | 27.5 (14.5) | 27.5 (14.1) | 24.9 (10.9) | 0.449 | 27.7 (15.0) | 27.2 (13.6) | 25.8 (11.9) | 0.676 |
| Total body fat content (%) | 31.6 (9.8) | 31.6 (9.7) | 30.6 (8.9) | 0.597 | 31.7 (10.1) | 31.4 (9.6) | 31.1 (9.0) | 0.893 |
| Waist circumference (cm) | 96.8 (17.6) | 96.7 (17.4) | 93.5 (15.5) | 0.255 | 96.2 (17.2) | 96.8 (17.3) | 94.6 (16.8) | 0.429 |
| Hip circumference (cm) | 103.4 (12.9) | 104.2 (13.3) | 100.7 (10.4) | 0.080 | 103.3 (12.6) | 104.0 (13.0) | 101.5 (12.2) | 0.189 |
| WHR | 0.933 (0.089) | 0.925 (0.085) | 0.925 (0.097) | 0.485 | 0.928 (0.089) | 0.927 (0.088) | 0.929 (0.089) | 0.980 |
| Visceral fat (cm3) | 109.6 (84.1) | 110.2 (81.3) | 101.7 (72.1) | 0.822 | 109.2 (78.3) | 110.3 (83.7) | 99.8 (72.5) | 0.648 |
| Visceral fat (%) | 37.3 (11.8) | 36.5 (12.0) | 38.2 (12.8) | 0.483 | 37.7 (11.8) | 36.9 (12.3) | 36.4 (12.0) | 0.617 |
| Subcutaneous fat (cm3) | 168.9 (85.4) | 173.4 (82.6) | 151.5 (70.0) | 0.124 | 168.6 (83.3) | 169.7 (82.4) | 160.9 (74.2) | 0.773 |
| Subcutaneous fat (%) | 62.7 (11.8) | 63.3 (12.6) | 61.9 (12.7) | 0.503 | 62.3 (11.8) | 63.0 (12.8) | 63.7 (11.8) | 0.598 |
| Visceral/subcutaneous fat ratio | 0.670 (0.410) | 0.655 (0.459) | 0.706 (0.472) | 0.486 | 0.689 (0.492) | 0.662 (0.421) | 0.641 (0.408) | 0.590 |
| Total cholesterol (mg/dL) | 195.3 (51.1) | 193.8 (42.3) | 196.9 (44.4) | 0.646 | 193.8 (40.1) | 191.7 (42.7) | 206.7 (62.7) | 0.029 |
| LDL (mg/dL) | 111.0 (39.6) | 111.1 (40.3) | 114.9 (41.7) | 0.487 | 111.0 (38.6) | 109.6 (38.7) | 119.8 (46.5) | 0.058 |
| HDL (mg/dL) | 60.2 (14.6) | 60.3 (15.8) | 58.3 (13.4) | 0.289 | 60.7 (14.7) | 59.2 (15.5) | 60.4 (13.8) | 0.176 |
| TG (mg/dL) | 120.5 (124.2) | 111.8 (67.8) | 118.6 (71.1) | 0.584 | 110.5 (58.7) | 114.6 (73.4) | 132.6 (170.6) | 0.689 |
| Frequency of prediabetes or diabetes | ||||||||
| 152 (49.5%) | 187 (49.6%) | 71 (53.0%) | 0.796 | 143 (53.0%) | 201 (49.5%) | 64 (46.4%) | 0.420 |
| 155 (50.5%) | 190 (50.4%) | 63 (47.0%) | 127 (47.0%) | 205 (50.5%) | 74 (53.6%) | |||
| Corrected insulin response level during OGTT (10 × mU × mL × mg−2) | ||||||||
| 0.8 (0.9) | 0.8 (0.7) | 0.9 (0.8) | 0.616 | 0.9 (0.9) | 0.8 (0.7) | 0.8 (0.7) | 0.618 |
| 0.6 (0.7) | 0.7 (0.8) | 0.7 (0.7) | 0.745 | 0.7 (0.7) | 0.7 (0.9) | 0.5 (0.5) | 0.194 | |
| 1.4 (1.4) | 1.2 (1.0) | 1.2 (1.0) | 0.949 | 1.3 (1.3) | 1.4 (1.0) | 1.1 (0.8) | 0.639 | |
| Daily energy intake (kcal) | 1786.5 (705.2) | 1825.7 (725.8) | 1720.7 (594.6) | 0.728 | 1775.7 (646.2) | 1792.7 (735.8) | 1816.0 (686.3) | 0.791 |
| Dietary fiber intake (g) | 18.1 (7.1) | 19.0 (8.5) | 17.9 (7.4) | 0.570 | 18.1 (7.4) | 18.8 (8.2) | 18.2 (7.4) | 0.598 |
| Daily physical activity level | ||||||||
| 22 (7.2%) | 30 (8.0%) | 8 (6.0%) | 0.736 | 23 (8.5%) | 26 (6.4%) | 10 (7.2%) | 0.060 |
| 68 (22.1%) | 81 (21.5%) | 24 (17.9%) | 55 (20.4%) | 77 (19.0%) | 41 (29.7%) | |||
| 217 (70.7%) | 266 (70.6%) | 102 (76.1%) | 192 (71.1%) | 303 (74.6%) | 87 (63.0%) | |||
| rs7190492 | ||||
|---|---|---|---|---|
| G/G | A/G | A/A | p Value | |
| N | 374 | 358 | 83 | |
| Genotype frequency | 0.46 | 0.44 | 0.10 | >0.05 |
| Age (years) | 40.4 (14.8) | 40.8 (14.4) | 40.4 (13.6) | 1.0 |
| BMI (kg/m2) | 28.7 (6.8) | 28.3 (6.5) | 28.1 (6.6) | 0.605 |
| 120 (32.5%) | 122 (34.8%) | 30 (36.1%) | 0.461 |
| 126 (34.1%) | 128 (36.5%) | 23 (27.7%) | ||
| 123 (33.3%) | 101 (28.8%) | 30 (36.1%) | ||
| Total body fat content (kg) | 27.8 (14.5) | 26.5 (13.4) | 26.6 (12.4) | 0.533 |
| Total body fat content (%) | 31.9 (10.0) | 30.8 (9.5) | 32.1 (8.8) | 0.303 |
| Waist circumference (cm) | 96.6 (17.3) | 96.2 (17.0) | 94.7 (17.6) | 0.585 |
| Hip circumference (cm) | 103.4 (12.7) | 103.6 (12.9) | 101.7 (12.5) | 0.588 |
| WHR | 0.930 (0.090) | 0.925 (0.086) | 0.927 (0.092) | 0.774 |
| Visceral fat (cm3) | 110.7 (83.0) | 106.6 (81.2) | 103.0 (67.1) | 0.777 |
| Visceral fat (%) | 37.4 (11.6) | 36.6 (12.5) | 37.5 (12.5) | 0.665 |
| Subcutaneous fat (cm3) | 170.4 (85.7) | 166.5 (78.9) | 160.7 (72.5) | 0.921 |
| Subcutaneous fat (%) | 62.4 (12.2) | 63.4 (12.5) | 62.7 (12.2) | 0.653 |
| Visceral/subcutaneous fat ratio | 0.674 (0.454) | 0.660 (0.434) | 0.676 (0.443) | 0.652 |
| Total cholesterol (mg/dL) | 192.9 (40.4) | 194.3 (51.0) | 206.0 (47.5) | 0.056 |
| LDL (mg/dL) | 110.1 (38.2) | 111.4 (41.0) | 119.6 (45.5) | 0.219 |
| HDL (mg/dL) | 60.4 (14.6) | 59.1 (15.4) | 61.0 (14.0) | 0.227 |
| TG (mg/dL) | 111.8 (62.8) | 118.6 (119.3) | 126.9 (86.2) | 0.422 |
| Frequency of prediabetes or diabetes | ||||
| 194 (51.9%) | 176 (49.2%) | 38 (45.8%) | 0.544 |
| 180 (48.1%) | 182 (50.8%) | 45 (54.2%) | ||
| Corrected insulin response level during OGTT (10 × mU × mL × mg−2) | ||||
| 0.8 (0.8) | 0.9 (0.7) | 0.9 (0.7) | 0.421 |
| 0.6 (0.6) | 0.8 (1.0) | 0.5 (0.4) | 0.428 | |
| 1.3 (1.3) | 1.3 (1.0) | 1.2 (0.9) | 0.828 | |
| Daily energy intake (kcal) | 1765.4 (660.6) | 1823.5 (728.6) | 1771.0 (744.1) | 0.609 |
| Dietary fiber intake (g) | 18.0 (6.8) | 18.8 (8.7) | 19.4 (8.4) | 0.586 |
| Daily physical activity level | ||||
| 29 (7.8%) | 23 (6.4%) | 8 (9.6%) | 0.626 |
| 75 (20.1%) | 75 (20.9%) | 21 (25.3%) | ||
| 270 (72.2%) | 260 (72.6%) | 54 (65.1%) | ||
3.1. General Characteristic of Studied Population Stratified by Genotypes
3.2. Assessment of Dietary Intake
3.3. Associations between rs3751812 Polymorphism, Anthropometric Measurements, Lipid Profile, and Dietary Fiber Intake
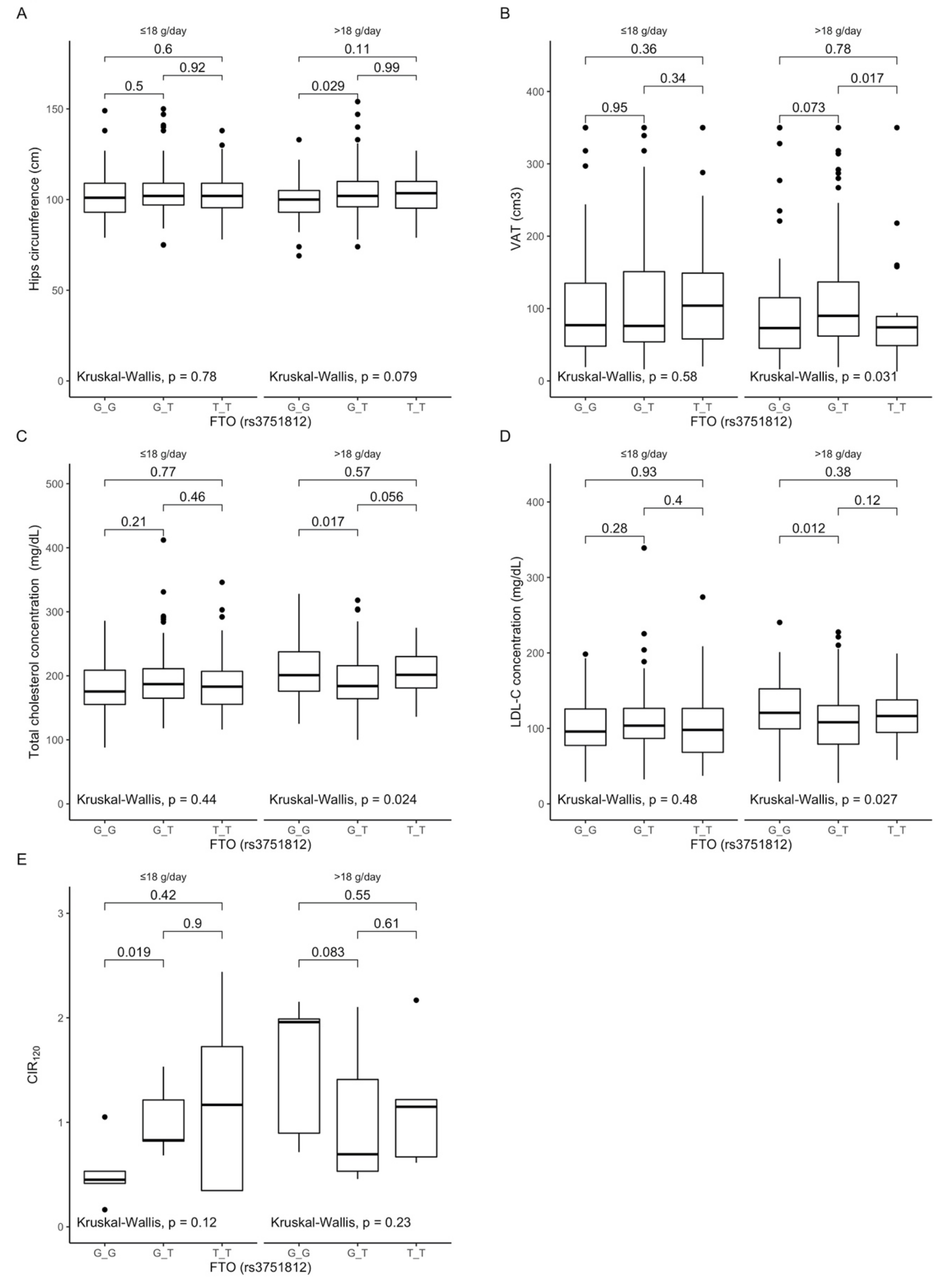
3.4. Associations between rs8050136 Polymorphism, Anthropometric Measurements, Lipid Profile, and Dietary Fiber Intake

3.5. Associations between rs6499640 Polymorphism, Anthropometric Measurements, Lipid Profile, and Dietary Fiber Intake
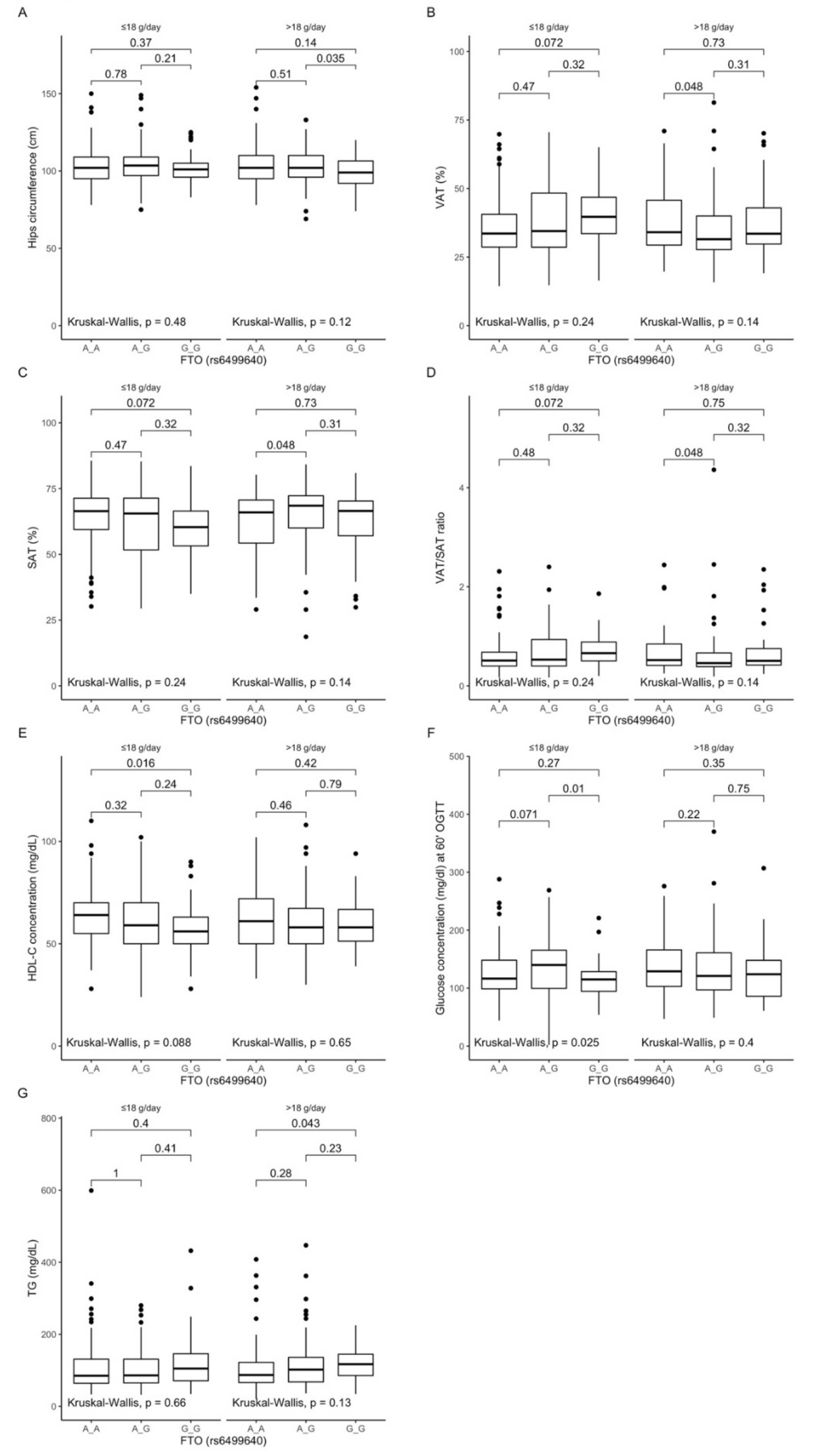
3.6. Associations between rs8044769 Polymorphism, Anthropometric Measurements, Lipid Profile, and Dietary Fiber Intake
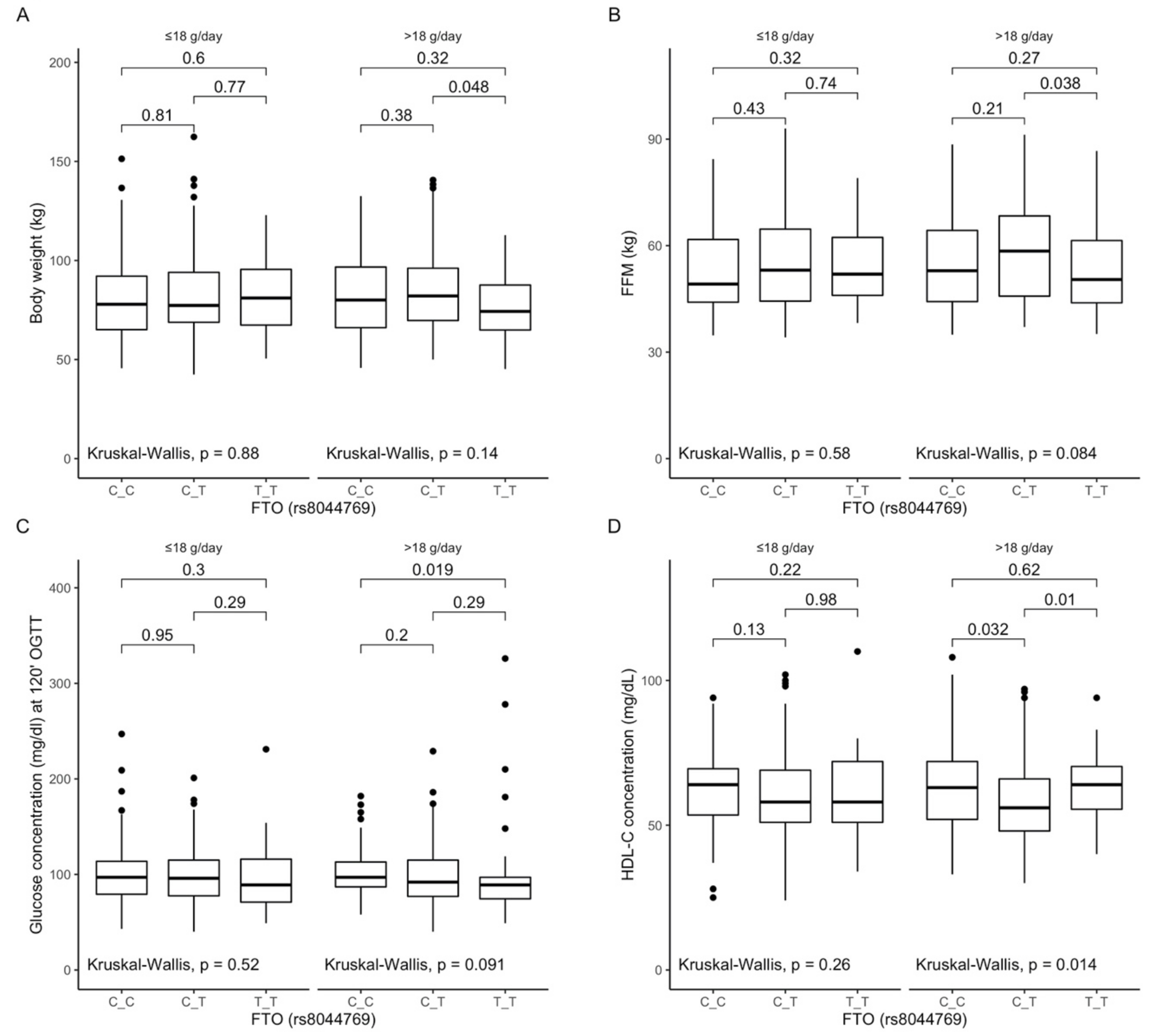
3.7. Associations between rs7190492 Polymorphism, Anthropometric Measurements, Lipid Profile, and Dietary Fiber Intake

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Loh, M.; Zhou, L.; Ng, H.K.; Chambers, J.C. Epigenetic disturbances in obesity and diabetes: Epidemiological and functional insights. Mol. Metab. 2019, 27, S33–S41. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Ryden, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.R.; Zhang, M.Y.; Ni, J.W.; Cheng, R.Q.; Zheng, Z.Q.; Xi, L.; Luo, F.H. Clinical characteristics and beta-cell function of Chinese children and adolescents with type 2 diabetes from 2009 to 2018. World J. Pediatr. 2019, 15, 405–411. [Google Scholar] [CrossRef]
- Tan, L.J.; Zhu, H.; He, H.; Wu, K.H.; Li, J.; Chen, X.D.; Zhang, J.G.; Shen, H.; Tian, Q.; Krousel-Wood, M.; et al. Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS ONE 2014, 9, e96149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steemburgo, T.; Azevedo, M.J.; Gross, J.L.; Milagro, F.I.; Campion, J.; Martinez, J.A. The rs9939609 polymorphism in the FTO gene is associated with fat and fiber intakes in patients with type 2 diabetes. J. Nutr. Nutr. 2013, 6, 97–106. [Google Scholar] [CrossRef]
- Czajkowski, P.; Adamska-Patruno, E.; Bauer, W.; Fiedorczuk, J.; Krasowska, U.; Moroz, M.; Gorska, M.; Kretowski, A. The Impact of FTO Genetic Variants on Obesity and Its Metabolic Consequences is Dependent on Daily Macronutrient Intake. Nutrients 2020, 12, 3255. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Jenkins, A.L. Dietary fiber and the glycemic response. Proc. Soc. Exp. Biol. Med. 1985, 180, 422–431. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Adamska-Patruno, E.; Goscik, J.; Czajkowski, P.; Maliszewska, K.; Ciborowski, M.; Golonko, A.; Wawrusiewicz-Kurylonek, N.; Citko, A.; Waszczeniuk, M.; Kretowski, A.; et al. The MC4R genetic variants are associated with lower visceral fat accumulation and higher postprandial relative increase in carbohydrate utilization in humans. Eur. J. Nutr. 2019, 58, 2929–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretowski, A.; Adamska, E.; Maliszewska, K.; Wawrusiewicz-Kurylonek, N.; Citko, A.; Goscik, J.; Bauer, W.; Wilk, J.; Golonko, A.; Waszczeniuk, M.; et al. The rs340874 PROX1 type 2 diabetes mellitus risk variant is associated with visceral fat accumulation and alterations in postprandial glucose and lipid metabolism. Genes Nutr. 2015, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska, K.; Adamska-Patruno, E.; Goscik, J.; Lipinska, D.; Citko, A.; Krahel, A.; Miniewska, K.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; et al. The Role of Muscle Decline in Type 2 Diabetes Development: A 5-Year Prospective Observational Cohort Study. Nutrients 2019, 11, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, E.; Vicente-Rodriguez, G.; Manios, Y.; Beghin, L.; Iliescu, C.; Censi, L.; Dietrich, S.; Ortega, F.B.; De Vriendt, T.; Plada, M.; et al. Harmonization process and reliability assessment of anthropometric measurements in a multicenter study in adolescents. Int. J. Obes. 2008, 32, S58–S65. [Google Scholar] [CrossRef] [Green Version]
- Hagstromer, M.; Oja, P.; Sjostrom, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bowler, E.; Cerezo, M.; Gil, L.; Hall, P.; Hastings, E.; Junkins, H.; McMahon, A.; Milano, A.; Morales, J.; et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, Y.; Xu, F.; Ren, X.; Li, X.; Lai, M. FTO gene polymorphisms and obesity risk: A meta-analysis. BMC Med. 2011, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Eastwood, M.A.; Kay, R.M. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am. J. Clin. Nutr. 1979, 32, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Che, D.; Qin, G.; Rui, H.; Sello, C.T.; Hailong, J. Interactions of Dietary Fibre with Nutritional Components on Gut Microbial Composition, Function and Health in Monogastrics. Curr. Protein Pept. Sci. 2018, 19, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Balkwill, A.; Cairns, B.J.; Appleby, P.N.; Green, J.; Reeves, G.K.; Key, T.J.; Beral, V.; Million Women Study, C.; Million Women Study, C. Source of dietary fibre and diverticular disease incidence: A prospective study of UK women. Gut 2014, 63, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Sanjoaquin, M.A.; Appleby, P.N.; Spencer, E.A.; Key, T.J. Nutrition and lifestyle in relation to bowel movement frequency: A cross-sectional study of 20630 men and women in EPIC-Oxford. Public Health Nutr. 2004, 7, 77–83. [Google Scholar] [CrossRef]
- Kritchevsky, D. Dietary fibre and lipid metabolism. Int. J. Obes. 1987, 11, 33–43. [Google Scholar]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordoni, L.; Petracci, I.; Zhao, F.; Min, W.; Pierella, E.; Assmann, T.S.; Martinez, J.A.; Gabbianelli, R. Nutrigenomics of Dietary Lipids. Antioxidants 2021, 10, 994. [Google Scholar] [CrossRef]
- Graff, M.; Gordon-Larsen, P.; Lim, U.; Fowke, J.H.; Love, S.A.; Fesinmeyer, M.; Wilkens, L.R.; Vertilus, S.; Ritchie, M.D.; Prentice, R.L.; et al. The influence of obesity-related single nucleotide polymorphisms on BMI across the life course: The PAGE study. Diabetes 2013, 62, 1763–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, G.; Tabassum, R.; Mahajan, A.; Dwivedi, O.P.; Mahendran, Y.; Kaur, I.; Nigam, S.; Dubey, H.; Varma, B.; Madhu, S.V.; et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J. Hum. Genet. 2011, 56, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Xu, J.; Zhang, Z.; Ren, J.; Li, Y.; Wang, J.; Cao, Y.; Rong, F.; Zhao, R.; Huang, X.; et al. Association of FTO polymorphisms with obesity and metabolic parameters in Han Chinese adolescents. PLoS ONE 2014, 9, e98984. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orru, M.; Usala, G.; et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007, 3, e115. [Google Scholar] [CrossRef]
- Villegas, R.; Goodloe, R.J.; McClellan, B.E., Jr.; Boston, J.; Crawford, D.C. Gene-carbohydrate and gene-fiber interactions and type 2 diabetes in diverse populations from the National Health and Nutrition Examination Surveys (NHANES) as part of the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) study. BMC Genet. 2014, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Wing, M.R.; Ziegler, J.; Langefeld, C.D.; Ng, M.C.; Haffner, S.M.; Norris, J.M.; Goodarzi, M.O.; Bowden, D.W. Analysis of FTO gene variants with measures of obesity and glucose homeostasis in the IRAS Family Study. Hum. Genet. 2009, 125, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Mirmiran, P.; Sedaghati-Khayat, B.; Azizi, F. The interaction of fat mass and obesity associated gene polymorphisms and dietary fiber intake in relation to obesity phenotypes. Sci. Rep. 2017, 7, 18057. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martinez-Gonzalez, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Aros, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Azorin, C.; Sorli, J.V.; Asensio, E.M.; Coltell, O.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Covas, M.I.; Aros, F.; Lapetra, J.; Serra-Majem, L.; et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc. Diabetol. 2012, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Axelsen, M.; Augustin, L.S.; Vuksan, V. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr. Opin. Lipidol. 2000, 11, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Mohlig, M.; Schofl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Freeland, K.R.; Wilson, C.; Wolever, T.M. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010, 103, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Sonestedt, E.; Roos, C.; Gullberg, B.; Ericson, U.; Wirfalt, E.; Orho-Melander, M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 2009, 90, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Lee, I.M.; Pare, G.; Chasman, D.I.; Rose, L.; Ridker, P.M.; Mora, S. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care 2011, 34, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt, A.; Thamer, C.; Machann, J.; Kirchhoff, K.; Stefan, N.; Tschritter, O.; Machicao, F.; Schick, F.; Haring, H.U.; Fritsche, A. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008, 16, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Hinney, A.; Scherag, A.; Nguyen, T.T.; Schreiner, F.; Schafer, H.; Hebebrand, J.; Roth, C.L.; Reinehr, T. “Fat mass and obesity associated” gene (FTO): No significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Med. Genet. 2008, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.; Pollock, N.K.; Bhagatwala, J.; Guo, D.H.; Gutin, B.; Zhu, H.; Dong, Y. Adolescent fiber consumption is associated with visceral fat and inflammatory markers. J. Clin. Endocrinol. Metab. 2012, 97, E1451–E1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.N.; Alexander, K.E.; Ventura, E.E.; Toledo-Corral, C.M.; Goran, M.I. Inverse relation between dietary fiber intake and visceral adiposity in overweight Latino youth. Am. J. Clin. Nutr. 2009, 90, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajkowski, P.; Adamska-Patruno, E.; Bauer, W.; Krasowska, U.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; Kretowski, A. Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile—A Cohort Study of a Caucasian Population of Polish Origin. Antioxidants 2021, 10, 1793. https://doi.org/10.3390/antiox10111793
Czajkowski P, Adamska-Patruno E, Bauer W, Krasowska U, Fiedorczuk J, Moroz M, Gorska M, Kretowski A. Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile—A Cohort Study of a Caucasian Population of Polish Origin. Antioxidants. 2021; 10(11):1793. https://doi.org/10.3390/antiox10111793
Chicago/Turabian StyleCzajkowski, Przemyslaw, Edyta Adamska-Patruno, Witold Bauer, Urszula Krasowska, Joanna Fiedorczuk, Monika Moroz, Maria Gorska, and Adam Kretowski. 2021. "Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile—A Cohort Study of a Caucasian Population of Polish Origin" Antioxidants 10, no. 11: 1793. https://doi.org/10.3390/antiox10111793
APA StyleCzajkowski, P., Adamska-Patruno, E., Bauer, W., Krasowska, U., Fiedorczuk, J., Moroz, M., Gorska, M., & Kretowski, A. (2021). Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile—A Cohort Study of a Caucasian Population of Polish Origin. Antioxidants, 10(11), 1793. https://doi.org/10.3390/antiox10111793







