Abstract
Excess epicardial adiposity, within a state of obesity and metabolic syndrome, is emerging as an important risk factor for the development of cardiovascular diseases (CVDs). Accordingly, increased epicardial fat thickness (EFT) implicates the exacerbation of pathological mechanisms involving oxidative stress and inflammation within the heart, which may accelerate the development of CVDs. This explains increased interest in targeting EFT reduction to attenuate the detrimental effects of oxidative stress and inflammation within the setting of metabolic syndrome. Here, we critically discuss clinical and preclinical evidence on the impact of physical exercise on EFT in correlation with reduced CVD risk within a setting of metabolic disease. This review also brings a unique perspective on the implications of oxidative stress and inflammation as major pathological consequences that link increased EFT to accelerated CVD risk in conditions of metabolic disease.
1. Introduction
Epicardial adipose tissue (EAT), due to its close proximity to the heart, is known to affect the cardiovascular system by releasing excess lipids such as low-density lipoproteins (LDL), as well as adipokines such as interleukin (IL)-6 that can elicit an undesired pro-inflammatory response, subsequently prompting atherosclerosis and vascular endothelial dysfunction [1,2,3]. In clinical research, measuring EAT volume or mass using non-invasive imaging procedures such as computed tomography or magnetic resonance imaging and echocardiography has proved essential to assess potential cardiovascular disease (CVD) risk [4]. Increased epicardial fat thickness (EFT), which may occur concurrently with elevated parameters of oxidative stress and inflammation, has been shown to be a useful marker for increased CVD risk in patients with metabolic syndrome [5,6,7,8,9]. This has increased research efforts to establish whether reducing EFT expansion may be a feasible strategy to lower CVD risk in individuals with metabolic abnormalities. Several reviews on the feasibility of using physical exercise to reduce EFT and protect against CVDs have been published. For example, in 2015, Rabkin and Campbell [10] conducted a systematic review and meta-analysis comparing interventions such as exercise, diet or bariatric surgery for their role in reducing EFT to lower CVD risk. They revealed that diet and bariatric surgery could markedly reduce EAT, but this was not achieved with exercise. Moreover, reduction in body mass index (BMI) was significantly associated with reduced EAT for diet-based interventions. In other review papers, inflammation plays a central role in the pathophysiological mechanisms linked with EAT [3,11].
More review articles published in 2020 [12] and early 2021 [13,14] have supported the notion that exercise, together with a restricted diet, bariatric surgery and some pharmaceutical interventions, can reduce EAT volume to improve cardiac function. Indeed, these reviews have further highlighted the impact of physical exercise on reducing EFT to attenuate pathological mechanisms, such as those involving inflammation, to improve cardiac function within a setting of metabolic disease. Here, we undertook a comprehensive approach to update and discuss evidence from both preclinical and clinical studies on the impact of physical exercise on EAT and associated CVD risk. Beyond giving a general overview on increased EAT mass and volume and its impact on CVDs, the current review brings a unique perspective on oxidative stress and inflammation as major pathological consequences that link increased EAT mass and volume to accelerated CVD risk in conditions of metabolic disease.
2. General Overview of EFT and Its Impact on CVDs
EAT is considered a visceral fat depot that surrounds 80% of the heart’s surface [15]. The multifaceted functional characteristics of EAT include mechanical, metabolic, thermogenic, and endocrine properties to support heart function [15]. In the physiological state, EAT plays a pivotal role in cardiac function, primarily due to its ability to take up and metabolize excess lipids, thus preventing atherosclerotic plaque formation [16]. However, during pathological conditions resulting from oxidative stress or inflammation, EAT may cause detrimental effects to the heart. Similar to other fat depots, EAT is affected by exercise [13,14]. Notably, beneficial effects of exercise on the heart include reducing the EAT mass and volume, which may be followed by increased mitochondrial biogenesis and anti-inflammatory effects that ultimately influence cardiac function [17]. Different exercise stress tests are often used to determine or monitor cardiac functional capacity, and these have been used as an independent indicator of cardiac events [18,19]. For example, treadmill exercise tests are regularly applied in sports and occupational medicine for disease stratification and to monitor response to treatment [19]. Negative exercise stress tests indicate absence of a condition, while positive tests point to diagnosis of coronary artery disease (CAD) [19,20]. Interestingly, it has been observed that patients with coronary slow flow phenomenon display negative exercise stress electrocardiography that correlates with enhanced left ventricular function, while those with a positive exercise stress test exhibit impaired left ventricular function [21]. Accordingly, several studies have reported on the link between EFT and CVD risk in response to the treadmill stress test (Table 1).
Briefly, in patients with established coronary microvascular dysfunction, Sengul et al. [22] found that increased EFT was associated with altered blood pressure responses to exercise stress testing, a risk factor for hypertension. Parsaei et al. [7] reported that EFT was significantly higher in patients with positive exercise test results, and this was associated with reduced levels of high-density lipoprotein (HDL) cholesterol. Moreover, Katlandur et al. [6] showed that EFT was significantly correlated with the severity and prevalence of CAD in positive exercise stress test patients. Türker Duyuler et al. [23] confirmed the relationship between increased EFT and raised blood pressure during stress testing, although these effects did not affect homocysteine levels. Plasma homocysteine levels are inversely correlated with HDL cholesterol [24] and have been shown to be an independent predictor of CVD by contributing to arterial damage and the formation of blood clots [25]. Gorter et al. [26] found that EFT positively correlated with increased BMI and right ventricular end-diastolic pressure and pulmonary vascular resistance, but inversely associated with maximum oxygen consumption rate (VO2-max) and exercise capacity in patients with preserved ejection fraction heart failure. Similarly, Haykowsky et al. [27] reported that patients with preserved ejection fraction heart failure had substantially lower EFT than healthy controls, which correlated with reduced peak oxygen uptake and decreased cardiac function. These findings suggest that increased EFT may not consistently indicate increased CVD risk, especially in older patients with metabolic disease and preserved ejection fraction heart failure. Summarized findings in Table 1 highlight the link between EFT and increased CVD risk in patients with metabolic syndrome or those already presenting with established cardiovascular complications.

Table 1.
Evidence of the link between epicardial fat thickness and cardiovascular disease risk in response to the treadmill stress test.
Table 1.
Evidence of the link between epicardial fat thickness and cardiovascular disease risk in response to the treadmill stress test.
| Study | Country | Participants | Main Findings |
|---|---|---|---|
| Sengul et al., 2011 [22] | Turkey | 32 patients with hypertension, with an average age of 49 years | Epicardial fat thickness (EFT) was associated with altered blood pressure responses to exercise stress testing, a risk factor for hypertension. |
| Parsaei et al., 2014 [7] | Iran | 62 patients with coronary microvascular dysfunction, with an average age of 52 years | EFT was significantly higher in patients with positive exercise test results. Moreover, high-density lipoprotein (HDL) cholesterol levels were significantly lower in patients with positive exercise test. |
| Fidan-Yaylali et al., 2016 [28] | Turkey | 114 obese subjects, with an average age of 41 years | EFT was not associated with autonomic nervous system dysfunction. |
| Katlandur et al., 2016 [6] | Turkey | 45 patients with severe coronary artery disease, with an average age of 62 years | EFT was significantly correlated with the severity and prevalence of coronary artery disease in positive exercise test patients. |
| Türker Duyuler et al., 2017 [23] | Turkey | 40 patients with hypertension, with an average age of 47 years | EFT positively correlated with high blood pressure in response to exercise stress testing, although homocysteine levels were not affected. |
| Haykowsky et al., 2018 [27] | United States | 100 obese patients with heart failure with preserved ejection fraction, with an average age of 66 years | Lower EFT was consistent with peak oxygen uptake during exercise stress testing and was negatively associated with decreased cardiac function. |
| Cho et al., 2017 [29] | South Korea | 64 patients with metabolic syndrome, with an average age of 52 years | Increased EFT was correlated with reduced heart rate recovery during exercise stress testing and severe liver steatosis. |
| Sugita et al., 2020 [30] | Japan | 176 patients with type 2 diabetes, with an average age of 65 | Increased EFT was positively associated with left ventricular structural and functional abnormalities and exercise intolerance. |
| Gorter et al., 2020 [26] | Netherlands | 75 patients with heart failure with preserved ejection fraction, with an average age of 74 years | EFT correlated with high body mass index, as well as increased right ventricular end-diastolic pressure and pulmonary vascular resistance, but inversely correlated with maximum oxygen consumption rate (VO2-max) and exercise capacity. |
3. Oxidative Stress and Inflammation as Major Pathological Factors Linking EFT to Increased CVD Risk
Understanding how EAT expansion during obesity or in patients with metabolic disease is related to increased CVD risk (as highlighted in Table 1) has garnered considerable interest in recent years. Key to this is the elucidation of the intricate pathological or molecular mechanisms that are implicated in cardiac abnormalities within EAT. Oxidative stress and inflammation are well-established pathological features that are associated with the development and progression of various metabolic complications, which may or may not be linked with EAT expansion [31,32,33]. The accumulation of triglycerides and lipid metabolites such as ceramides within EAT contributes to lipotoxicity and mitochondrial dysfunction, subsequently causing elevation in markers of oxidative stress and an undesired inflammation that drive cardiac hypertrophy, as represented in Figure 1. In fact, a number of studies have linked increased circulating levels of oxidative products such as thiobarbituric acid reactive substances (TBARS) and pro-inflammatory markers such as C-reactive protein (CRP) with CVD risk [34,35,36]. Likewise, several reviews have explored the pathological link between oxidative stress, inflammation and EFT in individuals at risk of CVD. In 2011, Sacks and Fain [37] proposed that increased EAT volume may be an indicator of acute myocardial infarction, and that increased inflammatory cells and pro-inflammatory actions within EAT are associated with oxidative stress in people with impaired metabolic function. In an expert review published in 2017, Wong et al. [38] discussed data from basic science and translational studies and concluded that an undesired pro-inflammatory response and raised markers of oxidative stress are involved in the pathology linking EAT with CVD risk. Others [39,40,41,42] have emphasized that lipid metabolism in cardiomyocytes appears to be primarily influenced by ectopic fat deposits, leading to endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress, with an exacerbated inflammatory response and cell death. A few authors [33,43,44] have suggested that increased cardiac oxidative stress and inflammation within an obese state may lead to heart failure, while therapies that are able to attenuate these conditions may diminish the risk of developing cardiovascular complications. This information highlights the importance of understanding the implications of oxidative stress and inflammation in driving the pathological impact of EAT in patients with metabolic syndrome.
Table 2 gives an overview of preclinical studies reporting on the link between oxidative stress, inflammation, EAT and increased CVD risk. Briefly, Company et al. [45] demonstrated that increased EAT volume was correlated with raised heart weight and enhanced messenger ribonucleic acid (mRNA) expression levels of oxidative stress markers, including down-regulation of glutathione peroxidase (GPx), heme oxygenase (HO-1), and superoxide dismutase (SOD) and up-regulation of endothelial nitric oxide synthase (eNOS) in peri-myocardial EAT of pigs with coronary atherosclerosis. Kang et al. [46] reported that EAT was significantly higher in rats fed a high fat diet (HFD), when compared to standard controls. Interestingly, this consequence was correlated with increased myocardial mRNA expression levels of mitochondrial oxidative phosphorylation (OXPHOS) subunit of NDUFB5 (NADH: Ubiquinone Oxidoreductase Subunit B5) and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1 α), and the mitochondrial DNA copy number. Similarly, there were also increased levels of 8-hydroxydeoxyguanosine within the myocardial tissue, which is a known marker consistent with oxidative damage. Besides reporting on the levels of oxidative stress, Xu et al. [47] indicated that increased EAT weight was associated with increased total cholesterol, enhanced expression of matrix metalloproteinase (MMP)2/9 in the aorta and left ventricle, as well as raised pro-inflammatory markers, including serum concentrations of high-sensitivity (hs)-CRP and IL-6 in New Zealand white rabbits fed HFD. Patel et al. [48] showed that loss of angiotensin-converting enzyme (ACE2), a key pathogenic mechanism involved in the development of CVDs, resulted in decreased weight gain but increased glucose intolerance and EAT inflammation, including polarization of EAT resident macrophages into a pro-inflammatory phenotype in mice fed HFD. Thus, from the brief preclinical evidence summarized in Table 2, EAT expansion appearing in conditions of HFD seems to occur consistent with raised markers of oxidative stress and inflammation.
Table 3 further evaluates any correlation between markers of oxidative stress and inflammation, as well as EAT expansion and CVD risk in clinical studies. Apparently, consistent with evidence from preclinical studies (presented in Table 2), clinical data indicate that EAT expansion may occur concomitant with raised markers of oxidative stress and inflammation in patients with metabolic syndrome or those at increased risk of CVD [34,49]. In fact, recently reviewed clinical evidence also infers that measuring EAT may provide an important and reproducible diagnostic tool to stratify patients at risk of heart failure [50]. In addition, elevated pro-inflammatory markers such as CRP and interleukin (IL)-6 are considered reliable biomarkers to indicate increased risk of myocardial injury in patients with T2D at increased risk of developing CVD [35,51,52]. Here, several studies support the notion that impaired lipid metabolism in EAT may be a central feature in the pathological mechanisms that connect EAT with mitochondrial dysfunction, oxidative stress, and an undesired pro-inflammatory response. For example, Sengul et al. [53] and Aydogdu et al. [5] reported that increased EFT correlated with increased waist circumference, elevated LDL-cholesterol, fasting glucose, triglyceride and hs-CRP concentrations and higher systolic and diastolic blood pressure levels in patients with metabolic syndrome. Sacks et al. [54] showed that expanded EAT was accompanied by increased expression of pro-inflammatory and oxidative stress markers such as GPx3 and HO-1 in EAT of patients with CAD. Notably, the increase in antioxidant defense genes with EAT expansion could indicate an essential adaptive response mechanism that is necessary to protect against oxidative stress, as reported elsewhere [55,56,57]. Nonetheless, it is evident that well-known markers of oxidative stress and inflammation such as TBARS, hs-CRP and IL-6 may play a significant role in linking EAT with enhanced CVD risk in conditions of metabolic disease, as reviewed elsewhere [38,58]. Moreover, Chechi et al. [59] observed that EAT biopsies from patients undergoing various heart surgeries display increased expression of genes involved in inflammation, such as tumor necrosis factor (TNF)-α, including the presence of adipose tissue expansion and thermogenic genes such as fatty acid binding protein-4 (FABP4) and uncoupling protein 1. Recently, Zhao et al. [60] showed that the expression of proteins such as serine proteinase inhibitor A3, which are involved in heart failure-related processes including inflammation, oxidative stress, and lipid metabolism, are altered in EAT of patients with heart failure. Ultimately, these findings highlight that EAT in the metabolic disease state is associated with impaired expression of genes involved in various pathological mechanisms such as inflammation, oxidative stress, and thermogenesis. Other studies mentioned in this review show that derangements in the molecular signature of EAT during metabolic disease are related to abnormalities in cardiac mitochondrial functional capacity, as well as myocardial structural and functional competencies that may accelerate CVD risk (Table 2 and Table 3). Overall, the current state of evidence suggests that exacerbated oxidative stress and inflammation within EAT during metabolic disorders are major therapeutic targets to improve cardiac function and reduce CVD risk (Figure 1).
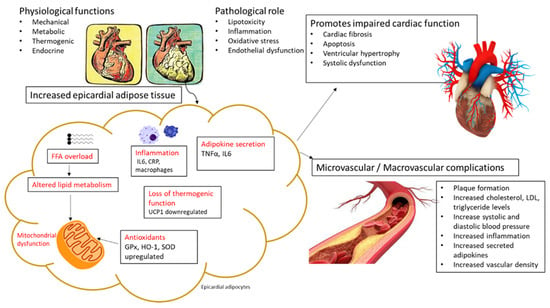
Figure 1.
An overview of pathological mechanisms linking increased epicardial adipose tissue (EAT) with the detrimental effects of oxidative stress and inflammation. Briefly, the expansion of EAT is consistent with raised heart weight, and this may be associated with free fatty acid (FFA) overload and impaired lipid metabolism and mitochondrial dysfunction, as evident in some preclinical and clinical studies summarized in the current review. As such, prominent markers of oxidative stress and inflammation that are consistently altered within EAT and that are also detected in the myocardial tissue and in circulation, include glutathione peroxidase (GPx), heme oxygenase 1 (HO-1), superoxide dismutase (SOD), C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor alpha (TNF-α). Notably, the expression levels of some antioxidant genes such as GPx, HO-1, and SOD were increased within EAT in some patients at risk of cardiovascular disease (CVD), indicating a possible adaptive response against oxidative damage. Overall, beyond the detrimental effects of oxidative stress and inflammation, other factors that may be induced through high fat diet feeding, such as dysfunctional adipose tissue and altered thermogenesis, can contribute to the development of cardiovascular complications and increased CVD risk in conditions of metabolic disease. Uncoupling protein 1 (UCP1), Low density lipoprotein (LDL).

Table 2.
An overview of preclinical studies reporting on the link between oxidative stress, inflammation, epicardial adipose tissue, and increased cardiovascular disease risk.
Table 2.
An overview of preclinical studies reporting on the link between oxidative stress, inflammation, epicardial adipose tissue, and increased cardiovascular disease risk.
| Author, Year | Country | Preclinical Model | Main Findings |
|---|---|---|---|
| Company et al., 2010 [45] | United States | 13 pigs with coronary atherosclerosis, with ages ranging between 10–11 months | Increased epicardial adipose tissue (EAT) weight was correlated with raised heart weight and enhanced mRNA expression levels of oxidative stress markers, including down-regulated glutathione peroxidase, heme oxygenase, superoxide dismutase, and up-regulated endothelial nitric oxide synthase in peri-myocardial EAT |
| Kang et al., 2015 [46] | Korea | 6 Wistar rats fed a high fat diet (HFD) for 10 weeks | EAT was significantly higher in the HFD group when compared to rats fed a standard diet. This correlated with increased myocardial expression levels of mitochondrial oxidative phosphorylation (OXPHOS) subunit NDUFB5 (NADH:Ubiquinone Oxidoreductase Subunit B5) and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α), and the mitochondrial DNA copy number. There were also increased levels of 8-hydroxydeoxyguanosine within the myocardial tissue, a known marker for oxidative damage |
| Xu et al., 2015 [47] | China | 6 New Zealand white rabbits fed HFD for 12 weeks | Increased EAT weight was associated with increased total cholesterol, enhanced expression of matrix metalloproteinase (MMP)2/9 in the aorta and left ventricle, as well as raised high-sensitivity CRP and IL-6 serum levels |
| Patel et al., 2016 [48] | Canada | Angiotensin-converting enzyme 2 (ACE2) knockout mice were fed HFD from weaning to 6 months of age | Loss of ACE2 resulted in decreased weight gain but increased glucose intolerance, EAT inflammation, and polarization of EAT resident macrophages into a pro-inflammatory phenotype in response to HFD |

Table 3.
An overview of clinical studies reporting on the link between oxidative stress, inflammation, epicardial adipose tissue, and increased cardiovascular disease risk.
Table 3.
An overview of clinical studies reporting on the link between oxidative stress, inflammation, epicardial adipose tissue, and increased cardiovascular disease risk.
| Author, Year | Country | Characteristic Features of Participants | Main Findings |
|---|---|---|---|
| Salgado-Somoza et al., 2010 [49] | Spain | 55 patients with metabolic syndrome undergoing heart surgery, with an average age of 71 ± 9 years | Higher reactive oxygen species production and differential expression of oxidative stress related genes catalase, glutathione S-transferase P, and protein disulfide isomerase in epicardial adipose tissue (EAT) compared to subcutaneous adipose tissue |
| Wilund et al., 2010 [34] | United States | 9 patients on maintenance hemodialysis at increased risk of cardiovascular disease, with an average age of 59 ± 4.9 years | Enhanced EAT correlated with increased serum markers of thiobarbituric acid reactive substances, a marker of oxidative stress. This was also consistent with increased serum lipids and inflammatory markers, C-reactive protein (CRP), and interleukin (IL)-6 |
| Sacks et al., 2011 [54] | United States | 16 patients with severe stable coronary artery disease, with age range between 40 and 60 years | A depot specific increase in expression of pro-inflammatory, redox, endothelial cell, and angiogenic genes; additionally, glutathione peroxidase 3, heme oxygenase, and IL-8 gene expression were increased in EAT |
| Sengul et al., 2011 [53] | Turkey | 40 patients with metabolic syndrome, with an average age of 48 years | Increased epicardial fat thickness (EFT) positively correlated with increased waist circumference, serum levels of total and low-density lipoprotein (LDL)-cholesterol, fasting glucose concentrations, triglycerides, systolic and diastolic blood pressure levels, hs-CRP, and insulin resistance |
| Aydogdu et al., 2015 [5] | Turkey | 30 patients with subclinical hypothyroidism, with an average age of 37 years | EFT and increased oxidative stress index correlated with body fat percentage, serum levels of total cholesterol, LDL, and diastolic blood pressure |
| Chechi et al., 2017 [59] | Canada | EAT biopsies from patients undergoing various heart surgeries, with an average age of 64 years | EAT biopsies revealed increased expression of genes involved in inflammation such as tumor necrosis factor-α (TNF-α), as well as the presence of adipose tissue expansion and thermogenic genes such as fatty acid binding protein-4 (FABP4) and uncoupling protein 1 (UCP1) |
| Zhao et al., 2020 [60] | China | 5 patients with heart failure | Proteomic analysis of EAT revealed increased levels of proteins involved in inflammation and lipid metabolism, including serine proteinase inhibitor A3 and fatty acid synthase |
4. Evidence on the Impact of Physical Exercise on EFT and CVD-Related Markers
The rising trends in metabolic disorders, especially the state of overweight and obesity, contribute significantly to impaired metabolic function through adipose tissue expansion [61,62]. A sedentary lifestyle, which is characterized by little or no physical exercise, is known to promote ectopic lipid accumulation and cardiac deterioration, partially through expansion of EAT [61,62]. Accordingly, it is suggested that interventions that can reduce excessive body fat accumulation, including EAT, can lower CVD risk [13,63]. As such, clinical studies have markedly increased reporting on the impact of moderate to intensive physical exercise on EFT in individuals at risk of developing cardiovascular complications. Such studies have predominantly involved adult obese subjects or those with established cardiovascular complications. Some of these studies have been published in developing countries such as India or China that are plagued by rapidly rising cases of noncommunicable diseases such as type 2 diabetes (T2D) [64], whereas most publications are from developed countries such as the United States and in Europe. Notably, there is limited literature, if any, reporting on the impact of physical exercise on EFT in Africa. Nonetheless, data summarized in Table 4 and Table 5 supports the beneficial effects of different forms of physical exercise, ranging from moderate to high intensity, in reducing EFT and improving metabolic parameters in patients at increased CVD risk.
Briefly, findings in obese subjects including those with a cluster of metabolic complications such as glucose intolerance, hypertension or CAD, support the beneficial effects of short-term physical exercise (<3 months) in reducing EFT, subsequently influencing markers that are essential in determining cardiac function (Table 4). For example, Kahl et al. [63] reported that exercise training for 6 weeks reduced EFT, along with improving metabolic factors such as body weight, BMI, and HDL levels in patients with metabolic syndrome, especially those characterized by major depressive disorder. Similarly, Wu et al. [65] demonstrated that aerobic steps that consisted of 3 to 5 sessions per week for 3 months significantly reduced EFT, BMI, waist circumference, and visceral fat in obese subjects. To build on these findings, Honkala et al. [66] reported that high-intensity interval training and moderate-intensity continuous training for 2 weeks effectively reduced EAT mass in subjects with defective glucose tolerance. Interestingly, this study showed that high intensity training appeared superior in improving aerobic capacity and whole-body insulin sensitivity. Other studies on the short-term impact of high intensity physical exercise, either for 3 weeks [67] or 8 weeks [68], demonstrated that reduced EFT was linked with muscular endurance and improved flow-mediated dilation in females with obesity or patients with hypertension, respectively.
Clinical evidence presented in Table 5 evaluated the impact of long-term (≥3 months) physical exercise on EFT expansion in correlation with CVD risk in patients with metabolic diseases (Table 5). Interestingly, although they did not observe a change in epicardial fat volume or cardiac function in diabetic individuals, Jonker et al. [69] reported that moderate to intensive exercise for 6 months could effectively reduce levels of hepatic triglycerides, visceral abdominal fat, and paracardial fat (a thoracic mesenchyme-derived fat pad located superior to the visceral pericardium and around the blood vessels). It is important to note that reduced hepatic triglycerides, visceral abdominal fat, and paracardial fat are associated with decreased CVD risk in diabetic individuals [2]. As such, Serrano-Ferrer et al. [70] demonstrated that 6 months of resistance and endurance exercise could reduce EFT and improve left ventricular strains and inflammatory profiles (increased adiponectin and reduced TNF-α) in adult patients with metabolic syndrome. Notably, also indicating the potential role of long-term physical exercise in targeting EFT to improve inflammatory status, others have reported that 3 months of aerobic exercise training [71] or resistance training [72] remains effective in decreasing EFT volume or EAT mass, concomitant with reducing the levels of hs-CRP as well as known CVD risk indices such as resting heart rate, LDL levels, and total cholesterol levels in patients with metabolic syndrome. Generally, these results suggest that enhanced adiposity and increased EFT in individuals with metabolic syndrome or already presenting with CAD are consistent with elevated inflammation and lower cardiac efficiency. In turn, by reducing body weight, vigorous physical exercise can significantly reduce EFT and, as a result, improve metabolic function, attenuate an undesired pro-inflammatory response, and enhance cardiac efficiency.
Additional clinical studies have also reported on the positive effects of the long-term effects of physical exercise on the reduction of EFT in patients with heart failure or diagnosed with CVDs (Table 5). Jo et al. [73] demonstrated that 3 months of exergame and treadmill exercise showed similar effects in reducing EFT and improving markers of cardiorespiratory fitness and endothelial function, including VO2-max and flow-mediated dilation in postmenopausal women with high CVD risk. Zhang et al. [74] revealed that 90 min of Tai Chi exercise daily for 3 months could reduce EFT, lower heart rate, and increase the quality of life of patients with heart disease. Mechanistically, this evidence was supported by reduced levels of microRNA (miR)-126, which is one of the prominent markers of inflammation related with elevated CVD risk [75,76]. Markers of inflammation, especially the emerging role of microRNAs in metabolic diseases and CVDs, are attracting interest to develop novel therapeutics [77,78]. Exercise has also been shown to decrease mitogen activated protein kinase (MAPK) activity, one of the signaling pathways involved in cardiac hypertrophy and heart failure, and inflammatory responses in aortas of adult male Sprague-Dawley rats [79]. Taken together, these findings highlight that regardless of the type of exercise intervention, in addition to reducing EFT, long-term (≥3 months) exercise has various beneficial effects on the cardiovascular system, in part by attenuating systematic inflammation and improving cardiac efficiency in conditions of metabolic syndrome. Interestingly, others indicated that physical activity in combination with caloric restriction for 4 months could also reduce EFT; however, there were no significant differences in cardiometabolic profile in patients with T2D [80]. However, such evidence is still limited, and additional studies are necessary to assess the therapeutic impact of combining physical exercise with caloric restriction.

Table 4.
An overview of studies on the impact of physical exercise for less than 3 months on epicardial adipose tissue thickness in individuals at risk of cardiovascular disease.
Table 4.
An overview of studies on the impact of physical exercise for less than 3 months on epicardial adipose tissue thickness in individuals at risk of cardiovascular disease.
| Study | Country | Study Size and Population | Main Findings |
|---|---|---|---|
| Kahl et al., 2016 [63] | Germany | 20 participants with major depressive disorder, with an average age of 44 years | Six weeks of moderate intensity exercise training reduced epicardial fat thickness (EFT) and improved metabolic factors such as body weight, body mass index, and high-density lipoprotein |
| Wu et al., 2016 [65] | Taiwan | 39 obese subjects, with an average age of 39 years | Aerobic steps that consisted of 3 to 5 sessions per week for 3 months significantly reduced EFT, body mass index, waist circumference, and visceral fat |
| Honkala et al., 2017 [66] | Finland | 16 subjects with defective glucose tolerance, with ages ranging between 40 and 55 years | High-intensity interval training and moderate-intensity continuous training for 2 weeks effectively reduced epicardial adipose tissue mass. However, high intensity training appeared superior in improving aerobic capacity and whole-body insulin sensitivity |
| Fernandez-del-Valle et al., 2018 [67] | United States | 6 young females with obesity, with an average age of 22 years | Epicardial and paracardial fat volumes were reduced by 3 weeks of high intensity, moderate-volume muscular endurance resistance training |
| Jo et al., 2020 [68] | South Korea | 17 patients with hypertension, with an average age of 50 years | High and moderate intensity training for 8 weeks significantly reduced EFT and improved flow-mediated dilation |

Table 5.
An overview of studies on the impact of physical exercise for 3 months or more on epicardial adipose tissue thickness in individuals at risk of cardiovascular disease.
Table 5.
An overview of studies on the impact of physical exercise for 3 months or more on epicardial adipose tissue thickness in individuals at risk of cardiovascular disease.
| Study | Country | Study Size and Population | Main Findings |
|---|---|---|---|
| Jonker et al., 2013 [69] | Netherlands | 12 patients with type 2 diabetes (T2D), with an average age of 46 years | Six months of moderate to intensive exercise decreased paracardial fat volume hepatic triglyceride content and visceral abdominal fat. However, cardiac function was unaffected |
| Serrano-Ferrer et al., 2016 [70] | France | 87 patients with metabolic syndrome, with an average age of 59 years | Six months of resistance and endurance exercise reduced epicardial fat thickness (EFT) and improved left ventricular strains and inflammatory profiles (increased adiponectin and reduced tumor necrosis factor alpha expression) |
| Bairapareddy et al., 2018 [71] | India | 66 overweight subjects, with age ranging between 20 and 45 years | Three months of aerobic exercise training resulted in a significant reduction in EFT and decreased body weight and high sensitivity C-reactive protein levels |
| Christensen et al., 2019 [72] | United States | 50 physically inactive participants with abdominal obesity, with an average age of 39 years | Three months of endurance and resistance training reduced epicardial adipose tissue (EAT) mass, resting heart rate as well as cardiometabolic parameters such as low-density lipoproteins and total cholesterol |
| Jo et al., 2020 [73] | South Korea | 44 postmenopausal women with high cardiovascular disease risk, with an age range between 57–62 years | Three months of exergame and treadmill exercise showed similar effects in reducing EFT and improving markers of cardiorespiratory fitness and endothelial function, including VO2-max and flow-mediated dilation |
| Zhang et al., 2020 [74] | China | 36 patients with heart disease, with an average age of 62 years | Ninety minutes of Tai Chi exercise daily for three months decreased EAT volume and heart rate and improved quality of life. This was concomitant with lowered levels of microRNA (miR)-126, mitogen-activated protein kinase, c-Jun N-terminal kinases, and extracellular signal-regulated kinase |
| Leroux-Stewart et al., 2021 [80] | Canada | 26 patients with T2D, with an average age of 58 years | Physical activity in combination with caloric restriction for 4 months was effective in reducing EFT and total fat mass; however, there were no significant differences in cardiometabolic profile |
5. Summary and Conclusions
Non-communicable diseases, especially metabolic syndrome, contribute significantly to the rising global disease burden [81]. Increasing research has been directed to identifying the precise pathophysiological mechanisms that are implicated in the development of diverse metabolic complications, including gestational diabetes, an important feature predominantly seen in pregnant patients with T2D [82,83]. Seemingly, determining this aspect remains crucial for early disease diagnosis, especially to identify potential therapeutics that could be effectively used to protect against or even slow disease progression. As such, excessive adiposity in major organs including the heart, usually seen in conditions of obesity or metabolic syndrome, has been consistently linked to CVD [16,84]. Accumulative research is currently underway to determine how EFT affects cardiac efficiency in individuals at risk of CVDs [5,42,85]. From the current review, we were able to summarize the use of treadmill exercise tests in identifying individuals at risk of CVD. Moreover, it was evident that increased EFT was constantly associated with reduced HDL-cholesterol, raised triglycerides, and left ventricular structural and functional abnormalities that occurred concurrent with the severity and prevalence of cardiovascular complications (Table 1). Inferring that increased EFT is strongly associated with increased CVD risk, it may be a reliable therapeutic target to improve cardiac function in individuals with metabolic syndrome.
Although currently used therapeutic drugs such as metformin and statins can effectively reduce blood glucose or lipid levels to prolong the lives of patients with metabolic syndrome, the long-term use of these drugs has been associated with several limitations including toxicity, ultimately leading to increased CVD risk [86,87,88]. Certainly, from evidence presented in the current review (Table 2 and Table 3), we hypothesize that increased EFT occurs concurrently with raised circulating levels of TBARS, hs-CRP, and IL-6, indicating oxidative stress and chronic low-grade inflammation. This consequence was associated with impaired lipid metabolism, mitochondrial dysfunction within the myocardium, reduced myocardial respiration, increased cardiac steatosis, and worsened cardiac function in conditions of metabolic syndrome [48,59]. These findings suggest that increased EFT is significantly associated with increased CVD risk; thus, it remains essential to develop therapies that target reduction of EFT beyond the whole-body fat, in an effort to lower CVD risk.
Similar to weight gain, it is currently understood that EFT can be exacerbated by several modifiable risk factors such as a sedentary lifestyle, stress, and an unbalanced diet [42,85]. Studies have demonstrated that physical activity or exercise intervention provides an effective non-invasive strategy for reducing EFT that may also exert beneficial effects on the cardiovascular system [14,71]. In agreement, data from clinical studies summarized in the current review (Table 4 and Table 5) support the notion that vigorous or endurance exercise can improve metabolic function by decreasing weight gain and BMI, and in the process also lower EFT in patients with metabolic syndrome. Moreover, it was observed that reduction in EFT was accompanied by a decrease in inflammatory markers TNF-α and CRP, a consequence that was linked with improved cardiac aerobic capacity and flow-mediated dilation (Table 5). Figure 2 gives an overview of how physical exercise may contribute to lowered CVD risk by improving metabolic function and myocardial maximal oxygen uptake, especially by reducing EFT in correlation with markers of inflammation in patients with obesity or metabolic syndrome.
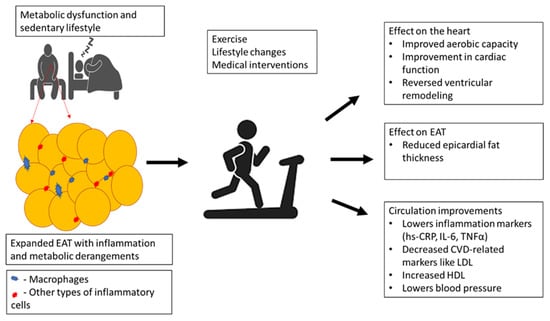
Figure 2.
An overview of how physical exercise may contribute to lowered cardiovascular disease (CVD) risk by improving metabolic function and myocardial maximal oxygen uptake, especially by reducing epicardial adipose tissue (EAT) thickness in correlation with markers of inflammation in patients with obesity or metabolic syndrome. Briefly, physical exercise can reduce EAT volume to improve myocardial aerobic capacity, lower blood pressure, and reverse the myocardial remodeling process, in part by blocking low-density lipoprotein (LDL) and enhancing high-density lipoprotein (HDL) levels. This is also consistent with decreasing markers of inflammation such as high sensitivity-C-reactive protein (hs-CRP), interleukin (IL)-6, and tumor necrosis factor alpha (TNF-α).
In conclusion, evidence from the current review supports the regular use of physical activity or exercise to alleviate complications linked with the progression of metabolic diseases, including reducing EFT, to attenuate the detrimental effects of oxidative stress and inflammation in those at risk of developing cardiovascular complications. Notably, while such beneficial effects of physical exercise are acknowledged, few individuals adhere to these interventions. Thus, research into novel therapies that could target EFT to improve cardiac function is still required.
Author Contributions
Conceptualization, T.A.N., C.P., S.E.M.-M. and P.V.D.; methodology, T.A.N., S.X.H.M., T.M.N. and B.B.N.; writing—original draft preparation, T.A.N., C.P., S.E.M.-M. and P.V.D. writing—review and editing, T.A.N., C.P., S.E.M.-M., S.X.H.M., T.M.N., B.B.N., H.S.-V.G., H.S., L.T. and P.V.D.; funding acquisition, C.P., S.E.M.-M. and P.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by baseline funding from the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) and the National Research Foundation (Grant number: 117829). The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Acknowledgments
Financial support for T.A.N. was provided under the DST-NRF Professional Development Programme (PDP). S.X.H.M. is funded by the SAMRC through its Division of Research Capacity Development under the internship scholarship program from funding received from the South African National Treasury. Grant holders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by SAMRC-supported research are those of the authors, and that the SAMRC accepts no liability whatsoever in this regard.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
Cardiovascular disease (CVD); epicardial fat thickness (EFT); epicardial adipose tissue (EAT); coronary artery disease (CAD); high-density lipoproteins (HDL); type 2 diabetes (T2D); tumor necrosis factor-alpha (TNF α); flow-mediated dilation (FMD); angiotensin-converting enzyme 2 (ACE2); C-reactive protein (CRP); interleukin 6 (IL6); low-density lipoprotein (LDL); thiobarbituric acid reactive substances (TBARS).
References
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.; Nakazato, R.; Li, D.; Berman, D.S. Epicardial and thoracic fat—Noninvasive measurement and clinical implications. Cardiovasc. Diagn. Ther. 2012, 2, 85–93. [Google Scholar]
- Aydogdu, A.; Karakas, E.Y.; Erkus, E.; Altıparmak, I.H.; Savık, E.; Ulas, T.; Sabuncu, T. Epicardial fat thickness and oxidative stress parameters in patients with subclinical hypothyroidism. Arch. Med. Sci. 2017, 13, 383–389. [Google Scholar] [CrossRef]
- Katlandur, H.; Ulucan, S.; Özdil, H.; Keser, A.; Kaya, Z.; Özbek, M.; Ülgen, S. Evaluation of echocardiographic epicardial fat thickness as a sign of cardiovascular risk in positive exercise test patients. Acta Cardiol. Sin. 2016, 32, 684–689. [Google Scholar]
- Parsaei, M.S.; Nabati, M.; Yazdani, J.; Bagheri, B.; Ghaemian, A.; Saffar, N. Relationship between epicardial fat and coronary microvascular dysfunction. Kardiol. Pol. 2014, 72, 417–424. [Google Scholar] [CrossRef]
- Colom, C.; Viladés, D.; Pérez-Cuellar, M.; Leta, R.; Rivas-Urbina, A.; Carreras, G.; Ordóñez-Llanos, J.; Pérez, A.; Sánchez-Quesada, J.L. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc. Diabetol. 2018, 17, 156. [Google Scholar] [CrossRef] [Green Version]
- Rabkin, S.W. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metab. Syndr. Relat. Disord. 2014, 12, 31–42. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Campbell, H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 406–415. [Google Scholar] [CrossRef]
- Matloch, Z.; Cinkajzlova, A.; Mraz, M.; Haluzik, M. The role of inflammation in epicardial adipose tissue in heart diseases. Curr. Pharm. Des. 2018, 24, 297–309. [Google Scholar] [CrossRef]
- Colonetti, T.; Grande, A.J.; Amaral, M.C.; Colonetti, L.; Uggioni, M.L.; Inês da Rosa, M.; Hernandez, A.V.; Tse, G.; Liu, T.; Nerlekar, N.; et al. Effect of exercise on epicardial adipose tissue in adults: A systematic review and meta-analyses. Heart Fail. Rev. 2021, 26, 1399–1411. [Google Scholar] [CrossRef]
- Saco-Ledo, G.; Valenzuela, P.L.; Castillo-García, A.; Arenas, J.; León-Sanz, M.; Ruilope, L.M.; Lucia, A. Physical exercise and epicardial adipose tissue: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2021, 22, e13103. [Google Scholar] [CrossRef]
- Launbo, N.; Zobel, E.H.; von Scholten, B.J.; Faerch, K.; Jørgensen, P.G.; Christensen, R.H. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13136. [Google Scholar] [CrossRef]
- Talman, A.H.; Psaltis, P.J.; Cameron, J.D.; Meredith, I.T.; Seneviratne, S.K.; Wong, D.T. Epicardial adipose tissue: Far more than a fat depot. Cardiovasc. Diagn. Ther. 2014, 4, 416–429. [Google Scholar]
- Matloch, Z.; Kotulák, T.; Haluzík, M. The role of epicardial adipose tissue in heart disease. Physiol. Res. 2016, 65, 23–32. [Google Scholar] [CrossRef]
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-intensity exercise and mitochondrial biogenesis: Current controversies and future research directions. Physiology 2019, 34, 56–70. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Liu, S.; Mu, L.; Li, G.; Yu, H.; Yang, J.; Ma, C. Value of exercise stress electrocardiography for stratification of exercise capacity and left ventricular systolic and diastolic function on coronary slow flow: Case-control study. BMC Cardiovasc. Disord. 2019, 19, 288. [Google Scholar] [CrossRef]
- Löllgen, H.; Leyk, D. Exercise testing in sports medicine. Dtsch. Arztebl. Int. 2018, 115, 409–416. [Google Scholar] [CrossRef]
- Kharabsheh, S.M.; Al-Sugair, A.; Al-Buraiki, J.; Al-Farhan, J. Overview of exercise stress testing. Ann. Saudi Med. 2006, 26, 1–6. [Google Scholar] [CrossRef]
- Löffler, A.I.; Perez, M.V.; Nketiah, E.O.; Bourque, J.M.; Keeley, E.C. Usefulness of achieving ≥10 METs with a negative stress electrocardiogram to screen for high-risk obstructive coronary artery disease in patients referred for coronary angiography after exercise stress testing. Am. J. Cardiol. 2018, 121, 289–293. [Google Scholar] [CrossRef]
- Sengul, C.; Ozveren, O.; Duman, D.; Eroglu, E.; Oduncu, V.; Tanboga, H.I.; Can, M.M.; Akgun, T.; Dindar, I. Echocardiographic epicardial fat thickness is related to altered blood pressure responses to exercise stress testing. Blood Press. 2011, 20, 303–308. [Google Scholar] [CrossRef]
- Türker Duyuler, P.; Duyuler, S.; Demir, M.; Uçar Elalmiş, Ö.; Güray, Ü.; İleri, M. Homocysteine, visceral adiposity-related novel cardiometabolic risk factors, and exaggerated blood pressure response to the exercise treadmill test. Blood Press. Monit. 2017, 22, 333–338. [Google Scholar] [CrossRef]
- Liao, D.; Tan, H.; Hui, R.; Li, Z.; Jiang, X.; Gaubatz, J.; Yang, F.; Durante, W.; Chan, L.; Schafer, A.I.; et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ. Res. 2006, 99, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barter, P.J.; Rye, K.A. Homocysteine and cardiovascular disease: Is HDL the link? Circ. Res. 2006, 99, 565–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorter, T.M.; van Woerden, G.; Rienstra, M.; Dickinson, M.G.; Hummel, Y.M.; Voors, A.A.; Hoendermis, E.S.; van Veldhuisen, D.J. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. Heart Fail. 2020, 8, 667–676. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Nicklas, B.J.; Brubaker, P.H.; Hundley, W.G.; Brinkley, T.E.; Upadhya, B.; Becton, J.T.; Nelson, M.D.; Chen, H.; Kitzman, D.W. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. Heart Fail. 2018, 6, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Fidan-Yaylali, G.; Yaylali, Y.T.; Erdogan, Ç.; Can, B.; Senol, H.; Gedik-Topçu, B.; Topsakal, S. The association between central adiposity and autonomic dysfunction in obesity. Med. Princ. Pract. 2016, 25, 442–448. [Google Scholar] [CrossRef]
- Cho, K.I.; Jo, E.A.; Cho, S.H.; Kim, B.H. The influence of epicardial fat and nonalcoholic fatty liver disease on heart rate recovery in metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 226–232. [Google Scholar] [CrossRef]
- Sugita, Y.; Ito, K.; Sakurai, S.; Sakai, S.; Kuno, S. Epicardial adipose tissue is tightly associated with exercise intolerance in patients with type 2 diabetes mellitus with asymptomatic left ventricular structural and functional abnormalities. J. Diabetes Complicat. 2020, 34, 107552. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galassetti, P. Inflammation and oxidative stress in obesity, metabolic syndrome, and diabetes. Exp. Diabetes Res. 2012, 2012, 943706. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Wilund, K.R.; Tomayko, E.J.; Wu, P.T.; Ryong Chung, H.; Vallurupalli, S.; Lakshminarayanan, B.; Fernhall, B. Intradialytic exercise training reduces oxidative stress and epicardial fat: A pilot study. Nephrol. Dial. Transplant. 2010, 25, 2695–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndevahoma, F.; Nkambule, B.B.; Dludla, P.V.; Mukesi, M.; Natanael, K.N.; Nyambuya, T.M. The effect of underlying inflammation on iron metabolism, cardiovascular risk and renal function in patients with type 2 diabetes. EJHaem 2021, 2, 357–365. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Sabbatinelli, J.; Matacchione, G.; Ramini, D.; Bonfigli, A.R.; Rippo, M.R.; de Candia, P.; Procopio, A.D.; Olivieri, F.; et al. Prevalence of residual inflammatory risk and associated clinical variables in patients with type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N. Human epicardial fat: What is new and what is missing? Clin. Exp. Pharmacol. Physiol. 2011, 38, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2017, 38, 1294–1302. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. J. Am. Coll. Cardiol. Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar]
- Gandoy-Fieiras, N.; Gonzalez-Juanatey, J.R.; Eiras, S. Myocardium metabolism in physiological and pathophysiological states: Implications of epicardial adipose tissue and potential therapeutic targets. Int. J. Mol. Sci. 2020, 21, 2641. [Google Scholar] [CrossRef] [Green Version]
- Anthony, S.R.; Guarnieri, A.R.; Gozdiff, A.; Helsley, R.N.; Phillip Owens, A.; Tranter, M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. 2019, 133, 2329–2344. [Google Scholar] [CrossRef]
- Packer, M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Rafeh, R.; Viveiros, A.; Oudit, G.Y.; El-Yazbi, A.F. Targeting perivascular and epicardial adipose tissue inflammation: Therapeutic opportunities for cardiovascular disease. Clin. Sci. 2020, 134, 827–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Company, J.M.; Booth, F.W.; Laughlin, M.H.; Arce-Esquivel, A.A.; Sacks, H.S.; Bahouth, S.W.; Fain, J.N. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: Relationship to visceral and subcutaneous fat. J. Appl. Physiol. 2010, 109, 1904–1912. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.W.; Kim, O.S.; Chin, J.Y.; Kim, W.H.; Park, S.H.; Choi, Y.J.; Shin, J.H.; Jung, K.T.; Lim, D.S.; Lee, S.K. Diastolic dysfunction induced by a high-fat diet is associated with mitochondrial abnormality and adenosine triphosphate levels in rats. Endocrinol. Metab. 2015, 30, 557–568. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Z.; Liu, L.; Luo, C.; Lu, G.; Li, Q.; Gao, X. Zinc regulates lipid metabolism and MMPs expression in lipid disturbance rabbits. Biol. Trace Elem. Res. 2015, 168, 411–420. [Google Scholar] [CrossRef]
- Patel, V.B.; Mori, J.; McLean, B.A.; Basu, R.; Das, S.K.; Ramprasath, T.; Parajuli, N.; Penninger, J.M.; Grant, M.B.; Lopaschuk, G.D.; et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 2016, 65, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, A.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef] [Green Version]
- Nyawo, T.A.; Dludla, P.V.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Nyambuya, T.M.; Nkambule, B.B.; Sadie-Van Gijsen, H.; Strijdom, H.; Pheiffer, C. A systematic review exploring the significance of measuring epicardial fat thickness in correlation to B-type natriuretic peptide levels as prognostic and diagnostic markers in patients with or at risk of heart failure. Heart Fail. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: Experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J. Am. Heart Assoc. 2017, 6, e005077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlangu, T.; Dludla, P.V.; Nyambuya, T.M.; Mxinwa, V.; Mazibuko-Mbeje, S.E.; Cirilli, I.; Marcheggiani, F.; Tiano, L.; Louw, J.; Nkambule, B.B. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine 2020, 126, 154892. [Google Scholar] [CrossRef]
- Sengul, C.; Cevik, C.; Ozveren, O.; Oduncu, V.; Sunbul, A.; Akgun, T.; Can, M.M.; Semiz, E.; Dindar, I. Echocardiographic epicardial fat thickness is associated with carotid intima-media thickness in patients with metabolic syndrome. Echocardiography 2011, 28, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N.; Cheema, P.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Wolford, D.; Samaha, J. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab. Syndr. Relat. Disord. 2011, 9, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Nono Nankam, P.A.; Nguelefack, T.B.; Goedecke, J.H.; Blüher, M. Contribution of adipose tissue oxidative stress to obesity-associated diabetes risk and ethnic differences: Focus on women of African ancestry. Antioxidants 2021, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The impact of oxidative stress on adipose tissue energy balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The interplay between adipose tissue and vasculature: Role of oxidative stress in obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef] [Green Version]
- Chechi, K.; Voisine, P.; Mathieu, P.; Laplante, M.; Bonnet, S.; Picard, F.; Joubert, P.; Richard, D. Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue. Sci. Rep. 2017, 7, 15566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Guo, Z.; Wang, P.; Zheng, M.; Yang, X.; Liu, Y.; Ma, Z.; Chen, M.; Yang, X. Proteomics of epicardial adipose tissue in patients with heart failure. J. Cell. Mol. Med. 2020, 24, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Guglielmi, V.; Sbraccia, P. Epicardial adipose tissue: At the heart of the obesity complications. Acta Diabetol. 2017, 54, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Overnutrition, ectopic lipid and the metabolic syndrome. J. Investig. Med. 2016, 64, 1082–1086. [Google Scholar] [CrossRef] [Green Version]
- Kahl, K.G.; Kerling, A.; Tegtbur, U.; Gützlaff, E.; Herrmann, J.; Borchert, L.; Ates, Z.; Westhoff-Bleck, M.; Hueper, K.; Hartung, D. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: A randomized pilot study. J. Affect. Disord. 2016, 192, 91–97. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). IDF Diabetes Atlas Nineth Edition. 2019. Available online: https://www.diabetesatlas.org/en/ (accessed on 3 August 2021).
- Wu, F.Z.; Huang, Y.L.; Wu, C.C.; Wang, Y.C.; Pan, H.J.; Huang, C.K.; Yeh, L.R.; Wu, M.T. Differential effects of bariatric surgery versus exercise on excessive visceral fat deposits. Medicine 2016, 95, e2616. [Google Scholar] [CrossRef]
- Honkala, S.M.; Motiani, K.K.; Eskelinen, J.J.; Savolainen, A.; Saunavaara, V.; Virtanen, K.A.; Löyttyniemi, E.; Kapanen, J.; Knuuti, J.; Kalliokoski, K.K.; et al. Exercise training reduces intrathoracic fat regardless of defective glucose tolerance. Med. Sci. Sports Exerc. 2017, 49, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-del-Valle, M.; Gonzales, J.U.; Kloiber, S.; Mitra, S.; Klingensmith, J.; Larumbe-Zabala, E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: A randomized pilot study. Eur. J. Appl. Physiol. 2018, 118, 1231–1240. [Google Scholar] [CrossRef]
- Jo, E.A.; Cho, K.I.; Park, J.J.; Im, D.S.; Choi, J.H.; Kim, B.J. Effects of high-intensity interval training versus moderate-intensity continuous training on epicardial fat thickness and endothelial function in hypertensive metabolic syndrome. Metab. Syndr. Relat. Disord. 2020, 18, 96–102. [Google Scholar] [CrossRef]
- Jonker, J.T.; de Mol, P.; de Vries, S.T.; Widya, R.L.; Hammer, S.; van Schinkel, L.D.; van der Meer, R.W.; Gans, R.O.; Webb, A.G.; Kan, H.E.; et al. Exercise and type 2 diabetes mellitus: Changes in tissue-specific fat distribution and cardiac function. Radiology 2013, 269, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ferrer, J.; Crendal, E.; Walther, G.; Vinet, A.; Dutheil, F.; Naughton, G.; Lesourd, B.; Chapier, R.; Courteix, D.; Obert, P. Effects of lifestyle intervention on left ventricular regional myocardial function in metabolic syndrome patients from the RESOLVE randomized trial. Metabolism 2016, 65, 1350–1360. [Google Scholar] [CrossRef]
- Bairapareddy, K.C.; Maiya, A.G.; Kumar, P.; Nayak, K.; Guddattu, V.; Nayak, V. Effect of aerobic exercise on echocardiographic epicardial adipose tissue thickness in overweight individuals. Diabetes Metab. Syndr. Obes. 2018, 11, 303–312. [Google Scholar]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of aerobic and resistance exercise on cardiac adipose tissues: Secondary analyses from a randomized clinical trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Jo, E.A.; Wu, S.S.; Han, H.R.; Park, J.J.; Park, S.; Cho, K.I. Effects of exergaming in postmenopausal women with high cardiovascular risk: A randomized controlled trial. Clin. Cardiol. 2020, 43, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, S.; Gu, Y.; Song, L.; Yu, S.; Feng, X. Tai chi improves coronary heart disease risk by inactivating MAPK/ERK pathway through serum miR-126. Evid.-Based Complement. Alternat. Med. 2020, 2020, 4565438. [Google Scholar] [CrossRef]
- Olivieri, F.; Bonafè, M.; Spazzafumo, L.; Gobbi, M.; Prattichizzo, F.; Recchioni, R.; Marcheselli, F.; La Sala, L.; Galeazzi, R.; Rippo, M.R.; et al. Age- and glycemia-related miR-126-3p levels in plasma and endothelial cells. Aging 2014, 6, 771–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamorro-Jorganes, A.; Araldi, E.; Suárez, Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Prattichizzo, F.; Matacchione, G.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; de Candia, P.; De Nigris, V.; Ceriello, A. Extracellular vesicle-shuttled miRNAs: A critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics 2021, 11, 1031–1045. [Google Scholar] [CrossRef]
- Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell. Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Z.; Zang, W.; Jiang, H.; Li, Y.; Wang, S.; Chen, S. Exercise training reduces insulin resistance in postmyocardial infarction rats. Physiol. Rep. 2015, 3, e12339. [Google Scholar] [CrossRef] [Green Version]
- Leroux-Stewart, J.; Elisha, B.; Tagougui, S.; Suppère, C.; Bernard, S.; Mircescu, H.; Desjardin, K.; Messier, V.; Iacobellis, G.; Rabasa-Lhoret, R. Effect of caloric restriction with or without physical activity on body composition and epicardial fat in type 2 diabetic patients: A pilot randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 921–929. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 2 August 2021).
- Dias, S.; Pheiffer, C.; Abrahams, Y.; Rheeder, P.; Adam, S. Molecular biomarkers for gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 2926. [Google Scholar] [CrossRef] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Ferrannini, E.; Iozzo, P.; Virtanen, K.A.; Honka, M.J.; Bucci, M.; Nuutila, P. Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: Role of blood flow and diabetes. Am. J. Clin. Nutr. 2018, 108, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.H.; von Scholten, B.J.; Lehrskov, L.L.; Rossing, P.; Jørgensen, P.G. Epicardial adipose tissue: An emerging biomarker of cardiovascular complications in type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820928824. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nyambuya, T.M.; Johnson, R.; Silvestri, S.; Orlando, P.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Mxinwa, V.; Mokgalaboni, K.; Tiano, L.; et al. Metformin and heart failure-related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2020, 26, 1437–1445. [Google Scholar] [CrossRef]
- Lee, M.M.Y.; Sattar, N.; McMurray, J.J.V.; Packard, C.J. Statins in the prevention and treatment of heart failure: A review of the evidence. Curr. Atheroscler. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).