Response to Antimony Toxicity in Dittrichia viscosa Plants: ROS, NO, H2S, and the Antioxidant System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Treatments
2.2. Determination of Sb and Mineral Content
2.3. Determination of Photosynthetic Pigment Contents and Photosynthetic Efficiency
2.4. Determination of Lipid Peroxidation and Reactive Oxygen Species (O2●− and H2O2), NO and H2S Content
2.5. Determination of Enzymatic Activities
2.6. Determination of Phenolics Content
2.7. Determination of the Components of the AsA/GSH Cycle
2.8. Visualization and Determination of ROS, RNS, and H2S
2.9. Statistical Analyses
3. Results
3.1. Effect of Sb on the Growth of D. viscosa Plants
3.2. Effect of Sb on the Accumulation of Sb and Other Mineral Elements
3.3. Effect of Sb on Photosynthetic Pigment Content and Photosynthetic Efficiency
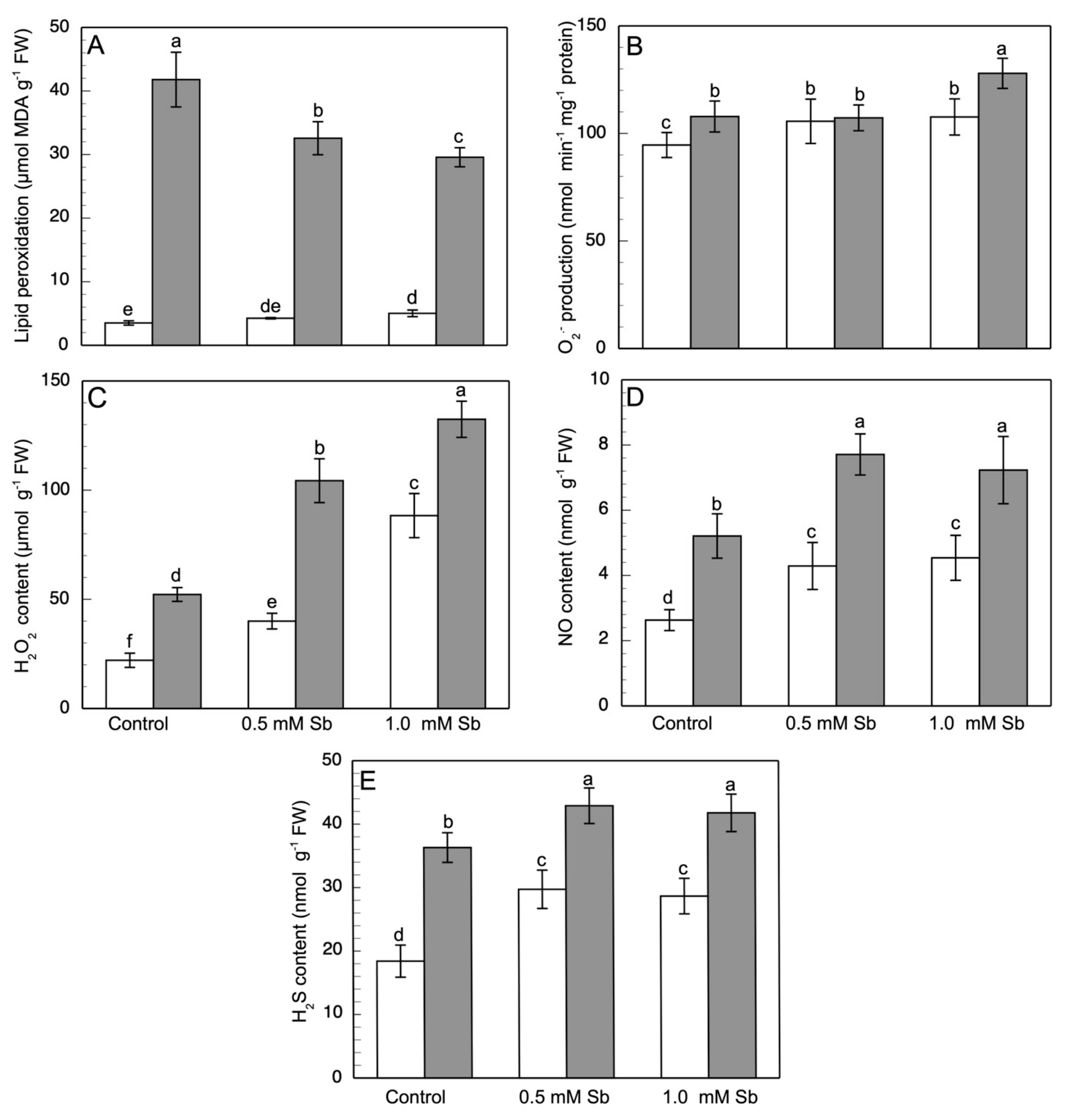
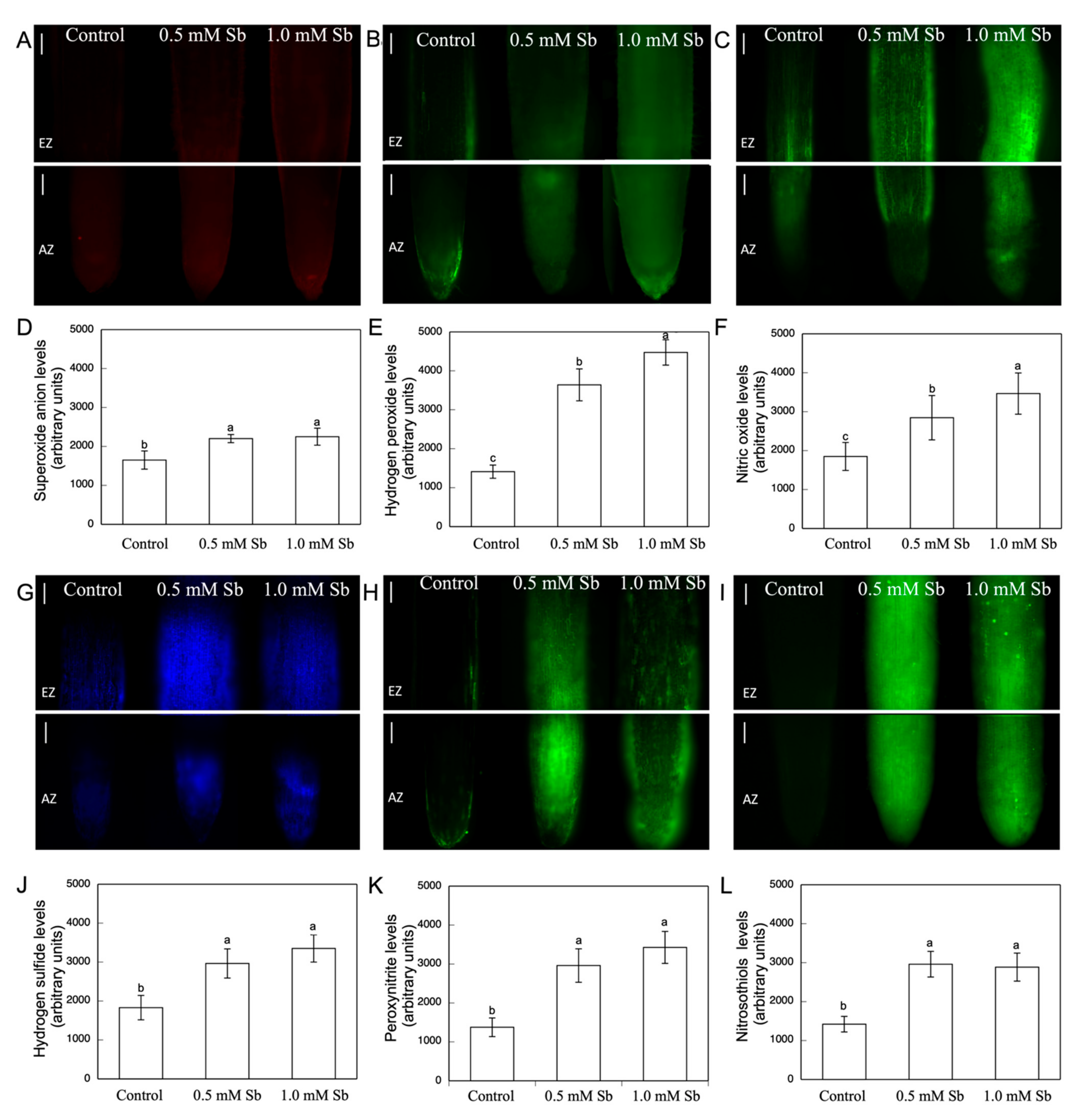
3.4. Lipid Peroxidation and the Content of ROS, NO, H2S, ONOO−, and RSNOs
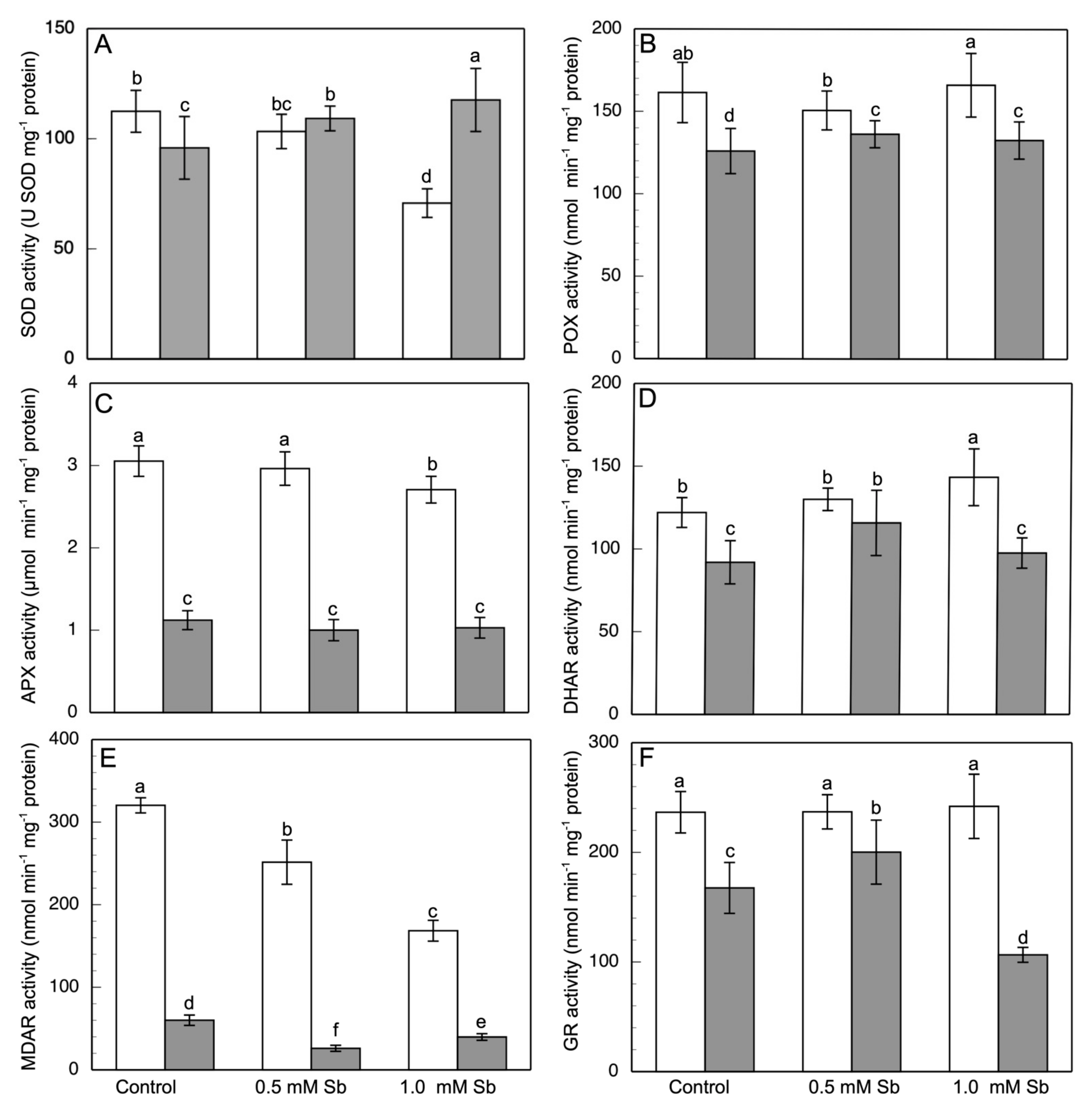
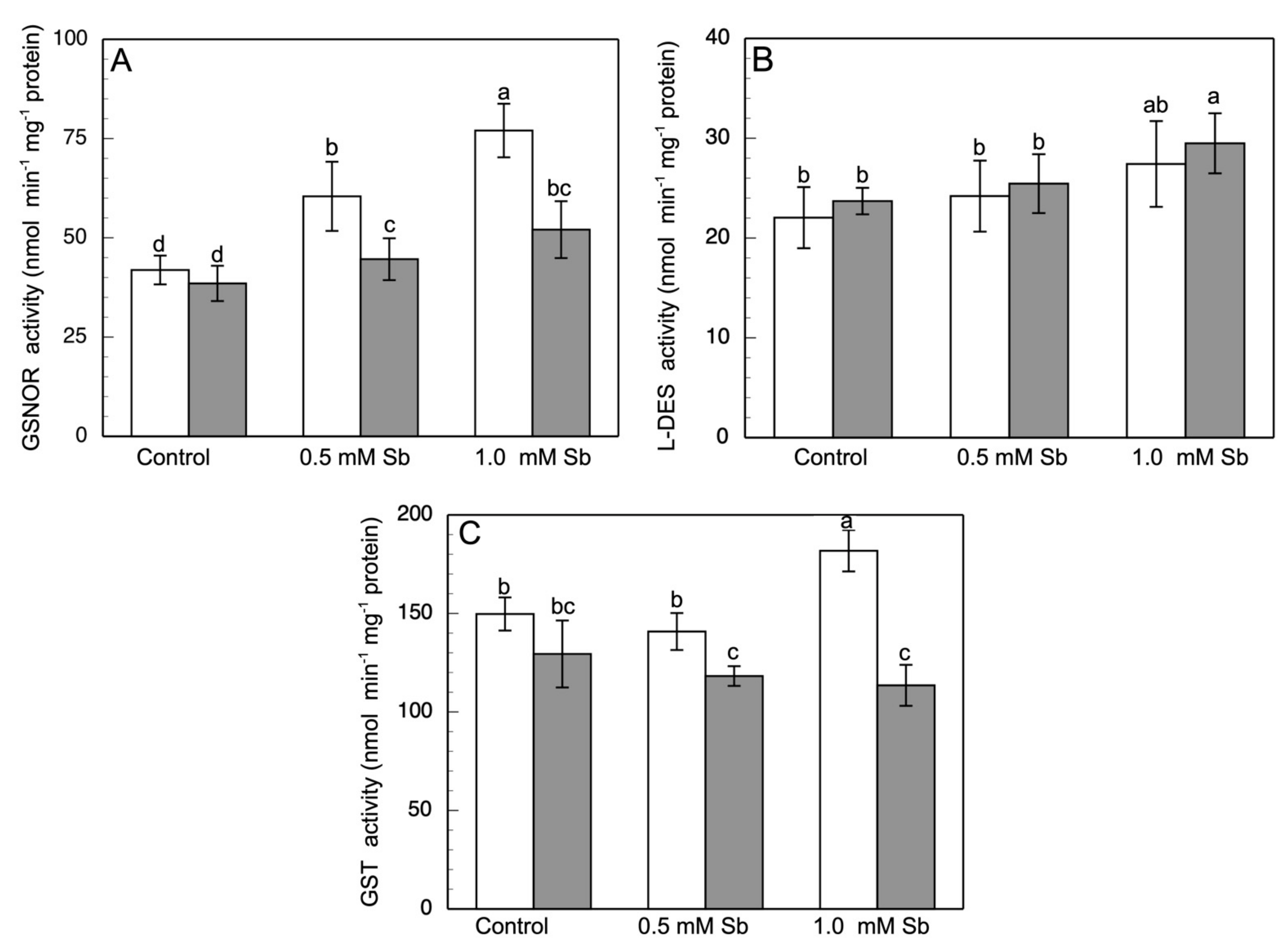
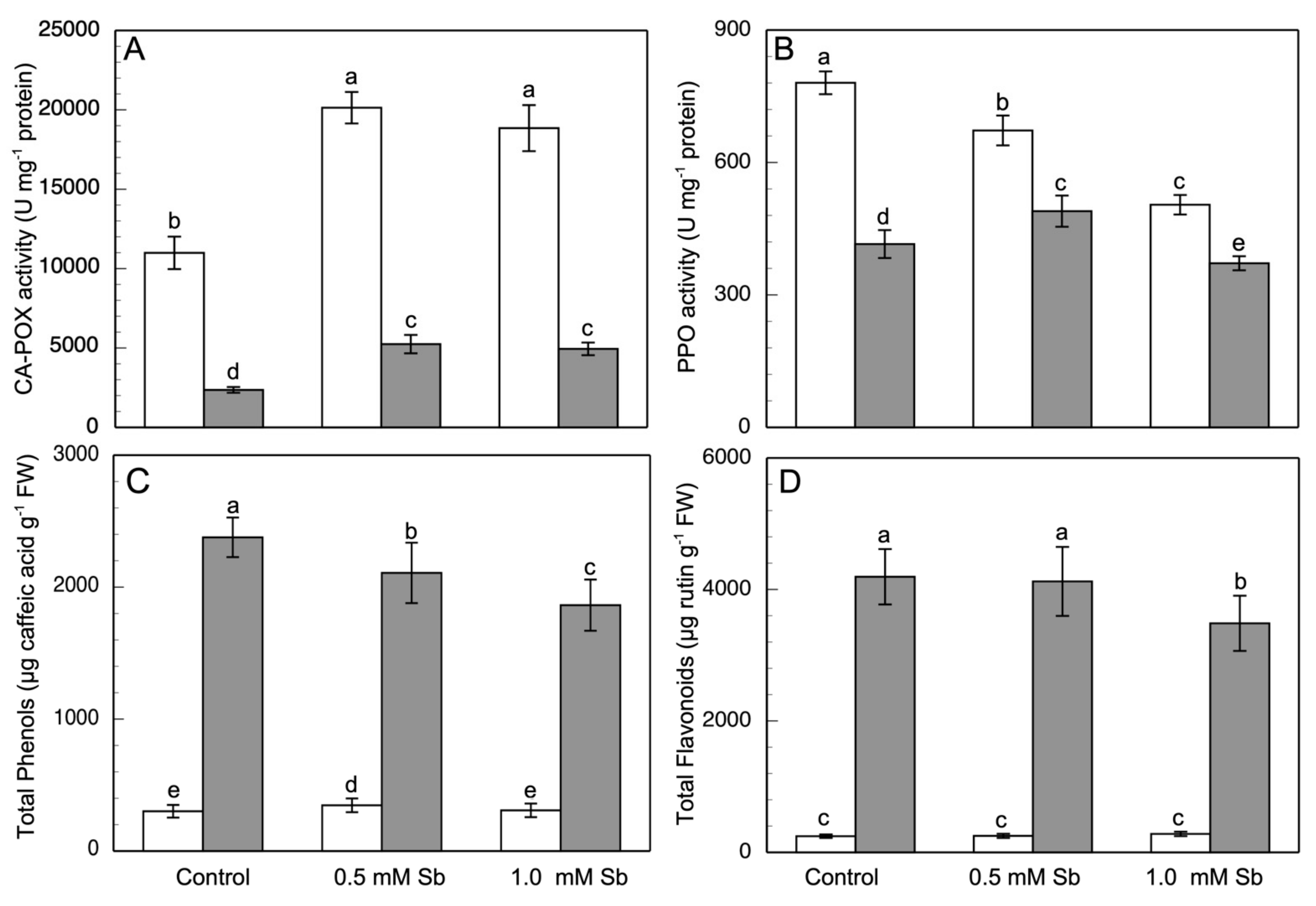
3.5. Effect of Sb on Enzymatic Activities
3.6. Effect of Sb on the Phenolic Compound Content
3.7. Effect of Sb on the Components of the AsA/GSH Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Wang, R.; Guo, J. The uptake and detoxification of antimony by plants: A review. Environ. Exp. Bot. 2013, 96, 28–34. [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; He, Y.; Zhou, Y.; Chen, X.; Ruan, S.; Yang, Y.; Dai, C.; Tang, L. Antimony contamination, consequences and removal techniques: A review. Ecotoxicol. Environ. Saf. 2018, 156, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, C.; Wang, Z.; Feng, G.; Du, E.; Liu, Y.; Wang, L.; Quiao, B.; Xu, Y.; Zhang, Z. Antimony enhances c-Myc stability in prostate cancer via activating CtBP2-ROCK1 signaling pathway. Ecotoxicol. Environ. Saf. 2018, 164, 61–68. [Google Scholar] [CrossRef]
- Tschan, M.; Robinson, B.H.; Schulin, R. Antimony in the soil-plant system—A review. Environ. Chem. 2009, 6, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Okkenhaug, G.; Zhu, Y.G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- García-Lorenzo, M.L.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; López-Sánchez, J.; Molina-Ruiz, J.; Tudela, M.L. Geogenic distribution of arsenic (as) and antimony (Sb) in soils of the Murcia region in Spain. Environ. Forensics. 2015, 16, 88–95. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Zhang, C.; Zeng, G.; Liu, Y.; Xu, W.; Wu, Y.; Lan, S. Spatial distribution and transport characteristics of heavy metals around an antimony mine area in central China. Chemosphere 2017, 170, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhou, J.; Zhou, A.; Liu, C.; Li, L. A review of antimony (Sb) isotopes analytical methods and application in environmental systems. Int. Biodeterior. Biodegrad. 2017, 128, 109–116. [Google Scholar] [CrossRef]
- Wu, T.L.; Cui, X.D.; Cui, P.X.; Ata-UI-Karim, S.T.; Sun, Q.; Liu, C.; Fan, T.T.; Gong, H.; Zhou, D.M.; Wang, Y.J. Speciation and location of arsenic and antimony in rice samples around antimony mining area. Environ. Pollut. 2019, 252, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, X.; Guo, X.; He, M. A review of removal technology for antimony in aqueous solution. J. Environ. Sci. 2020, 90, 189–204. [Google Scholar] [CrossRef]
- Baroni, F.; Boscagli, A.; Protano, G.; Riccobono, F. Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environ. Pollut. 2000, 109, 347–352. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Niemelä, M.; Perämäki, P. Effects of soil amendments on antimony uptake by wheat. J. Soils Sediments 2013, 14, 679–686. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Dumat, C.; Pierart, A.; Niazi, N.K. Biogeochemistry of antimony in soil-plant system: Ecotoxicology and human health. Appl. Geochem. 2019, 106, 45–59. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Wang, L.; Lin, Z.; Dai, J.; Wang, R.; Yu, Y.; Liu, H.; Rensing, C.; Feng, R. Factors influencing the uptake and speciation transformation of antimony in the soil-plant system, and the redistribution and toxicity of antimony in plants. Sci. Total Environ. 2020, 738, 140232. [Google Scholar] [CrossRef]
- Chai, L.Y.; Mubarak, H.; Yang, Z.H.; Yong, W.; Tang, C.J.; Mirza, N. Growth, photosynthesis and defense mechanism of antimnoy (Sb)-contaminated Boehmeria nivea L. Environ. Sci. Pollut. Res. 2016, 23, 7470–7481. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Singh, B.; Bali, R.; Ayrault, S.; Timofeev, S. Antimony Accumulation in Wheat Seedlings Grown in Soil and Water. Commun. Soil Sci. Plant Anal. 2014, 457, 968–983. [Google Scholar] [CrossRef]
- Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Effects of Antimony on Reactive Oxygen and Nitrogen Species (ROS and RNS) and Antioxidant Mechanisms in Tomato Plants. Front. Plant Sci. 2020, 11, 674. [Google Scholar] [CrossRef]
- Feng, R.; Lei, L.; Su, J.; Zhang, R.; Zhu, Y.; Chen, W.; Wang, L.; Wang, R.; Dai, J.; Lin, Z.; et al. Toxicity of different forms of antimony to rice plant: Effects on root exudates, cell wall components, endogenous hormones and antioxidant system. Sci. Total Environ. 2020, 711, 134589. [Google Scholar] [CrossRef]
- Zhong, Q.; Ma, C.; Chu, J.; Wang, X.; Liu, X.; Ouyang, W.; Lin, C.; He, M. Toxicity and bioavailability of antimony in edible amaranth (Amaranthus tricolor Linn.) cultivated in two agricultural soil types. Environ. Pollut. 2020, 257, 113642. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Gruhlke, M.C. Reactive Sulfur Species. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 715–728. [Google Scholar] [CrossRef]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Kapoor, D.; Kumar, S.; Singh, S.; Dhanjal, D.S.; Datta, S.; Samuel, J.; Dey, P.; Wang, S.; et al. Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol. Plant 2019, 168, 301–317. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Loake, G.J.; Chu, C. Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front. Plant Sci. 2013, 4, 314. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 2017, 67, 39–52. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. Abiotech 2021, 2, 32–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S.; Wu, F.; Yang, L. Antimony accumulation and antioxidative responses in four fern plants. Plant Soil 2008, 317, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zhang, D.; Chen, X.; Bao, A.; Li, L. Antimony Accumulation, Growth Performance, Antioxidant Defense System and Photosynthesis of Zea mays in Response to Antimony Pollution in Soil. Water Air Soil Pollut. 2011, 215, 517–523. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Peroxynitrite (ONOO−) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Ann. Bot. 2013, 113, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Ruiz, M.R.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Karacan, M.S.; Rodionova, M.V.; Tunç, T.; Venedik, K.B.; Mamaş, S.; Shitov, A.V.; Zharmukhamedov, S.K.; Klimov, V.V.; Karacan, N.; Allakhverdiev, S.I. Characterization of nineteen antimony(III) complexes as potent inhibitors of photosystem II, carbonic anhydrase, and glutathione reductase. Photosynth. Res. 2016, 130, 167–182. [Google Scholar] [CrossRef]
- Ma, C.; He, M.; Zhong, Q.; Ouyang, W.; Lin, C.; Liu, X. Uptake, translocation and phytotoxicity of antimonite in wheat (Triticum aestivum). Sci. Total Environ. 2019, 669, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Garrido, I.; Casimiro, I.; Espinosa, F. Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS ONE 2017, 12, e0183991. [Google Scholar] [CrossRef] [Green Version]
- Santisree, P.; Adimulam, S.S.; Sharma, K.; Bhatnagar-Mathur, P.; Sharma, K.K. Insights into the nitric oxide mediated stress tolerance in plants. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 385–406. [Google Scholar] [CrossRef]
- Ahsan, N.; Renaut, J.; Komatsu, S. Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 2009, 9, 2602–2621. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Zhang, F.-Q.; Wang, Y.-S.; Lou, Z.-P.; Dong, J.-D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef]
- Qiao, W.; Li, C.; Fan, L.-M. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ. Exp. Bot. 2014, 100, 84–93. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. BioMetals 2012, 25, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen sulfide decreases Cd translocation from root to shoot through increasing Cd accumulation in cell wall and decreasing Cd2+ influx in Isatis indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.K.; Singh, V.P. Glutathione and hydrogen sulfide are required for sulfur-mediated mitigation of Cr(VI) toxicity in tomato, pea and brinjal seedlings. Physiol. Plant 2019, 168, 406–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.K.; Gautam, A. Stress responsive gene regulation in relation to hydrogen sulfide in plants under abiotic stress. Physiol. Plant 2020, 168, 511–525. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Calderón-Urrea, A.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Tang, Z. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil 2020, 449, 1–10. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Fontes, E.; Modolo, L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017, 256, 148–159. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, A.; Ansary, M.U.; Watanabe, A.; Fujita, M.; Tran, L.-S. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant 2019, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Murciego, A.M.; Sánchez, A.G.; González, M.R.; Gil, E.P.; Gordillo, C.T.; Fernández, J.C.; Triguero, T.B. Antimony distribution and mobility in topsoils and plants (Cytisus striatus, Cistus ladanifer and Dittrichia viscosa) from polluted Sb-mining areas in Extremadura (Spain). Environ. Pollut. 2007, 145, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Ortega, A.; Hernández, M.; Fernández-Pozo, L.; Cabezas, J.; Espinosa, F. Effect of antimony in soils of an Sb mine on the photosynthetic pigments and antioxidant system of Dittrichia viscosa leaves. Environ. Geochem. Health 2021, 43, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Guarino, F.; Conte, B.; Improta, G.; Sciarrillo, R.; Castiglione, S.; Cicatelli, A.; Guarino, C. Genetic characterization, micropropagation, and potential use for arsenic phytoremediation of Dittrichia viscosa (L.) Greuter. Ecotoxicol. Environ. Saf. 2018, 148, 675–683. [Google Scholar] [CrossRef]
- Papadia, P.; Barozzi, F.; Angilé, F.; Migoni, D.; Piro, G.; Fanizzi, F.P.; Di Sansebastiano, G.-P. Evaluation of Dittrichia viscosa performance in substrates with moderately low levels of As and Cd contamination. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 154, 983–989. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Pető, A.; Feigl, G.; Ördög, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.A. Simple Microdetermination of Kjeldahl Nitrogen in Biological Materials. Anal. Chem. 1958, 30, 1692–1694. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv-/Fm-; without measuring Fo-. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Garrido, I.; García-Sánchez, M.; Casimiro, I.; Casero, P.J.; García-Romera, I.; Ocampo, J.A.; Espinosa, F. Oxidative Stress Induced in Sunflower Seedling Roots by Aqueous Dry Olive-Mill Residues. PLoS ONE 2012, 7, e46137. [Google Scholar] [CrossRef] [Green Version]
- Sergiev, I.; Alxieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comp. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Zhou, B.; Guo, Z.; Xing, J.; Huang, B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005, 56, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hu, L.-Y.; Hu, K.-D.; He, Y.-D.; Wang, S.-H.; Luo, J.-P. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and Ozone-Induced Biochemical Changes in Antioxidant Enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- De Gara, L.; De Pinto, M.C.; Moliterni, V.M.C.; D’Egidio, M.G. Redox regulation and storage processes during maturation in kernels of Triticum durum. J. Exp. Bot. 2003, 54, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate Reductase in Spinach Chloroplasts and Its Participation in Regeneration of Ascorbate for Scavenging Hydrogen Peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar] [CrossRef]
- Sakamoto, A.; Ueda, M.; Morikawa, H. Arabidopsisglutathione-dependent formaldehyde dehydrogenase is anS-nitrosoglutathione reductase. FEBS Lett. 2002, 515, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Hossain, Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53–64. [Google Scholar]
- Riemenschneider, A.; Nikiforova, V.; Hoefgen, R.; De Kok, L.J.; Papenbrock, J. Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol. Biochem. 2005, 43, 473–483. [Google Scholar] [CrossRef]
- Thipyapong, P.; Hunt, M.D.; Steffens, J.C. Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry 1995, 40, 673–676. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Troudsale, E. Caftaric acid disappearance and conversion to products of enzymatic oxidation in grape must and wine. Am. J. Enol. Viticul. 1985, 36, 50–56. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- De Pinto, M.C.; Francis, D.; De Gara, L. The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 1999, 209, 90–97. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Ocaña, A.M.F.; Chaki, M.; Luque, F.; Rodríguez, M.V.G.; Colmenero-Varea, P.; Del Río, L.A.; Barroso, J.B. Nitrosative stress in plants. FEBS Lett. 2007, 581, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Carreras, A.; Esteban, F.J.; Chaki, M.; Valderrama, R.; Del Río, L.A.; Barroso, J.B. Localization of S-Nitrosothiols and Assay of Nitric Oxide Synthase and S-Nitrosoglutathione Reductase Activity in Plants. Methods Enzymol. 2008, 437, 561–574. [Google Scholar] [CrossRef]
- Liu, D.; Xu, S.; Hu, H.; Pan, J.; Li, P.; Shen, W. Endogenous Hydrogen Sulfide Homeostasis Is Responsible for the Alleviation of Senescence of Postharvest Daylily Flower via Increasing Antioxidant Capacity and Maintained Energy Status. J. Agric. Food Chem. 2017, 65, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Rodríguez-Serrano, M.; del Rio, L.A. Reactive oxygen species and signalling in cadmium toxicity. In Reactive Oxygen Species in Plant Signaling; del Rio, L.A., Puppo, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 175–189. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2017, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, C.; Zhu, P.; Liu, F. Effects of Antimony Stress on Photosynthesis and Growth of Acorus calamus. Front. Plant Sci. 2018, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Ma, L.Q.; Sun, H.; Cai, F.; Luo, J. Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci. Total Environ. 2014, 475, 83–89. [Google Scholar] [CrossRef]
- Ji, Y.; Mestrot, A.; Schulin, R.; Tandy, S. Uptake and Transformation of Methylated and Inorganic Antimony in Plants. Front. Plant Sci. 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhamdi, A.; Bentellis, A.; Rached, O.; Du Laing, G.; Mechakra, A. Effects of Antimony and Arsenic on Antioxidant Enzyme Activities of Two Steppic Plant Species in an Old Antimony Mining Area. Biol. Trace Element Res. 2014, 158, 96–104. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Sánchez, M.J.M.; Martínez-López, S.; Bech, J.; Bolan, N. Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscosa growing in mining-affected semiarid soils in southeast Spain. J. Geochem. Explor. 2012, 123, 128–135. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Steinnes, E.; Lierhagen, S. Uptake of different forms of antimony by wheat and rye seedlings. Environ. Sci. Pollut. Res. 2011, 19, 502–509. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Bali, R.; Harris, A. Bioavailability and toxicity of antimony. J. Geochem. Explor. 2011, 110, 40–45. [Google Scholar] [CrossRef]
- Ruiz, M.R.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, X.; Mu, G.; Wang, J. Toxic effects of antimony on photosystem II of Synechocystis sp. as probed by in vivo chlorophyll fluorescence. Environ. Boil. Fishes 2010, 22, 479–488. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Li, S.; Gao, M.; Shi, S.; Chang, E.; Wei, Y.; Yao, X.; Jiang, Z.; Liu, J. Comparative proteomic analysis in Miscanthus sinensis exposed to antimony stress. Environ. Pollut. 2015, 201, 150–160. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Jain, M.; Tiwary, S.; Gadre, R. Sorbitol-induced changes in various growth and biochemici parameters in maize. Plant Soil Environ. 2010, 56, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Chai, L.-Y.; Wang, Y.; Yang, Z.-H.; Mubarak, H.; Mirza, N. Physiological characteristics of Ficus tikoua under antimony stress. Trans. Nonferrous Met. Soc. China 2017, 27, 939–945. [Google Scholar] [CrossRef]
- Anderson, J.M.; Aro, E.-M. Grana stacking and protection of Photosystem II in thylakoid membranes of higher plant leaves under sustained high irradiance: An hypothesis. Photosynth. Res. 1994, 41, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S. Grana formation: Entropy-assisted local order in chloroplasts? Funct. Plant Biol. 1999, 26, 641–647. [Google Scholar] [CrossRef]
- Simkin, A.J.; Moreau, H.; Kuntz, M.; Pagny, G.; Lin, C.; Tanksley, S.; McCarthy, J. An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. J. Plant Physiol. 2008, 165, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Di Toppi, L.S.; Castagna, A.; Andreozzi, E.; Careri, M.; Predieri, G.; Vurro, E.; Ranieri, A. Occurrence of different inter-varietal and inter-organ defence strategies towards supra-optimal zinc concentrations in two cultivars of Triticum aestivum L. Environ. Exp. Bot. 2009, 66, 220–229. [Google Scholar] [CrossRef]
- Palm, E.; Nissim, W.G.; Giordano, C.; Mancuso, S.; Azzarello, E. Root potassium and hydrogen flux rates as potential indicators of plant response to zinc, copper and nickel stress. Environ. Exp. Bot. 2017, 143, 38–50. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant 2019, 168, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Wei, C.; Tu, S.; Tang, S.; Wu, F. Simultaneous hyperaccumulation of arsenic and antimony in Cretan brake fern: Evidence of plant uptake and subcellular distributions. Microchem. J. 2011, 97, 38–43. [Google Scholar] [CrossRef]
- Corrales, I.; Barceló, J.; Bech, J.; Poschenrieder, C. Antimony accumulation and toxicity tolerance mechanisms in Trifolium species. J. Geochem. Explor. 2014, 147, 167–172. [Google Scholar] [CrossRef]
- Vaculíková, M.; Vaculík, M.; Šimková, L.; Fialová, I.; Kochanová, Z.; Sedláková, B.; Luxová, M. Influence of silicon on maize roots exposed to antimony—Growth and antioxidative response. Plant Physiol. Biochem. 2014, 83, 279–284. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Lv, H.; Chen, W.; Wang, L.; Shi, S.; Yang, J.; Zhao, P.; Li, Y.; Christopher, R.; et al. Toxicity of different forms of antimony to rice plants: Effects on reactive oxidative species production, antioxidative systems, and uptake of essential elements. Environ. Pollut. 2020, 263, 114544. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 2015, 252, 1217–1229. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Ge, Y. Response of Glutathione and Glutathione S-transferase in Rice Seedlings Exposed to Cadmium Stress. Rice Sci. 2008, 15, 73–76. [Google Scholar] [CrossRef]
- Hou, X.; Tan, L.; Tang, S.-F. Molecular mechanism study on the interactions of cadmium (II) ions with Arabidopsis thaliana glutathione transferase Phi8. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Tran, L.-S. Hydrogen Sulfide Regulates Salt Tolerance in Rice by Maintaining Na+/K+ Balance, Mineral Homeostasis and Oxidative Metabolism Under Excessive Salt Stress. Front. Plant Sci. 2015, 6, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen Sulfide Alleviates Aluminum Toxicity via Decreasing Apoplast and Symplast Al Contents in Rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant 2019, 168, 256–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaraj, S.M.; Vats, S.; Bhat, J.A.; Dhakte, P.; Goyal, V.; Khatri, P.; Kumawat, S.; Singh, A.; Prasad, M.; Sonah, H.; et al. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol. Plant 2019, 168, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, L.; Tian, S.; Li, S.; Liu, X.; Gao, X.; Zhou, W.; Lin, X. Cadmium-induced nitric oxide burst enhances Cd tolerance at early stage in roots of a hyperaccumulator Sedum alfredii partially by altering glutathione metabolism. Sci. Total Environ. 2019, 650, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Ali, H.M.; Khan, M.I.R.; Singh, V.P.; Siddiqui, M.H. Exogenous nitric oxide requires endogenous hydrogen sulfide to induce the resilience through sulfur assimilation in tomato seedlings under hexavalent chromium toxicity. Plant Physiol. Biochem. 2020, 155, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant 2019, 168, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Garcia, B.M.; Queval, G.; Foyer, C. Glutathione in plants: An integrated overview. Plant Cell Environ. 2011, 35, 454–484. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.K.; Ali, H.M.; Siddiqui, M.H.; Singh, V.P. Nitric oxide-mediated regulation of sub-cellular chromium distribution, ascorbate–glutathione cycle and glutathione biosynthesis in tomato roots under chromium (VI) toxicity. J. Biotechnol. 2020, 318, 68–77. [Google Scholar] [CrossRef]
- Cui, W.; Chen, H.; Zhu, K.; Jin, Q.; Xie, Y.; Cui, J.; Xia, Y.; Zhang, J.; Shen, W. Cadmium-Induced Hydrogen Sulfide Synthesis Is Involved in Cadmium Tolerance in Medicago sativa by Reestablishment of Reduced (Homo)glutathione and Reactive Oxygen Species Homeostases. PLoS ONE 2014, 9, e109669. [Google Scholar] [CrossRef]
- Aslam, S.; Gul, N.; Mir, M.A.; Asgher, M.; Al-Sulami, N.; Abulfaraj, A.A.; Qari, S. Role of Jasmonates, Calcium, and Glutathione in Plants to Combat Abiotic Stresses Through Precise Signaling Cascade. Front. Plant Sci. 2021, 12, 149–181. [Google Scholar] [CrossRef]
- Sun, H.; Yan, S.C.; Cheng, W.S. Interaction of antimony tartrate with the tripeptide glutathione. JBIC J. Biol. Inorg. Chem. 2000, 267, 5450–5457. [Google Scholar] [CrossRef]
- Wojas, S.; Clemens, S.; Skłodowska, A.; Antosiewicz, D.M. Arsenic response of AtPCS1- and CePCS-expressing plants—Effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. J. Plant Physiol. 2010, 167, 169–175. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon L. Pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Per, T.S.; Masood, A.; Khan, N.A. Nitric oxide improves S-assimilation and GSH production to prevent inhibitory effects of cadmium stress on photosynthesis in mustard (Brassica juncea L.). Nitric Oxide 2017, 68, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Kostetski, I.; Chu, S.H.; Siau, J.L.; Bhatia, M.; Moore, P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-M.; Dawood, M.; Wang, N.-H.; Wu, F. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019, 89, 227–237. [Google Scholar] [CrossRef]

| Treatments | Length (cm) | FW (mg) | DW (mg) | Biomass Production (%) | |||
|---|---|---|---|---|---|---|---|
| Roots | Stems | Roots | Stems | Roots | Stems | ||
| Control | 47.11 ± 1.95a | 29.41 ± 1.05a | 3460 ± 210a | 5930 ± 243a | 232 ± 18a | 817 ± 51a | 100a |
| 0.5 mM Sb | 34.32± 1.11b | 23.43 ± 1.15b | 2620 ± 200b | 3853 ± 240b | 167 ± 13b | 687 ± 39b | 75.5 ± 3.1b |
| 1.0 mM Sb | 29.68 ± 1.50c | 24.13 ± 0.66b | 2250 ± 200c | 2404 ± 87c | 126 ± 14c | 506 ± 25c | 47.0 ± 2.8c |
| Treatments | Sb (µg Sb g−1 FW) | BF | TF | ||
|---|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | ||
| Control | 4.1 ± 1.1c | -- | -- | -- | -- |
| 0.5 mM Sb | 13,503 ± 225b | 810 ± 52.5b | 224a | 12.1a | 0.060a |
| 1.0 mM Sb | 24,450 ± 356a | 1547 ± 75.0a | 202a | 12.8a | 0.063a |
| Treatments | N (mg g−1 DW) | P (mg g−1 DW) | S (mg g−1 DW) | K (mg g−1 DW) | Ca (mg g−1 DW) | Mg (mg g−1 DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | |
| Control | 38.0 ± 0.45a | 39.2 ± 5.1a | 37.6 ± 4.0a | 32.3 ± 3.8a | 7.5 ± 1.0a | 4.4 ± 0.9a | 43.2 ± 5.0a | 39.0 ± 5.8a | 20.5 ± 3.0a | 17.4 ± 2.5a | 9.7 ± 1.5a | 7.2 ± 1.1a |
| 0.5 mM Sb | 27.4 ± 0.38b | 28.8 ± 4.0b | 38.6 ± 4.1a | 32.4 ± 4.2a | 4.9 ± 1.2b | 3.7 ± 0.7a | 31.0 ± 4.5b | 28.9 ± 3.3b | 14.1 ± 2.2b | 17.0 ± 1.9a | 4.7 ± 0.8b | 6.0 ± 1.5a |
| 1.0 mM Sb | 26.9 ± 0.30b | 23.4 ± 3.3b | 37.2 ± 4.7a | 30.8 ± 5.0a | 5.2 ± 1.1b | 3.2 ± 0.9b | 31.5 ± 4.b1 | 27.4 ± 3.5b | 13.9 ± 2.7b | 16.3 ± 2.7a | 4.6 ± 1.0b | 6.1 ± 1.3a |
| Treatments | Fe (μg g−1 DW) | Mn (μg g−1 DW) | Cu (μg g−1 DW) | Zn (μg g−1 DW) | B (μg g−1 DW) | |||||||
| roots | leaves | roots | leaves | roots | leaves | roots | leaves | roots | leaves | |||
| Control | 2298.3 ± 176.4a | 631.4 ± 81.4a | 753.3 ± 60.5a | 245.5 ± 31.3a | 94.8 ± 10.1a | 63.9 ± 6.1a | 55.4 ± 6.2b | 78.0 ± 8.5a | 165.2 ± 19.8a | 239.2 ± 18.5b | ||
| 0.5 mM Sb | 878.6 ± 22.6b | 526.7 ± 64.5b | 769.2 ± 91.5b | 260.6 ± 33.5a | 39.6 ± 4.2c | 55.8 ± 6.2b | 80.2 ± 7.4a | 64.4 ± 5.7b | 123.6 ± 13.4b | 288.0 ± 25.5a | ||
| 1.0 mM Sb | 620.9 ± 68.5c | 469.0 ± 55.3b | 440.6 ± 52.8c | 208.8 ± 34.0b | 49.1 ± 5.3b | 44.5 ± 5.6c | 48.5 ± 5.8b | 63.5 ± 5.1b | 56.9 ± 5.8c | 285.4 ± 25.9a | ||
| Treatments | Chl a (μg g−1 FW) | Chl b (μg g−1FW) | Chl a+b (μg g−1 FW) | Chl a/chl b | Carotenoids (μg g−1 FW) | Carotenoids/Total chl | Fv/Fm |
|---|---|---|---|---|---|---|---|
| Control | 1773.5 ± 42.6a | 838.7 ±46.4a | 2617.8a | 2.11c | 145.9 ± 7.1b | 0.056c | 0.789 ± 0.018a |
| 0.5 mM Sb | 1512.8 ±57.2b | 672.1 ± 34.5b | 2188.3b | 2.26b | 157.5 ± 6.3a | 0.072b | 0.783 ± 0.026a |
| 1.0 mM Sb | 1144.0 ± 34.1c | 488.2 ± 28.6c | 1630.8c | 2.35a | 155.1 ± 7.4a | 0.094a | 0.713 ± 0.031b |
| Treatments | AsA (nmol g−1 FW) | DHA (nmol g−1 FW) | AsA + DHA (nmol g−1 FW) | AsA/DHA | ||||
|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | |
| Control | 76.86 ± 7.63a | 185.30 ± 15.61b | 308.23 ± 18.57a | 1827.49 ± 129.10a | 385.06 ± 21.14a | 2012.72 ± 92.75a | 0.249 ± 0.030a | 0.101 ± 0.009c |
| 0.5 mM Sb | 70.30 ± 8.53a | 210.21 ± 10.17a | 339.24± 28.13a | 1288.89 ± 52.30b | 409.54 ± 32.17a | 1499.10 ± 105.11b | 0.210 ± 0.024a | 0.164 ± 0.009b |
| 1.0 mM Sb | 64.55 ± 8.05a | 213.97 ± 22.80a | 243.41 ± 13.10b | 1021.50 ± 100.13c | 307.92 ± 18.50b | 1237.47 ± 107.98c | 0.264 ± 0.027a | 0.224 ± 0.044a |
| Treatments | GSH (nmol g−1 FW) | GSSG (nmol g−1 FW) | GSH + GSSG (nmol g−1 FW) | GSH/GSSG | ||||
| roots | leaves | roots | leaves | roots | leaves | roots | leaves | |
| Control | 2.81 ± 0.32a | 5.01 ± 0.72a | 0.34 ± 0.04a | 10.10 ± 1.82a | 3.15 ± 0.30a | 15.11 ± 1.53a | 8.36 ± 1.3a | 0.492 ± 0.035a |
| 0.5 mM Sb | 0.90 ± 0.12b | 3.48 ± 0.16b | 0.23 ± 0.11b | 10.25 ± 0.86a | 1.13 ± 0.21b | 13.73 ± 1.10ab | 3.91 ± 0.8b | 0.342 ± 0.014b |
| 1.0 mM Sb | 0.54 ± 0.23c | 2.60 ± 0.29c | 0.14 ± 0.08c | 8.31 ± 0.49b | 0.68 ± 0.24c | 10.91 ± 0.57b | 3.85 ± 0.9b | 0.316 ± 0.044b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Response to Antimony Toxicity in Dittrichia viscosa Plants: ROS, NO, H2S, and the Antioxidant System. Antioxidants 2021, 10, 1698. https://doi.org/10.3390/antiox10111698
Espinosa-Vellarino FL, Garrido I, Ortega A, Casimiro I, Espinosa F. Response to Antimony Toxicity in Dittrichia viscosa Plants: ROS, NO, H2S, and the Antioxidant System. Antioxidants. 2021; 10(11):1698. https://doi.org/10.3390/antiox10111698
Chicago/Turabian StyleEspinosa-Vellarino, Francisco Luis, Inmaculada Garrido, Alfonso Ortega, Ilda Casimiro, and Francisco Espinosa. 2021. "Response to Antimony Toxicity in Dittrichia viscosa Plants: ROS, NO, H2S, and the Antioxidant System" Antioxidants 10, no. 11: 1698. https://doi.org/10.3390/antiox10111698
APA StyleEspinosa-Vellarino, F. L., Garrido, I., Ortega, A., Casimiro, I., & Espinosa, F. (2021). Response to Antimony Toxicity in Dittrichia viscosa Plants: ROS, NO, H2S, and the Antioxidant System. Antioxidants, 10(11), 1698. https://doi.org/10.3390/antiox10111698







