A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism
Abstract
1. Introduction: The Need for Oxygen Sensing
2. O2 Sensing Systems
3. Definition of an Oxygen Sensor
4. Metabolism of H2S as an O2 Sensing Mechanism
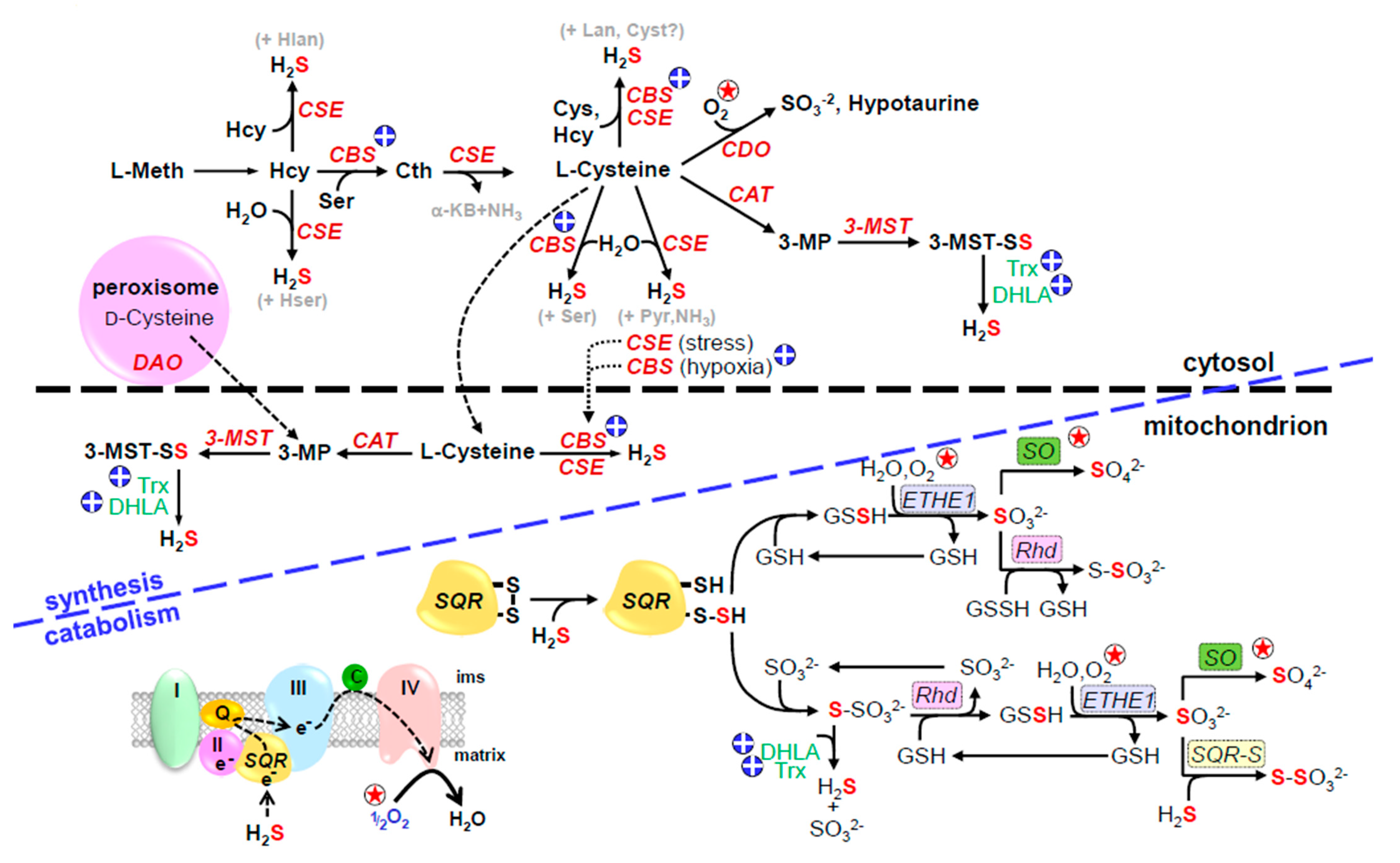
4.1. H2S Production and O2-Dependent Catabolism
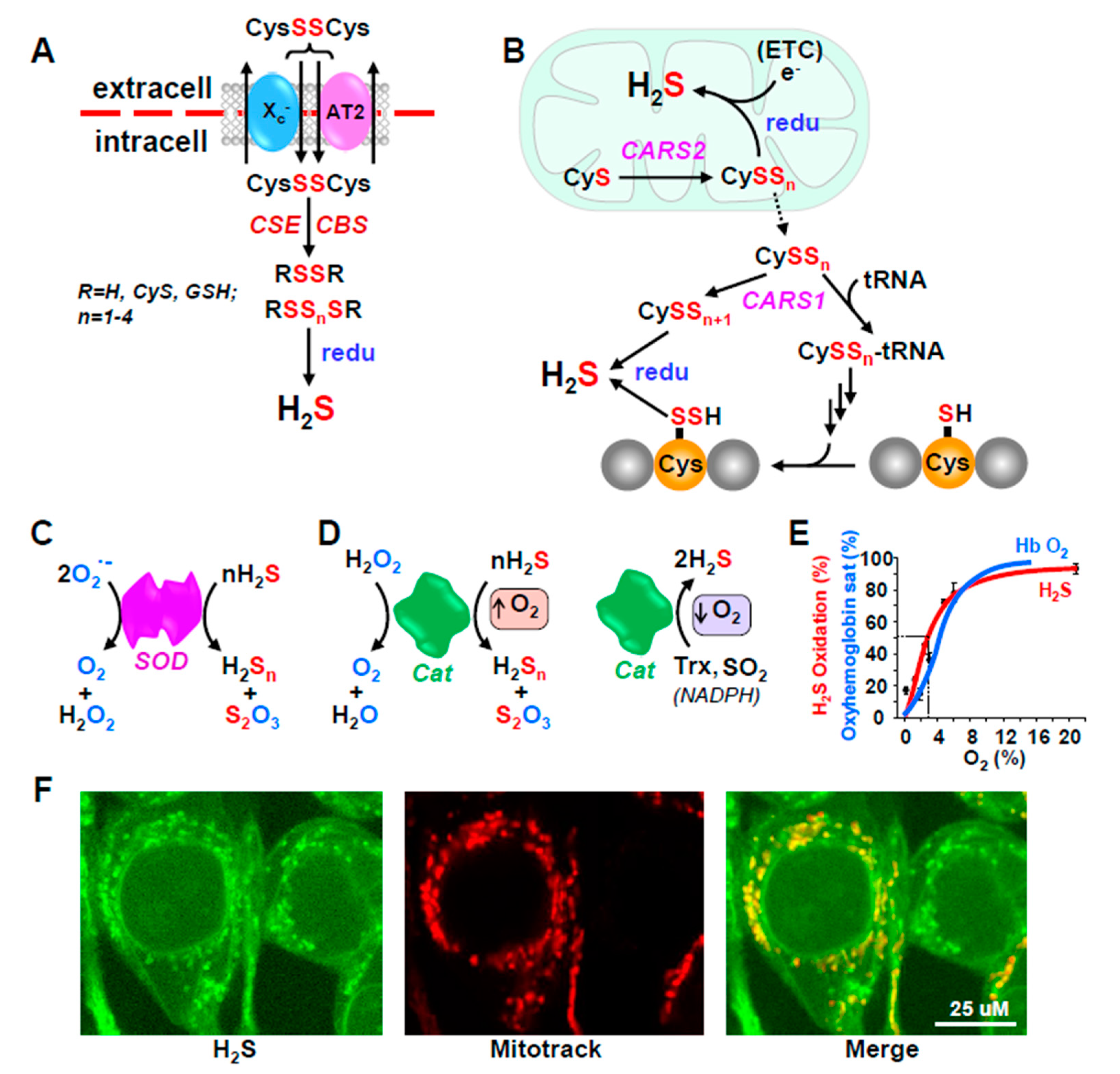
4.1.1. H2S Production from Cysteine and Methionine
4.1.2. H2S Production from Thiosulfate and Polysulfides
4.2. H2S Metabolism (Inactivation)
4.2.1. Conventional Pathways
4.2.2. Unconventional Pathways
5. Inverse and Po2-Dependent Relationship between O2 and H2S
6. Multiple Effectors of H2S Metabolism and Signaling Provide a Broad Timeline for O2 Sensing
6.1. H2S Signaling via Persulfidation
6.2. H2S Signaling via Reactions with Nitrogenous Compounds
6.3. H2S Signaling via Carbon Monoxide
6.4. Timescale of H2S/O2 Signaling
6.4.1. Rapid Responders
H2S Metabolism
Rapid Responding Effectors
6.4.2. Medium and Long-Term Responders
H2S Metabolism
Slow-Responding Effectors
7. Evidence for H2S Mediated O2 Sensing in Various Organ Systems and Tissues
7.1. Cardiovascular System
7.1.1. Blood Vessels
7.1.2. Heart
7.1.3. Central Cardiovascular Regulation
7.1.4. Ischemia/Reperfusion Injury
7.2. Respiratory System
7.2.1. General Effects on Respiration
7.2.2. H2S and Central Respiratory Centers
7.2.3. H2S Mediation of Peripheral Chemoreceptors, Carotid Body and Neuroepithelial Cells
7.2.4. H2S Mediation of O2 Sensing by Adrenal Medulla
7.2.5. Airway Receptors
7.2.6. Mechanical Effects on Airway Smooth Muscle
7.3. Kidney
7.4. Genitourinary Tract
7.5. H2S-HIF Interactions
8. Pathophysiological Consequences of the H2S/O2 Axis
8.1. Cerebral Ischemia and Stroke
8.2. High Altitude Pulmonary Edema (HAPE)
8.3. HAPE and Down Syndrome
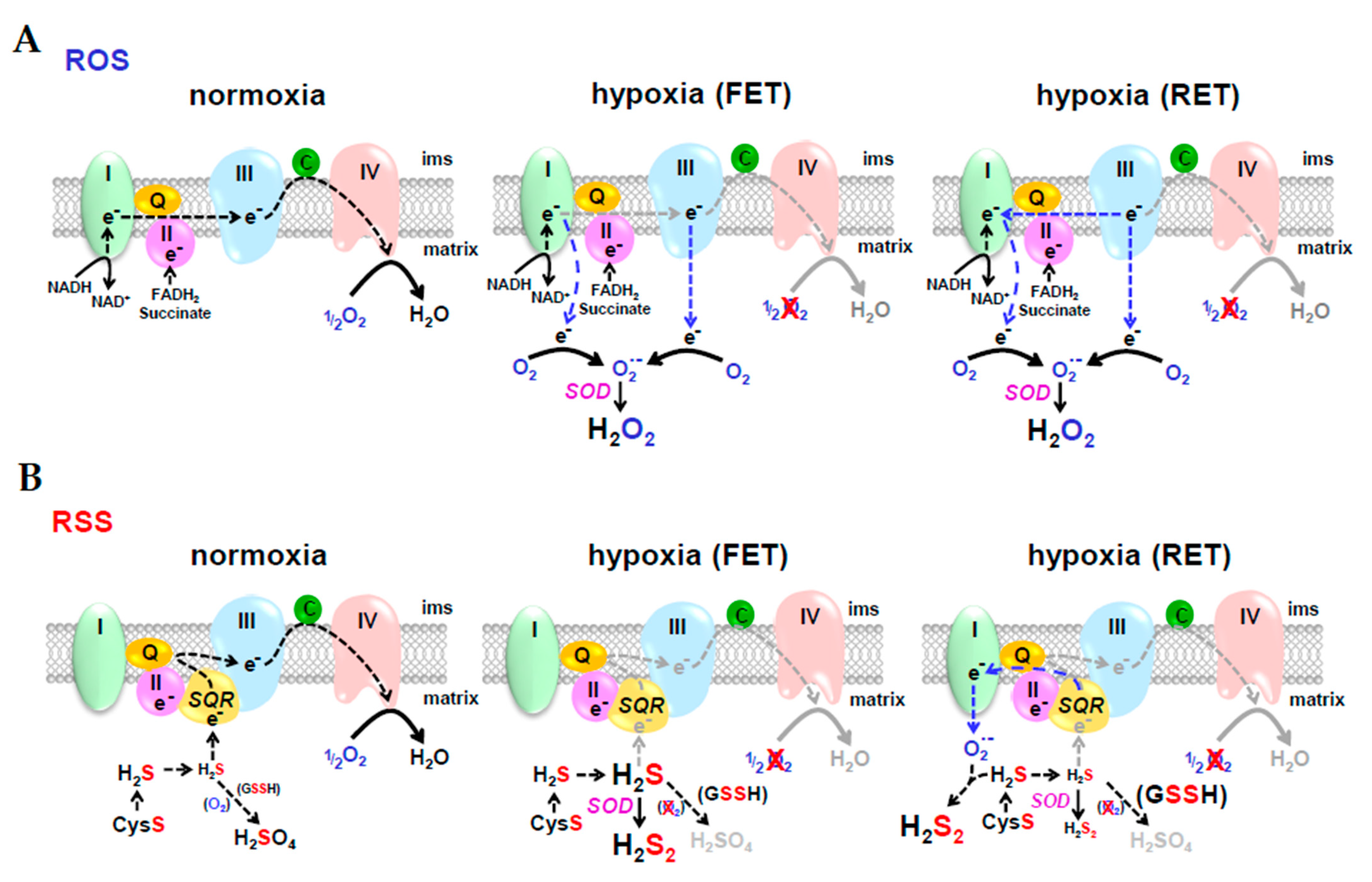
9. Resolving Differences between Competitive Theories of O2 Sensing; Reactive Oxygen Species (ROS) vs. Reactive Sulfur Species (RSS)
9.1. Chemical Similarities between ROS and RSS
9.2. ROS or RSS?
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Farrell, A.; Brauner, C. Hypoxia; Academic Press: London, UK, 2009. [Google Scholar]
- Milsom, W.K.; Burleson, M.L. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir. Physiol. Neurobiol. 2007, 157, 4–11. [Google Scholar] [CrossRef]
- Jonz, M.G.; Nurse, C.A. Peripheral chemoreceptors in air- versus water- breathers. Adv. Exp. Med. Biol. 2012, 758, 19–27. [Google Scholar] [PubMed]
- Kemp, P.J.; Lewis, A.; Hartness, M.E.; Searle, G.J.; Miller, P.; O’Kelly, I.; Peers, C. Airway chemotransduction: From oxygen sensor to cellular effector. Am. J. Respir. Crit. Care Med. 2002, 166, S17–S24. [Google Scholar] [CrossRef]
- Nurse, C.A.; Buttigieg, J.; Thompson, R.; Zhang, M.; Cutz, E. Oxygen sensing in neuroepithelial and adrenal chromaffin cells. Novartis. Found. Symp. 2006, 272, 106–114. [Google Scholar]
- Perry, S.F.; Montpetit, C.J.; Borowska, M. The effects of acute hypoxia on chemically or neuronally induced catecholamine secretion in rainbow trout (Oncorhynchus mykiss) in situ and in vivo. J. Exp. Biol. 2000, 203, 1487–1495. [Google Scholar] [CrossRef]
- Brinks, L.; Moonen, R.M.; Moral-Sanz, J.; Barreira, B.; Kessels, L.; Perez-Vizcaino, F.; Cogolludo, A.; Villamor, E. Hypoxia-induced contraction of chicken embryo mesenteric arteries: Mechanisms and developmental changes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R858–R869. [Google Scholar] [CrossRef]
- Mohammed, R.; Salinas, C.E.; Giussani, D.A.; Blanco, C.E.; Cogolludo, A.L.; Villamor, E. Acute hypoxia-reoxygenation and vascular oxygen sensing in the chicken embryo. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St. Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef]
- Russell, M.J.; Dombkowski, R.A.; Olson, K.R. Effects of hypoxia on vertebrate blood vessels. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, N.; Abe, A.S.; Andrade, D.V.; Wang, T. Hypoxic pulmonary vasoconstriction in reptiles: A comparative study of four species with different lung structures and pulmonary blood pressures. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1280–R1288. [Google Scholar] [CrossRef]
- Villamor, E.; Moreno, L.; Mohammed, R.; Perez-Vizcaino, F.; Cogolludo, A. Reactive oxygen species as mediators of oxygen signaling during fetal-to-neonatal circulatory transition. Free Radic. Biol. Med. 2019, 142, 82–96. [Google Scholar] [CrossRef]
- Agren, P.; Cogolludo, A.L.; Kessels, C.G.; Perez-Vizcaino, F.; de Mey, J.G.; Blanco, C.E.; Villamor, E. Ontogeny of chicken ductus arteriosus response to oxygen and vasoconstrictors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R485–R496. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A., 2nd; Altimiras, J. Cardiovascular development in embryos of the American alligator Alligator mississippiensis: Effects of chronic and acute hypoxia. J. Exp. Biol. 2005, 208, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bennie, R.E.; Packer, C.S.; Powell, D.R.; Jin, N.; Rhoades, R.A. Biphasic contractile response of pulmonary artery to hypoxia. Am. J. Physiol. 1991, 261, L156–L163. [Google Scholar] [CrossRef]
- Evans, A.M.; Hardie, D.G.; Peers, C.; Mahmoud, A. Hypoxic pulmonary vasoconstriction: Mechanisms of oxygen-sensing. Curr. Opin. Anaesthesiol. 2011, 24, 13–20. [Google Scholar] [CrossRef]
- Clanton, T.L.; Hogan, M.C.; Gladden, L.B. Regulation of cellular gas exchange, oxygen sensing, and metabolic control. Compr. Physiol. 2013, 3, 1135–1190. [Google Scholar] [PubMed]
- Kennel, K.B.; Burmeister, J.; Schneider, M.; Taylor, C.T. The PHD1 oxygen sensor in health and disease. J. Physiol. 2018, 596, 3899–3913. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 2017, 37, 115–121. [Google Scholar] [CrossRef]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox. Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox. Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.K.; Araujo, T.S.; Sousa, N.A.; Sousa, F.B.; Nogueira, K.M.; Nicolau, L.A.; Medeiros, J.V. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric. Oxide. 2017, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bearden, S.E.; Beard, R.S., Jr.; Pfau, J.C. Extracellular Transsulfuration Generates Hydrogen Sulfide from Homocysteine and Protects Endothelium from Redox Stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1568–H1576. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide: From Brain to Gut. Antioxid. Redox. Signal. 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Niu, W.; Wang, J.; Qian, J.; Wang, M.; Wu, P.; Chen, F.; Yan, S. Allosteric control of human cystathionine beta-synthase activity by a redox active disulfide bond. J. Biol. Chem. 2018, 293, 2523–2533. [Google Scholar] [CrossRef]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Ogasawara, Y.; Kimura, H. Hydrogen sulfide is produced by cystathionine gamma-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem. Biophys. Res. Commun. 2013, 431, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef]
- D’Imprima, E.; Mills, D.J.; Parey, K.; Brandt, U.; Kuhlbrandt, W.; Zickermann, V.; Vonck, J. Cryo-EM structure of respiratory complex I reveals a link to mitochondrial sulfur metabolism. Biochim. Biophys. Acta 2016, 1857, 1935–1942. [Google Scholar] [CrossRef]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human Sulfide:Quinone Oxidoreductase Catalyzes the First Step in Hydrogen Sulfide Metabolism and Produces a Sulfane Sulfur Metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef]
- Koj, A.; Frendo, J.; Wojtczak, L. Subcellular distribution and intramitochondrial localization of three sulfurtransferases in rat liver. FEBS Lett. 1975, 57, 42–466. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A Readily Accessible Source of Hydrogen Sulfide in Oxygen Sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef]
- Villarejo, M.; Westley, J. Mechanism of rhodanase catalysis of thiosulfate-lipoate oxidation-reduction. J. Biol. Chem. 1963, 238, 4016–4020. [Google Scholar] [CrossRef]
- Villarejo, M.; Westley, J. Rhodanese-catalyzed reduction of thiosulfate by reduced lipoic acid. J. Biol. Chem. 1963, 238, 1185–1186. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; et al. Stahelin, Extended hypoxia-mediated H2 S production provides for long-term oxygen sensing. Acta Physiol. 2020, 228, e13368. [Google Scholar] [CrossRef] [PubMed]
- Doka, E.; Pader, I.; Biro, A.; Johansson, K.; Cheng, Q.; Ballago, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Doka, E.; Fabian, I.; Nagy, P. Kinetic and thermodynamic studies on the disulfide-bond reducing potential of hydrogen sulfide. Nitric. Oxide 2015, 46, 93–101. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Jamurtas, A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- Wlodek, P.J.; Iciek, M.B.; Milkowski, A.; Smolenski, O.B. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin. Chim. Acta 2001, 304, 9–18. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc(−) cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Sims, B.; Clarke, M.; Francillion, L.; Kindred, E.; Hopkins, E.S.; Sontheimer, H. Hypoxic preconditioning involves system Xc− regulation in mouse neural stem cells. Stem Cell Res. 2012, 8, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L. Transport of H2S and HS(−) across the human red blood cell membrane: Rapid H2S diffusion and AE1-mediated Cl(−)/HS(−) exchange. Am. J. Physiol. Cell Physiol. 2013, 305, C941–C950. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Missner, A.; Kugler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef]
- Olson, K.R. A theoretical examination of hydrogen sulfide metabolism and its potential in autocrine/paracrine oxygen sensing. Respir. Physiol. Neurobiol. 2013, 186, 173–179. [Google Scholar] [CrossRef]
- Kohl, J.B.; Mellis, A.T.; Schwarz, G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 2019, 176, 554–570. [Google Scholar] [CrossRef]
- Jackson, M.R.; Loll, P.J.; Jorns, M.S. X-Ray Structure of Human Sulfide:Quinone Oxidoreductase: Insights into the Mechanism of Mitochondrial Hydrogen Sulfide Oxidation. Structure 2019, 27, 794–805.e794. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Zhang, M.; Huang, C.; Song, Y.; Xu, F.; Zhang, J.; Li, J.; He, M.; Li, Y.; et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020, 6, eaaz5752. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, E.; Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Blachier, F.; Bouillaud, F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta 2010, 1797, 1500–1511. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox. Biol. 2017, 15, 74–85. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox. Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Skora, N.C.; Whelan, J.; Villa, B.P.; Yuan, X.; Mannam, V.; Howard, S.; et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox Biol. 2020, 37, 101731. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Skora, N.C.; Whelan, J.; Villa, B.P.; Straub, K.D. ‘Antioxidant’ berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021, 165, 67–78. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Straub, K.D. Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. Int. J. Mol. Sci. 2021, 22, 961. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H2S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef]
- Doeller, J.E.; Isbell, T.S.; Benavides, G.; Koenitzer, J.; Patel, H.; Patel, R.P.; Lancaster, J.R., Jr.; Darley-Usmar, V.M.; Kraus, D.W. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 2005, 341, 40–51. [Google Scholar] [CrossRef]
- Kraus, D.W.; Doeller, J.E. Sulfide consumption by mussel gill mitochondria is not strictly tied to oxygen reduction: Measurements using a novel polarographic sulfide sensor. J. Exp. Biol. 2004, 207, 3667–3679. [Google Scholar] [CrossRef] [PubMed]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Dombkowski, R.A.; Naylor, M.G.; Shoemaker, E.; Smith, M.; DeLeon, E.R.; Stoy, G.F.; Gao, Y.; Olson, K.R. Hydrogen sulfide (H2S) and hypoxia inhibit salmonid gastrointestinal motility: Evidence for H2S as an oxygen sensor. J. Exp. Biol. 2011, 214, 4030–4040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drucker, N.A.; Winkel, J.P.T.; Shelley, W.C.; Olson, K.R.; Markel, T.A. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J. Pediatric Surg. 2019, 54, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Furne, J.; Stoltz, G.J.; Abdel-Rehim, M.S.; Levitt, M.D.; Szurszewski, J.H. Sulfide quinone reductase contributes to hydrogen sulfide metabolism in murine peripheral tissues but not in the central nervous system. Br. J. Pharmacol. 2011, 165, 2178–2190. [Google Scholar] [CrossRef]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012, 112, 411–418. [Google Scholar] [CrossRef]
- Olson, K.R.; Healy, M.J.; Qin, Z.; Skovgaard, N.; Vulesevic, B.; Duff, D.W.; Whitfield, N.L.; Yang, G.; Wang, R.; Perry, S.F. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R669–R680. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Whitfield, N.L. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid. Redox Signal. 2010, 12, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1930–R1937. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.P. Oxygen sensors in context. Biochim. Biophys. Acta 2008, 1777, 1–14. [Google Scholar] [CrossRef]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013, 591, 3549–3563. [Google Scholar] [CrossRef]
- Peng, Y.J.; Nanduri, J.; Raghuraman, G.; Souvannakitti, D.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. USA 2010, 107, 10719–10724. [Google Scholar] [CrossRef]
- Olson, K.R.; Russell, M.J.; Forster, M.E. Hypoxic vasoconstriction of cyclostome systemic vessels: The antecedent of hypoxic pulmonary vasoconstriction? Am. J. Physiol Regul. Integr. Comp. Physiol. 2001, 280, R198–R206. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Moller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Butler, A.R.; Woollins, J.D.; Feelisch, M. Inorganic sulfur-nitrogen compounds: From gunpowder chemistry to the forefront of biological signaling. Dalton. Trans. 2016, 45, 5908–5919. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020, 177, 720–733. [Google Scholar] [CrossRef]
- Kuschman, H.P.; Palczewski, M.B.; Thomas, D.D. Nitric oxide and hydrogen sulfide: Sibling rivalry in the family of epigenetic regulators. Free Radic. Biol. Med. 2021, 170, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Miller, C.G.; Schmidt, E.E. Sulfur Metabolism Under Stress. Antioxid. Redox Signal. 2020, 33, 1158–1173. [Google Scholar] [CrossRef]
- Paul, D.B.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol 2021, 38, 101772. [Google Scholar] [CrossRef]
- Suarez, S.A.; Vargas, P.; Doctorovich, F.A. Updating NO(*)/HNO interconversion under physiological conditions: A biological implication overview. J. Inorg. Biochem. 2021, 216, 111333. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Nie, X.W.; Huang, D.J.; Sun, M.T.; Bian, J.S. Role of nitroxyl (HNO) in cardiovascular system: From biochemistry to pharmacology. Pharmacol. Res. 2020, 159, 104961. [Google Scholar] [CrossRef] [PubMed]
- Doka, E.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020, 6, eaax8358. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef]
- Ondrias, K.; Stasko, A.; Cacanyiova, S.; Sulova, Z.; Krizanova, O.; Kristek, F.; Malekova, L.; Knezl, V.; Breier, A. H2S and HS(−) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflug. Arch. 2008, 457, 271–279. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.; Allgauer, A.; Chaurio, R.; Shubina, T.; Herrmann, M.; Ivanovic-Burmazovic, I. Biochemical insight into physiological effects of H2S: Reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012, 441, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Pardue, S.; Kolluru, G.K.; Shen, X.; Lewis, S.E.; Saffle, C.B.; Kelley, E.E.; Kevil, C.G. Hydrogen sulfide stimulates xanthine oxidoreductase conversion to nitrite reductase and formation of NO. Redox Biol. 2020, 34, 101447. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Yuan, G.; Vasavda, C.; Peng, Y.J.; Makarenko, V.V.; Raghuraman, G.; Nanduri, J.; Gadalla, M.M.; Semenza, G.L.; Kumar, G.K.; Snyder, S.H.; et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci. Signal. 2015, 8, ra37. [Google Scholar] [CrossRef]
- Gridina, A.; Su, X.; Khan, S.A.; Peng, Y.J.; Wang, B.; Nanduri, J.; Fox, A.P.; Prabhakar, N.R. Gaseous transmitter regulation of hypoxia-evoked catecholamine secretion from murine adrenal chromaffin cells. J. Neurophysiol. 2021, 125, 1533–1542. [Google Scholar] [CrossRef]
- Peng, Y.J.; Makarenko, V.V.; Gridina, A.; Chupikova, I.; Zhang, X.; Kumar, G.K.; Fox, A.P.; Prabhakar, N.R. H2S mediates carotid body response to hypoxia but not anoxia. Respir. Physiol. Neurobiol. 2019, 259, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Yuan, G.; Nanduri, J. Reactive oxygen radicals and gaseous transmitters in carotid body activation by intermittent hypoxia. Cell Tissue Res. 2018, 372, 427–431. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Sensing hypoxia: Physiology, genetics and epigenetics. J. Physiol. 2013, 591, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Drousiotou, A.; DiMeo, I.; Mineri, R.; Georgiou, T.; Stylianidou, G.; Tiranti, V. Ethylmalonic encephalopathy: Application of improved biochemical and molecular diagnostic approaches. Clin. Genet. 2011, 79, 385–390. [Google Scholar] [CrossRef]
- Giordano, C.; Viscomi, C.; Orlandi, M.; Papoff, P.; Spalice, A.; Burlina, A.; Di, M.I.; Tiranti, V.; Leuzzi, V.; d’Amati, G.; et al. Morphologic evidence of diffuse vascular damage in human and in the experimental model of ethylmalonic encephalopathy. J. Inherit. Metab. Dis. 2011, 35, 451–458. [Google Scholar] [CrossRef]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di, M.I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Rajapakshe, A.; Tollin, G.; Enemark, J.H. Kinetic and thermodynamic effects of mutations of human sulfite oxidase. Chem. Biodivers. 2012, 9, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Irreverre, F.; Laster, L. Sulfite oxidase deficiency in man: Demonstration of the enzymatic defect. Science 1967, 156, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Marks, J.D.; Guzy, R.; Mungai, P.T.; Schriewer, J.; Dokic, D.; Schumacker, P.T. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 2010, 106, 526–535. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Koenitzer, J.R.; Isbell, T.S.; Patel, H.D.; Benavides, G.A.; Dickinson, D.A.; Patel, R.P.; Darley-Usmar, V.M.; Lancaster, J.R., Jr.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2007, 297, H1953–H1960. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.N.; Gusakova, S.V.; Smaglii, L.V.; Koltsova, S.V.; Sidorenko, S.V. Vasoconstriction triggered by hydrogen sulfide: Evidence for Na(+),K(+),2Cl(-)cotransport and L-type Ca(2+) channel-mediated pathway. Biochem. Biophys. Rep. 2017, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Paredes, D.A.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ. Res. 2011, 108, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef]
- Lee, S.W.; Cheng, Y.; Moore, P.K.; Bian, J.S. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2007, 358, 1142–1147. [Google Scholar] [CrossRef]
- Miyamoto, R.; Koike, S.; Takano, Y.; Shibuya, N.; Kimura, Y.; Hanaoka, K.; Urano, Y.; Ogasawara, Y.; Kimura, H. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci. Rep. 2017, 7, 45995. [Google Scholar] [CrossRef]
- Dunn, W.R.; Alexander, S.P.; Ralevic, V.; Roberts, R.E. Effects of hydrogen sulphide in smooth muscle. Pharmacol. Ther. 2016, 158, 101–113. [Google Scholar] [CrossRef]
- Nagpure, B.V.; Bian, J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell Longev. 2016, 2016, 6904327. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxid. Med. Cell Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Tang, C.; Du, J.; Zhang, Z. Sulfur-containing gaseous signal molecules, ion channels and cardiovascular diseases. Br. J. Pharmacol. 2018, 175, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shen, X.; Kevil, C.G. Beyond a Gasotransmitter: Hydrogen Sulfide and Polysulfide in Cardiovascular Health and Immune Response. Antioxid. Redox Signal. 2017, 27, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Dromparis, P.; Michelakis, E.D. Mitochondria in vascular health and disease. Annu. Rev. Physiol. 2013, 75, 95–126. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I.; Dominy, J.E., Jr.; Simmons, C.R.; Hirschberger, L.L. Cysteine dioxygenase: A robust system for regulation of cellular cysteine levels. Amino. Acids 2009, 37, 55–63. [Google Scholar] [CrossRef]
- Ueki, I.; Roman, H.B.; Valli, A.; Fieselmann, K.; Lam, J.; Peters, R.; Hirschberger, L.L.; Stipanuk, M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol Endocrinol. Metab. 2011, 301, E668–E684. [Google Scholar] [CrossRef]
- Roman, H.B.; Hirschberger, L.L.; Krijt, J.; Valli, A.; Kozich, V.; Stipanuk, M.H. The Cysteine Dioxgenase Knockout Mouse: Altered Cysteine Metabolism in Nonhepatic Tissues Leads to Excess H2S/HS Production and Evidence of Pancreatic and Lung Toxicity. Antioxid. Redox. Signal. 2013, 19, 1321–1336. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric. Oxide 2014, 41, 11–26. [Google Scholar] [CrossRef]
- Baragatti, B.; Ciofini, E.; Sodini, D.; Luin, S.; Scebba, F.; Coceani, F. Hydrogen sulfide in the mouse ductus arteriosus: A naturally occurring relaxant with potential EDHF function. Am. J. Physiol Heart Circ. Physiol. 2013, 304, H927–H934. [Google Scholar] [CrossRef]
- Derwall, M.; Francis, R.C.; Kida, K.; Bougaki, M.; Crimi, E.; Adrie, C.; Zapol, W.M.; Ichinose, F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care 2011, 15, R51. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, N.; Olson, K.R. Hydrogen sulfide mediates hypoxic vasoconstriction through a production of mitochondrial ROS in trout gills. Am. J. Physiol Regul. Integr. Comp. Physiol. 2012, 303, R487–R494. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Swathi, Y.; Yeo, A.L.; Shoon, M.L.; Moore, P.K.; Bhatia, M. Hydrogen sulfide: Regulatory role on blood pressure in hyperhomocysteinemia. Vascul. Pharmacol. 2010, 53, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lloret, J.; Aaronson, P.I. Potentiation of Hypoxic Pulmonary Vasoconstriction by Hydrogen Sulfide Precursors 3-Mercaptopyruvate and D-Cysteine Is Blocked by the Cystathionine gamma Lyase Inhibitor Propargylglycine. Adv. Exp. Med. Biol. 2015, 860, 81–87. [Google Scholar] [PubMed]
- Prieto-Lloret, J.; Shaifta, Y.; Ward, J.P.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in isolated rat pulmonary arteries is not inhibited by antagonists of H2S-synthesizing pathways. J. Physiol. 2015, 593, 385–401. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Barrera, A.; Morales-Loredo, H.; Garcia, J.M.; Fregoso, G.; Pace, C.E.; Mendiola, P.J.; Naik, J.S.; Bosc, L.V.G.; Kanagy, N.L. Simulated sleep apnea alters hydrogen sulfide regulation of blood flow and pressure. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H511–H519. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Yu, W.; Chen, L.; Wang, Z.; Zhang, T. Expression of pulmonary arterial elastin in rats with hypoxic pulmonary hypertension using H2S. J. Recept. Signal. Transduct. Res. 2020, 40, 383–387. [Google Scholar] [CrossRef]
- Dongo, E.; Beliczai-Marosi, G.; Dybvig, A.S.; Kiss, L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide. 2018, 81, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2021, 27, 85–97. [Google Scholar] [CrossRef]
- Arndt, S.; Baeza-Garza, C.D.; Logan, A.; Rosa, T.; Wedmann, R.; Prime, T.A.; Martin, J.L.; Saeb-Parsy, K.; Krieg, T.; Filipovic, M.R.; et al. Assessment of H2S in vivo using the newly developed mitochondria-targeted mass spectrometry probe Mito A. J. Biol. Chem. 2017, 292, 7761–7773. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens 2011, 20, 107–112. [Google Scholar] [CrossRef]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef]
- LaPenna, K.B.; Polhemus, D.J.; Doiron, J.E.; Hidalgo, H.A.; Li, Z.; Lefer, D.J. Hydrogen Sulfide as a Potential Therapy for Heart Failure-Past, Present, and Future. Antioxidants 2021, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Polhemus, D.J.; Lefer, D.J. Evolution of Hydrogen Sulfide Therapeutics to Treat Cardiovascular Disease. Circ. Res. 2018, 123, 590–600. [Google Scholar] [CrossRef]
- Ufnal, M.; Nowinski, A. Central Administration of H2S Donors for Studying Cardiovascular Effects of H2S in Rats. Methods Mol. Biol. 2019, 2007, 167–172. [Google Scholar]
- Duan, X.C.; Liu, S.Y.; Guo, R.; Xiao, L.; Xue, H.M.; Guo, Q.; Jin, S.; Wu, Y.M. Cystathionine-beta-Synthase Gene Transfer into Rostral Ventrolateral Medulla Exacerbates Hypertension via Nitric Oxide in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2015, 28, 1106–1113. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Liu, L.; Li, X.; Li, X.Y.; Sun, X.; Li, K.X.; Zha, R.R.; Wang, H.D.; Zhang, M.D.; et al. The baroreflex afferent pathway plays a critical role in H2S-mediated autonomic control of blood pressure regulation under physiological and hypertensive conditions. Acta Pharmacol. Sin. 2021, 42, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Sikora, M. The role of brain gaseous transmitters in the regulation of the circulatory system. Curr. Pharm. Biotechnol. 2011, 12, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.P.; Traslavina, G.A.; Branco, L.G. Role of central hydrogen sulfide on ventilatory and cardiovascular responses to hypoxia in spontaneous hypertensive rats. Respir. Physiol. Neurobiol. 2016, 231, 21–27. [Google Scholar] [CrossRef]
- Sabino, J.P.; Soriano, R.N.; Donatti, A.F.; Fernandez, R.R.; Kwiatkoski, M.; Francescato, H.D.; Coimbra, T.M.; Branco, L.G. Involvement of endogenous central hydrogen sulfide (H2S) in hypoxia-induced hypothermia in spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2017, 95, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, S.; Hu, K.; Zhang, Z.; Liu, P.; Luo, Y.; Kang, J.; Xu, L. DL-Propargylglycine protects against myocardial injury induced by chronic intermittent hypoxia through inhibition of endoplasmic reticulum stress. Sleep Breath 2018, 22, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Zicola, E.; Arrigo, E.; Mancardi, D. H2S Pretreatment Is Promigratory and Decreases Ischemia/Reperfusion Injury in Human Microvascular Endothelial Cells. Oxid. Med. Cell Longev. 2021, 2021, 8886666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox. Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Citi, V.; Piragine, E.; Testai, L.; Breschi, M.C.; Calderone, V.; Martelli, A. The Role of Hydrogen Sulfide and H2S-donors in Myocardial Protection Against Ischemia/Reperfusion Injury. Curr. Med. Chem. 2018, 25, 4380–4401. [Google Scholar] [CrossRef]
- Ertugrul, I.A.; van Suylen, V.; Damman, K.; de Koning, M.L.Y.; van Goor, H.; Erasmus, M.E. Donor Heart Preservation with Hydrogen Sulfide: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 5737. [Google Scholar] [CrossRef]
- Jensen, A.R.; Drucker, N.A.; Khaneki, S.; Ferkowicz, M.J.; Yoder, M.C.; DeLeon, E.R.; Olson, K.R.; Markel, T.A. Hydrogen Sulfide: A Potential Novel Therapy for the Treatment of Ischemia. Shock 2017, 48, 511–524. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Cheng, J. H2S-based therapies for ischaemic stroke: Opportunities and challenges. Stroke Vasc. Neurol. 2019, 4, 63–66. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Bice, J.S.; Baxter, G.F. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: A systematic review and meta-analysis. Basic Res. Cardiol. 2018, 113, 6. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Z.; Wang, J.; Wang, H. Exogenous Hydrogen Sulfide Plays an Important Role Through Regulating Autophagy in Ischemia/Reperfusion Injury. Front. Mol. Biosci. 2021, 8, 681676. [Google Scholar] [CrossRef]
- Narne, P.; Pandey, V.; Phanithi, P.B. Role of Nitric Oxide and Hydrogen Sulfide in Ischemic Stroke and the Emergent Epigenetic Underpinnings. Mol. Neurobiol. 2019, 56, 1749–1769. [Google Scholar] [CrossRef]
- Roorda, M.; Miljkovic, J.L.; van Goor, H.; Henning, R.H.; Bouma, H.R. Spatiotemporal regulation of hydrogen sulfide signaling in the kidney. Redox. Biol. 2021, 43, 101961. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Wang, X.Y.; Bian, J.S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Wang, W.L.; Ge, T.Y.; Chen, X.; Mao, Y.; Zhu, Y.Z. Advances in the Protective Mechanism of NO, H2S, and H2 in Myocardial Ischemic Injury. Front. Cardiovasc. Med. 2020, 7, 588206. [Google Scholar] [CrossRef]
- Zhang, M.L.; Peng, W.; Ni, J.Q.; Chen, G. Recent advances in the protective role of hydrogen sulfide in myocardial ischemia/reperfusion injury: A narrative review. Med. Gas. Res. 2021, 11, 83–87. [Google Scholar]
- Klentz, R.D.; Fedde, M.R. Hydrogen sulfide: Effects on avian respiratory control and intrapulmonary CO2 receptors. Respir. Physiol. 1978, 32, 355–367. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxico. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Haggard, H.W.; Henderson, Y. The influence of hydrogensulphide upon respiration. Am. J. Physiol. 1922, 61, 289–297. [Google Scholar] [CrossRef]
- Haouzi, P.; Bell, H.J.; Notet, V.; Bihain, B. Comparison of the metabolic and ventilatory response to hypoxia and H2S in unsedated mice and rats. Respir. Physiol. Neurobiol. 2009, 167, 316–322. [Google Scholar] [CrossRef]
- Haouzi, P. Ventilatory and metabolic effects of exogenous hydrogen sulfide. Respir. Physiol. Neurobiol. 2012, 184, 170–177. [Google Scholar] [CrossRef]
- Haouzi, P.; Bell, H.; Philmon, M. Hydrogen sulfide oxidation and the arterial chemoreflex: Effect of methemoglobin. Respir. Physiol. Neurobiol. 2011, 177, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Van de Louw, A.; Haouzi, P. Inhibitory effects of hyperoxia and methemoglobinemia on H2S induced ventilatory stimulation in the rat. Respir. Physiol. Neurobiol. 2012, 181, 326–334. [Google Scholar] [CrossRef]
- Liu, W.Q.; Chai, C.; Li, X.Y.; Yuan, W.J.; Wang, W.Z.; Lu, Y. The cardiovascular effects of central hydrogen sulphide are related to K(ATP) channels activation. Physiol. Res. 2011, 60, 729–738. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Ding, Y.; Li, H.; Nie, L.; Zhou, H.; Tang, Y.; Zheng, Y. Site-specific hydrogen sulfide-mediated central regulation of respiratory rhythm in medullary slices of neonatal rats. Neuroscience 2013, 233, 118–126. [Google Scholar] [CrossRef]
- Hu, H.; Shi, Y.; Chen, Q.; Yang, W.; Zhou, H.; Chen, L.; Tang, Y.; Zheng, Y. Endogenous hydrogen sulfide is involved in regulation of respiration in medullary slice of neonatal rats. Neuroscience 2008, 156, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.G.; Hu, H.Y.; Zhang, J.; Zhou, H.; Chen, L.; Tang, Y.H.; Zheng, Y. Protective effect of hydrogen sulfide on hypoxic respiratory suppression in medullary slice of neonatal rats. Respir. Physiol. Neurobiol. 2010, 171, 181–186. [Google Scholar] [CrossRef]
- Pan, J.G.; Zhang, J.; Zhou, H.; Chen, L.; Tang, Y.H.; Zheng, Y. Protective action of endogenously generated H2S on hypoxia-induced respiratory suppression and its relation to antioxidation and down-regulation of c-fos mRNA in medullary slices of neonatal rats. Respir. Physiol. Neurobiol. 2011, 178, 230–234. [Google Scholar] [CrossRef]
- Li, M.; Nie, L.; Hu, Y.; Yan, X.; Xue, L.; Chen, L.; Zhou, H.; Zheng, Y. Chronic intermittent hypoxia promotes expression of 3-mercaptopyruvate sulfurtransferase in adult rat medulla oblongata. Auton. Neurosci. 2013, 179, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Donatti, A.F.; Soriano, R.N.; Sabino, J.P.; Branco, L.G. Involvement of endogenous hydrogen sulfide (H2S) in the rostral ventrolateral medulla (RVLM) in hypoxia-induced hypothermia. Brain Res. Bull. 2014, 108, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Donatti, A.F.; Soriano, R.N.; Sabino, J.P.; Branco, L.G. Endogenous hydrogen sulfide in the rostral ventrolateral medulla/Botzinger complex downregulates ventilatory responses to hypoxia. Respir. Physiol. Neurobiol. 2014, 200, 97–104. [Google Scholar] [CrossRef]
- Buckler, K.J. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012, 463, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, B.; Wang, X.; Jin, Z.; Zhou, Y.; Dong, L.; Jiang, L.H.; Rong, W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox. Signal. 2010, 12, 1179–1189. [Google Scholar] [CrossRef]
- Makarenko, V.V.; Nanduri, J.; Raghuraman, G.; Fox, A.P.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am. J. Physiol. Cell Physiol. 2012, 303, C916–C923. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D.; Del, R.R.; Ding, Y.; Marcus, N.J. Role of neurotransmitter gases in the control of the carotid body in heart failure. Respir. Physiol. Neurobiol. 2012, 184, 197–203. [Google Scholar] [CrossRef]
- Fitzgerald, R.S.; Shirahata, M.; Chang, I.; Kostuk, E.; Kiihl, S. The impact of hydrogen sulfide (H2S) on neurotransmitter release from the cat carotid body, Respir. Physiol. Neurobiol. 2011, 176, 80–89. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhang, X.; Gridina, A.; Chupikova, I.; McCormick, D.L.; Thomas, R.J.; Scammell, T.E.; Kim, G.; Vasavda, C.; Nanduri, J.; et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl. Acad. Sci. USA 2017, 114, 1413–1418. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Recent advances in understanding the physiology of hypoxic sensing by the carotid body. F1000Research 2018, 7, 1900. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J. Oxygen Sensing by the Carotid Body: Past and Present. Adv. Exp. Med. Biol. 2017, 977, 3–8. [Google Scholar] [PubMed]
- Wu, B.; Teng, H.; Zhang, L.; Li, H.; Li, J.; Wang, L.; Li, H. Interaction of Hydrogen Sulfide with Oxygen Sensing under Hypoxia. Oxid. Med. Cell Longev. 2015, 2015, 758678. [Google Scholar] [CrossRef] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.; Muller, C.T.; Riccardi, D.; Kemp, P.J. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009, 648, 65–72. [Google Scholar] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.H.; Wilkinson, W.J.; Riccardi, D.; Kemp, P.J. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir. Physiol. Neurobiol. 2010, 172, 169–178. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.; Wang, J.; White, C.; Carroll, J.L. Hydrogen sulfide and hypoxia-induced changes in TASK (K2P3/9) activity and intracellular Ca(2+) concentration in rat carotid body glomus cells. Respir. Physiol. Neurobiol. 2015, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hogan, J.O.; Wang, R.; White, C.; Kim, D. Role of cystathionine-gamma-lyase in hypoxia-induced changes in TASK activity, intracellular [Ca(2+)] and ventilation in mice. Respir. Physiol. Neurobiol. 2017, 246, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Porteus, C.S.; Abdallah, S.J.; Pollack, J.; Kumai, Y.; Kwong, R.W.; Yew, H.M.; Milsom, W.K.; Perry, S.F. The role of hydrogen sulphide in the control of breathing in hypoxic zebrafish (Danio rerio). J. Physiol. 2014, 592, 3075–3088. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, X.; Sun, J.; Li, J.; Ma, X.; Yao, W. H2S induces catecholamine secretion in rat adrenal chromaffin cells. Toxicology 2012, 302, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; McNeill, B.; Elia, E.; Nagpal, A.; Vulesevic, B. Hydrogen sulfide stimulates catecholamine secretion in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol Regul. Integr. Comp. Physiol. 2009, 296, R133–R140. [Google Scholar] [CrossRef][Green Version]
- Kemp, P.J.; Searle, G.J.; Hartness, M.E.; Lewis, A.; Miller, P.; Williams, S.; Wootton, P.; Adriaensen, D.; Peers, C. Acute oxygen sensing in cellular models: Relevance to the physiology of pulmonary neuroepithelial and carotid bodies. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 270, 41–50. [Google Scholar] [CrossRef]
- Chung, C.L.; Lin, Y.S.; Chan, N.J.; Chen, Y.Y.; Hsu, C.C. Hypersensitivity of Airway Reflexes Induced by Hydrogen Sulfide: Role of TRPA1 Receptors. Int. J. Mol. Sci. 2020, 21, 3929. [Google Scholar] [CrossRef]
- Huang, J.; Luo, Y.L.; Hao, Y.; Zhang, Y.L.; Chen, P.X.; Xu, J.W.; Chen, M.H.; Luo, Y.F.; Zhong, N.S.; Xu, J.; et al. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: Role of BKCa. Eur. J. Pharmacol. 2014, 741, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Doe, I.; Kurokawa, Y.; Kawabata, A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J. Pharmacol. Sci. 2007, 104, 392–396. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, P.P.; Wang, X.M.; He, Y.J.; Yao, W.Z.; Qi, Y.F.; Tang, C.S. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 2011, 53, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Castro-Piedras, I.; Perez-Zoghbi, J.F. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J. Physiol. 2013, 591, 5999–6015. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Patacchini, R.; Nicoletti, P.; Gatti, R.; Gazzieri, D.; Lissi, N.; Zagli, G.; Creminon, C.; Geppetti, P.; Harrison, S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br. J. Pharmacol. 2005, 145, 1123–1131. [Google Scholar] [CrossRef]
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018, 70, 196–205. [Google Scholar] [CrossRef]
- Beltowski, J. Hypoxia in the renal medulla: Implications for hydrogen sulfide signaling. J. Pharmacol. Exp. Ther. 2010, 334, 358–363. [Google Scholar] [CrossRef]
- Bianca, R.D.D.; Fusco, F.; Mirone, V.; Cirino, G.; Sorrentino, R. The Role of the Hydrogen Sulfide Pathway in Male and Female Urogenital System in Health and Disease. Antioxid. Redox Signal. 2017, 27, 654–668. [Google Scholar] [CrossRef]
- Shen, F.; Zhao, C.S.; Shen, M.F.; Wang, Z.; Chen, G. The role of hydrogen sulfide in gastric mucosal damage. Med. Gas. Res. 2019, 9, 88–92. [Google Scholar] [CrossRef]
- Verbeure, W.; van Goor, H.; Mori, H.; van Beek, A.P.; Tack, J.; van Dijk, P.R. The Role of Gasotransmitters in Gut Peptide Actions. Front Pharmacol. 2021, 12, 720703. [Google Scholar] [CrossRef]
- Dombkowski, R.A.; Doellman, M.M.; Head, S.K.; Olson, K.R. Hydrogen sulfide mediates hypoxia-induced relaxation of trout urinary bladder smooth muscle. J. Exp. Biol. 2006, 209, 3234–3240. [Google Scholar] [CrossRef]
- Battino, M.; Bompadre, S.; Politi, A.; Fioroni, M.; Rubini, C.; Bullon, P. Antioxidant status (CoQ10 and Vit. E levels) and immunohistochemical analysis of soft tissues in periodontal diseases. Biofactors 2005, 25, 213–217. [Google Scholar] [CrossRef]
- Fusco, F.; d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Cirino, G.; Sorrentino, R.; Mirone, V. Sildenafil Effect on the Human Bladder Involves the L-cysteine/Hydrogen Sulfide Pathway: A Novel Mechanism of Action of Phosphodiesterase Type 5 Inhibitors. Eur. Urol. 2012, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, J.; Li, D.; Li, Z.; Liu, H.; Ding, M.; Cai, Z.; Liang, X.; Yang, Q.; Long, Z.; et al. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF-1alpha/MAPK signaling pathway. Int. Immunopharmacol. 2020, 81, 105979. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Tanaka, T.; Daijo, H.; Harada, H.; Kishimoto, S.; Suzuki, K.; Takabuchi, S.; Takenaga, K.; Fukuda, K.; Hirota, K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid. Redox. Signal. 2012, 16, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.F.; Wang, J.; Guan, J.; Zhou, L.; Sheng, Y.; Zhao, J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 2013, 169, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Teng, H.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1alpha. Br. J. Pharmacol. 2012, 167, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.W.; Roth, M.B. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol. Biol. Cell 2010, 21, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Agbor, T.A.; Motta, J.P.; Ferraz, J.G.; Wang, R.; Buret, A.G.; Wallace, J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1alpha. FASEB J. 2015, 29, 1591–1602. [Google Scholar] [CrossRef]
- Ling, K.; Xu, A.; Chen, Y.; Chen, X.; Li, Y.; Wang, W. Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1alpha signaling pathway. Int. J. Mol. Med. 2019, 43, 1299–1310. [Google Scholar]
- Liu, X.; Pan, L.; Zhuo, Y.; Gong, Q.; Rose, P.; Zhu, Y. Hypoxia-inducible factor-1alpha is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol. Pharm. Bull. 2010, 33, 1550–1554. [Google Scholar] [CrossRef]
- Lohninger, L.; Tomasova, L.; Praschberger, M.; Hintersteininger, M.; Erker, T.; Gmeiner, B.M.; Laggner, H. Hydrogen sulphide induces HIF-1alpha and Nrf2 in THP-1 macrophages. Biochimie 2015, 112, 187–195. [Google Scholar] [CrossRef]
- Ma, D.K.; Vozdek, R.; Bhatla, N.; Horvitz, H.R. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron 2012, 73, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1alpha activation. Biochem. Pharmacol. 2020, 172, 113775. [Google Scholar] [CrossRef] [PubMed]
- Uba, T.; Matsuo, Y.; Sumi, C.; Shoji, T.; Nishi, K.; Kusunoki, M.; Harada, H.; Kimura, H.; Bono, H.; Hirota, K. Polysulfide inhibits hypoxia-elicited hypoxia-inducible factor activation in a mitochondria-dependent manner. Mitochondrion 2021, 59, 255–266. [Google Scholar] [CrossRef]
- Biermann, J.; Lagreze, W.A.; Schallner, N.; Schwer, C.I.; Goebel, U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol. Vis. 2011, 17, 1275–1286. [Google Scholar]
- Hu, Y.; Li, R.; Yang, H.; Luo, H.; Chen, Z. Sirtuin 6 Is Essential for Sodium Sulfide-mediated Cytoprotective Effect in Ischemia/Reperfusion-Stimulated Brain Endothelial Cells. J. Stroke Cerebrovasc. Dis. 2015, 24, 601–609. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Zheng, Q.; Wan, X.; Ouyang, S.; Yin, Y.; Sui, X.; Liu, J.; Yang, X. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem. Biophys. Res. Commun. 2012, 425, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xie, X.; Chen, D.; Zhang, J.; Zhou, Y.; Yang, G. Protective and biogenesis effects of sodium hydrosulfide on brain mitochondria after cardiac arrest and resuscitation. Eur. J. Pharmacol. 2014, 741, 74–82. [Google Scholar] [CrossRef]
- Qin, H.; Gu, L.Z.; Gao, L.; Guo, J. Protective effect of H2S pretreatment on cerebral ischemia-reperfusion injury and its mechanisms in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 249–253. [Google Scholar] [PubMed]
- Shen, Y.; Shen, Z.; Guo, L.; Zhang, Q.; Wang, Z.; Miao, L.; Wang, M.; Wu, J.; Guo, W.; Zhu, Y. MiR-125b-5p is involved in oxygen and glucose deprivation injury in PC-12 cells via CBS/H2S pathway. Nitric. Oxide. 2018, 78, 11–21. [Google Scholar] [CrossRef]
- Wen, X.; Qi, D.; Sun, Y.; Huang, X.; Zhang, F.; Wu, J.; Fu, Y.; Ma, K.; Du, Y.; Dong, H.; et al. H(2)S attenuates cognitive deficits through Akt1/JNK3 signaling pathway in ischemic stroke. Behav. Brain Res. 2014, 269, 6–14. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, Y.Q.; Wang, W.S.; Wang, X.Q. Excessive nNOS/NO/AMPK signaling activation mediated by the blockage of the CBS/H2S system contributes to oxygenglucose deprivationinduced endoplasmic reticulum stress in PC12 cells. Int. J. Mol. Med. 2017, 40, 549–557. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Wu, X.; Xu, Y.; He, M.; Yang, J.; Li, J.; Li, Y.; Ao, G.; Cheng, J.; Jia, J. The cystathionine beta-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain Behav. Immun. 2017, 66, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Wang, X.; Ding, Y.; Ren, Z.; Fang, J.; Sun, T.; Guo, Y.; Chen, Z.; Wen, J. CSE-Derived H2S Inhibits Reactive Astrocytes Proliferation and Promotes Neural Functional Recovery after Cerebral Ischemia/Reperfusion Injury in Mice Via Inhibition of RhoA/ROCK2 Pathway. ACS Chem. Neurosci. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Chen, C.P.; Halliwell, B.; Moore, P.K.; Wong, P.T. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 2006, 37, 889–893. [Google Scholar] [CrossRef]
- Marutani, E.; Morita, M.; Hirai, S.; Kai, S.; Grange, R.M.H.; Miyazaki, Y.; Nagashima, F.; Traeger, L.; Magliocca, A.; Ida, T.; et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 2021, 12, 3108. [Google Scholar] [CrossRef]
- Abou-Hamdan, A.; Guedouari-Bounihi, H.; Lenoir, V.; Andriamihaja, M.; Blachier, F.; Bouillaud, F. Oxidation of H2S in mammalian cells and mitochondria. Methods Enzym. 2015, 554, 201–228. [Google Scholar]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Kandel, R.S.; Mishra, R.; Gautam, J.; Alaref, A.; Hassan, A.; Jahan, N. Patchy Vasoconstriction Versus Inflammation: A Debate in the Pathogenesis of High Altitude Pulmonary Edema. Cureus 2020, 12, e10371. [Google Scholar]
- Swenson, E.R. Early hours in the development of high-altitude pulmonary edema: Time course and mechanisms. J. Appl. Physiol. 2020, 128, 1539–1546. [Google Scholar] [CrossRef]
- Agne, A.M.; Baldin, J.P.; Benjamin, A.R.; Orogo-Wenn, M.C.; Wichmann, L.; Olson, K.R.; Walters, D.V.; Althaus, M. Hydrogen sulfide decreases beta-adrenergic agonist stimulated lung liquid clearance by inhibiting ENaC-mediated transepithelial sodium absorption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R636–R649. [Google Scholar] [CrossRef][Green Version]
- Althaus, M.; Urness, K.D.; Clauss, W.G.; Baines, D.L.; Fronius, M. The gasotransmitter hydrogen sulphide decreases Na(+) transport across pulmonary epithelial cells. Br. J. Pharmacol. 2012, 166, 1946–1963. [Google Scholar] [CrossRef]
- Krause, N.C.; Kutsche, H.S.; Santangelo, F.; DeLeon, E.R.; Dittrich, N.P.; Olson, K.R.; Althaus, M. Hydrogen sulfide contributes to hypoxic inhibition of airway transepithelial sodium absorption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R607–R617. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. The re-emerging pathophysiological role of the cystathionine-beta-synthase—Hydrogen sulfide system in Down syndrome. FEBS J. 2020, 287, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Pecze, L.; Randi, E.B.; Szabo, C. Meta-analysis of metabolites involved in bioenergetic pathways reveals a pseudohypoxic state in Down syndrome. Mol. Med. 2020, 26, 102–128. [Google Scholar] [CrossRef]
- Durmowicz, A.G. Pulmonary edema in 6 children with Down syndrome during travel to moderate altitudes. Pediatrics 2001, 108, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; Chenivesse, C.; Larmignat, P.; Meille, L. High altitude pulmonary edema, down syndrome, and obstructive sleep apneas. High Alt. Med. Biol. 2008, 9, 179–181. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lloret, J.; Snetkov, V.A.; Shaifta, Y.; Docio, I.; Connolly, M.J.; MacKay, C.E.; Knock, G.A.; Ward, J.P.T.; Aaronson, P.I. Role of reactive oxygen species and sulfide-quinone oxoreductase in hydrogen sulfide-induced contraction of rat pulmonary arteries. Am. J. Physiol. Lung. Cell Mol. Physiol. 2018, 314, L670–L685. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Tielens, A.G.M.; Mentel, M. Mitochondria and Anaerobic Energy Metabolism in Eukaryotes; Walter de Gruyter: Berlin, Germany, 2021. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, K.R. A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism. Antioxidants 2021, 10, 1650. https://doi.org/10.3390/antiox10111650
Olson KR. A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism. Antioxidants. 2021; 10(11):1650. https://doi.org/10.3390/antiox10111650
Chicago/Turabian StyleOlson, Kenneth R. 2021. "A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism" Antioxidants 10, no. 11: 1650. https://doi.org/10.3390/antiox10111650
APA StyleOlson, K. R. (2021). A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism. Antioxidants, 10(11), 1650. https://doi.org/10.3390/antiox10111650





