Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Cell Differentials and Morphology
2.2. Plasma Ascorbate and Myeloperoxidase Analysis
2.3. Erythrocyte Isolation and Ascorbate Analysis
2.4. Neutrophil Isolation and Morphology Microscopy
2.5. Ex Vivo Ascorbate Uptake by Neutrophils
2.6. Ex Vivo Stimulation of Neutrophil Oxidative Burst
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Septic Participants
3.2. Neutrophil Ascorbate Content Relative to Plasma Concentrations
3.3. Erythrocyte Ascorbate Content Relative to Plasma Concentrations
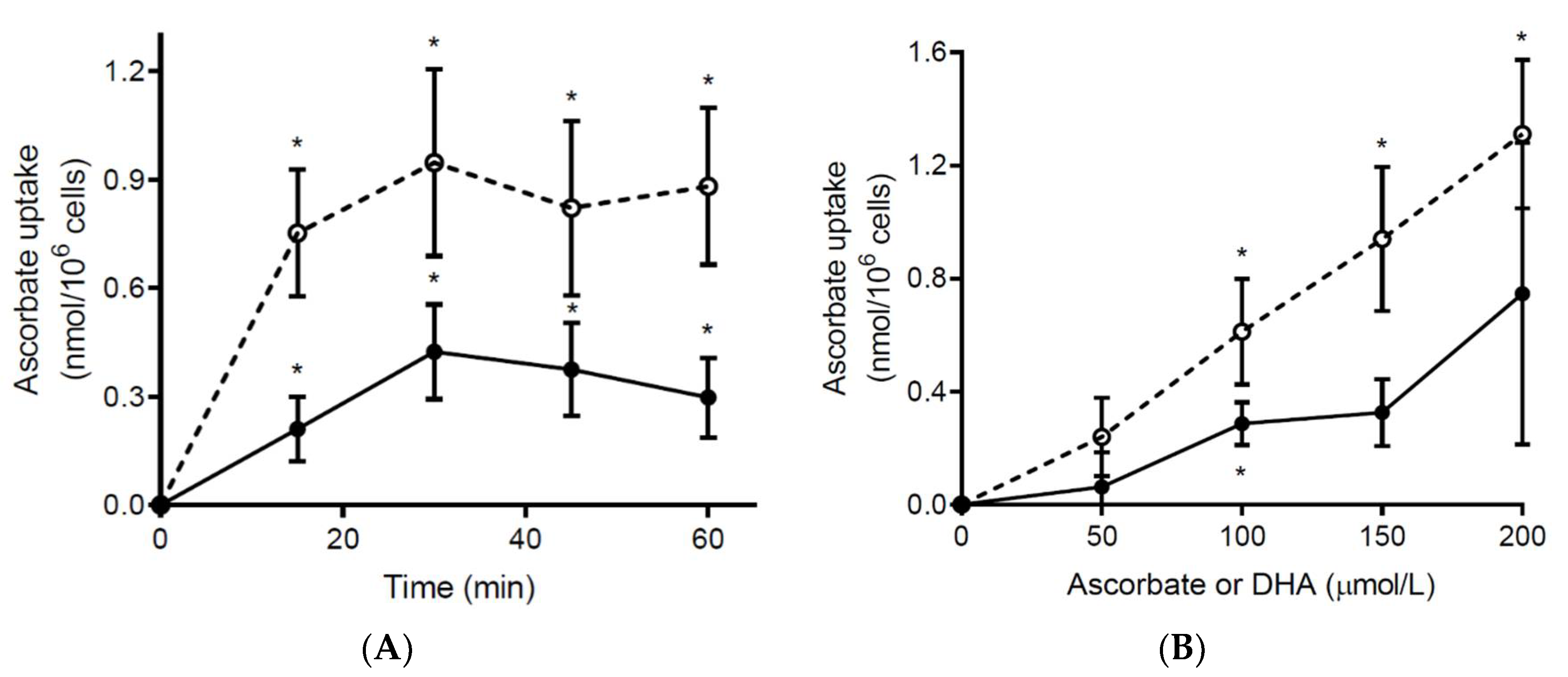
3.4. Ex Vivo Ascorbate Uptake by Neutrophils from Septic Patients
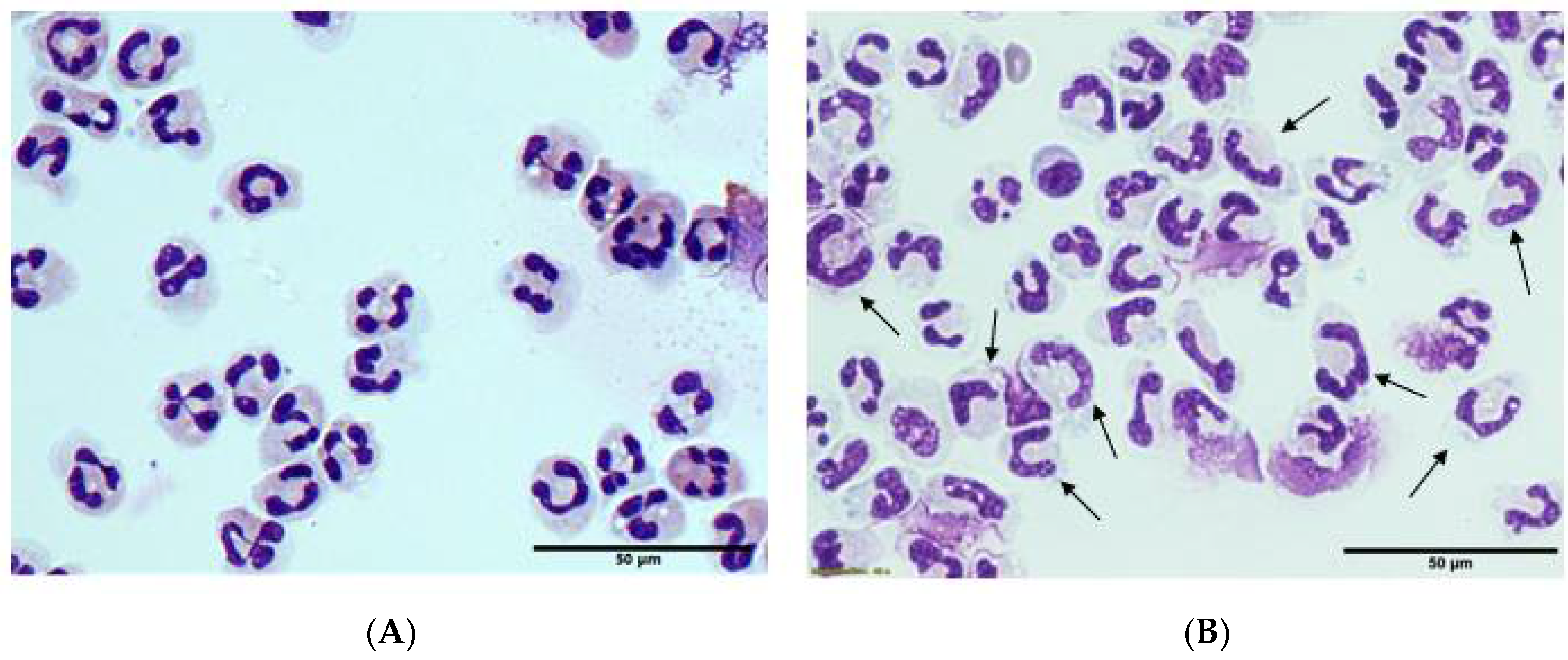
3.5. Morphology and Ex Vivo Activation of Neutrophils from Septic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Mackle, D.; Hunt, A.; Judd, H.; Mehrtens, J.; Parker, K.; Stockwell, Z.; Gale, C.; Beaumont, M.; et al. The effect of conservative oxygen therapy on systemic biomarkers of oxidative stress in critically ill patients. Free Radic. Biol. Med. 2020. under consideration. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washko, P.W.; Wang, Y.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, E1211. [Google Scholar] [CrossRef] [Green Version]
- Hume, R.; Weyers, E. Changes in leucocyte ascorbic acid during the common cold. Scott. Med. J. 1973, 18, 3–7. [Google Scholar] [CrossRef]
- Tanzer, F.; Ozalp, I. Leucocyte ascorbic acid concentration and plasma ascorbic acid levels in children with various infections. Mater. Med. Pol. 1993, 25, 5–8. [Google Scholar]
- Bozonet, S.M.; Carr, A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Pullar, J.M.; Moran, S.; Vissers, M.C. Bioavailability of vitamin C from kiwifruit in non-smoking males: Determination of ‘healthy’ and ‘optimal’ intakes. J. Nutr. Sci. 2012, 1, e14. [Google Scholar] [CrossRef] [Green Version]
- Pullar, J.; Dunham, S.; Dachs, G.; Vissers, M.; Carr, A.C. Erythrocyte ascorbate is a potential indicator of steady-state plasma ascorbate concentrations in healthy non-fasting individuals. Nutrients 2020, 12, 418. [Google Scholar] [CrossRef] [Green Version]
- Dachs, G.U.; Gandhi, J.; Wohlrab, C.; Carr, A.C.; Morrin, H.R.; Pullar, J.M.; Bayer, S.B.; Eglinton, T.W.; Robinson, B.A.; Vissers, M.C.M. Vitamin C administration by intravenous infusion increases tumor ascorbate content in patients with colon cancer: A clinical intervention study. Front. Oncol. 2020, in press. [Google Scholar]
- Bateman, R.M.; Sharpe, M.D.; Singer, M.; Ellis, C.G. The effect of sepsis on the erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef] [Green Version]
- Tu, H.; Li, H.; Wang, Y.; Niyyati, M.; Wang, Y.; Leshin, J.; Levine, M. Low red blood cell vitamin C concentrations induce red blood cell fragility: A link to diabetes via glucose, glucose transporters, and dehydroascorbic acid. EBioMedicine 2015, 2, 1735–1750. [Google Scholar] [CrossRef] [Green Version]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968, 97, 77–89. [Google Scholar] [PubMed]
- Vissers, M.C.; Day, W.A.; Winterbourn, C.C. Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachment. Blood 1985, 66, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Hawkins, C.L.; Thomas, S.R.; Stocker, R.; Frei, B. Relative reactivities of N-chloramines and hypochlorous acid with human plasma constituents. Free Radic. Biol. Med. 2001, 30, 526–536. [Google Scholar] [CrossRef]
- Tu, H.; Wang, Y.; Li, H.; Brinster, L.R.; Levine, M. Chemical transport knockout for oxidized vitamin C, dehydroascorbic acid, reveals its functions in vivo. EBioMedicine 2017, 23, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenisch, C.; Fladerer, P.; Patruta, S.; Krause, R.; Horl, W. Assessment of neutrophil function in patients with septic shock: Comparison of methods. Clin. Diagn. Lab. Immunol. 2001, 8, 178–180. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with community acquired pneumonia exhibit depleted vitamin C status and elevated oxidative stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, B.; Banerjee, S. Dehydroascorbic acid level in blood of patients suffering from various infectious diseases. Proc. Soc. Exp. Biol. Med. 1955, 88, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Bayer, S.; Carr, A.C. Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin C to dehydroascorbic acid. Antioxidants 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef]
- Drifte, G.; Dunn-Siegrist, I.; Tissieres, P.; Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013, 41, 820–832. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Hoskin, T.S.; Rosengrave, P.; Kettle, A.J.; Shaw, G. Circulating myeloperoxidase is elevated in septic shock and is associated with systemic organ failure and mortality in critically ill patients. Free Radic. Biol. Med. 2020, 152, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef]
- Savini, I.; Rossi, A.; Catani, M.V.; Ceci, R.; Avigliano, L. Redox regulation of vitamin C transporter SVCT2 in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2007, 361, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Chakravorty, N.K.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam. Nutr. Res. 1994, 64, 212–219. [Google Scholar] [PubMed]
- Subramanian, V.S.; Teafatiller, T.; Agrawal, A.; Kitazawa, M.; Marchant, J.S. Effect of lipopolysaccharide and TNFα on neuronal ascorbic acid uptake. Mediat. Inflamm. 2021, 2021, 4157132. [Google Scholar] [CrossRef]
- Carr, A.C. Vitamin C in pneumonia and sepsis. In Vitamin C: New Biochemical and Functional Insights; Chen, Q., Vissers, M., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxford, UK, 2020; pp. 115–135. [Google Scholar]
- Carr, A.C.; Rowe, S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Cao, K.; Zhao, Y.; Du, J. Targeting neutrophils in sepsis: From mechanism to translation. Front. Pharmacol. 2021, 12, 644270. [Google Scholar] [CrossRef] [PubMed]
- Ferron-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.M.; Bueno, P.; Ferron, J.A. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 2009, 153, 224–230. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Septic Patients (n = 18) |
|---|---|
| Male sex, n (%) | 14 (78) 1 |
| Age, years | 66 (62, 77) |
| Source of sepsis, n (%): | |
| Abdominal | 9 (50) |
| Pulmonary | 5 (28) |
| Skin | 2 (11) |
| Blood | 2 (11) |
| SAPS 2 II | 50 (44, 56) |
| APACHE 3 III | 79 (70, 98) |
| SOFA 4 score | 9 (8, 11) |
| Mechanical ventilation, n (%) | 16 (89) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carr, A.C.; Bozonet, S.; Pullar, J.; Spencer, E.; Rosengrave, P.; Shaw, G. Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu. Antioxidants 2021, 10, 1607. https://doi.org/10.3390/antiox10101607
Carr AC, Bozonet S, Pullar J, Spencer E, Rosengrave P, Shaw G. Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu. Antioxidants. 2021; 10(10):1607. https://doi.org/10.3390/antiox10101607
Chicago/Turabian StyleCarr, Anitra C., Stephanie Bozonet, Juliet Pullar, Emma Spencer, Patrice Rosengrave, and Geoff Shaw. 2021. "Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu" Antioxidants 10, no. 10: 1607. https://doi.org/10.3390/antiox10101607
APA StyleCarr, A. C., Bozonet, S., Pullar, J., Spencer, E., Rosengrave, P., & Shaw, G. (2021). Neutrophils Isolated from Septic Patients Exhibit Elevated Uptake of Vitamin C and Normal Intracellular Concentrations despite a Low Vitamin C Milieu. Antioxidants, 10(10), 1607. https://doi.org/10.3390/antiox10101607







