The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications
Abstract
:1. Introduction
2. ROS Generation in Sickle Red Blood Cells
3. Endogenous Oxidative Stress and Sickle Red Blood Cell Structures
4. The Role of Sickle RBC ROS in the Adhesion to Endothelium and Vaso-Occlusion
5. The Contribution of Sickle RBC ROS to Hemolysis
6. The Contribution of ROS in Sickle RBCs to Inflammation and Vascular Damage
7. The Effect of ROS in Sickle RBCs on Hypercoagulation
8. The Role of ROS in Sickle RBCs in the Activation of the Complement System
9. Targeting Oxidative Stress in Sickle Red Blood Cells
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 2010, 1, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Chadwick, R.S.; Schechter, A.N. Influence of sickle hemoglobin polymerization and membrane properties on deformability of sickle erythrocytes in the microcirculation. Biophys. J. 1992, 63, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wood, D.K.; Higgins, J.M. Deoxygenation reduces sickle cell blood flow at arterial oxygen tension. Biophys. J. 2016, 110, 2751–2758. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.P.; Abidi, S.Z.; Chang, H.Y.; Li, X.; Kato, G.J.; Karniadakis, G.E.; Suresh, S.; Dao, M. Simultaneous polymerization and adhesion under hypoxia in sickle cell disease. Proc. Natl. Acad. Sci. USA 2018, 115, 9473–9478. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, G.P. Overview of pathophysiology and rationale for treatment of sickle cell anemia. Semin. Hematol. 1997, 34, 2–7. [Google Scholar]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Nagababu, E.; Mohanty, J.G.; Friedman, J.S.; Rifkind, J.M. Role of peroxiredoxin-2 in protecting RBCs from hydrogen peroxide-induced oxidative stress. Free Radic. Res. 2013, 47, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Nur, E.; Biemond, B.J.; Otten, H.M.; Brandjes, D.P.; Schnog, J.J.; Group, C.S. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am. J. Hematol. 2011, 86, 484–489. [Google Scholar] [CrossRef]
- De Franceschi, L.; Bertoldi, M.; Matte, A.; Santos Franco, S.; Pantaleo, A.; Ferru, E.; Turrini, F. Oxidative stress and beta-thalassemic erythroid cells behind the molecular defect. Oxid. Med. Cell. Longev. 2013, 2013, 985210. [Google Scholar] [CrossRef] [Green Version]
- Hebbel, R.P.; Morgan, W.T.; Eaton, J.W.; Hedlund, B.E. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl. Acad. Sci. USA 1988, 85, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.; Pushkaran, S.; Konstantinidis, D.G.; Koochaki, S.; Malik, P.; Mohandas, N.; Zheng, Y.; Joiner, C.H.; Kalfa, T.A. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 2013, 121, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKinney, A.; Woska, E.; Spasojevic, I.; Batinic-Haberle, I.; Zennadi, R. Disrupting the vicious cycle created by NOX activation in sickle erythrocytes exposed to hypoxia/reoxygenation prevents adhesion and vasoocclusion. Redox Biol. 2019, 25, 101097. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.; Auclair, C.; Voisin, E.; Gautero, H.; Dhermy, D.; Boivin, P. Superoxide dismutase, catalase, and glutathione peroxidase in red blood cells from patients with malignant diseases. Cancer Res. 1984, 44, 4137–4139. [Google Scholar]
- Nagababu, E.; Chrest, F.J.; Rifkind, J.M. Hydrogen-peroxide-induced heme degradation in red blood cells: The protective roles of catalase and glutathione peroxidase. Biochim. Biophys. Acta 2003, 1620, 211–217. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.U.; Yu, S.L.; Kim, S.H.; Park, D.S.; Moon, H.B.; Dho, S.H.; Kwon, K.S.; Kwon, H.J.; Han, Y.H.; et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 2003, 101, 5033–5038. [Google Scholar] [CrossRef] [Green Version]
- Tsantes, A.E.; Bonovas, S.; Travlou, A.; Sitaras, N.M. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid. Redox Signal. 2006, 8, 1205–1216. [Google Scholar] [CrossRef]
- Umbreit, J. Methemoglobin—It’s not just blue: A concise review. Am. J. Hematol. 2007, 82, 134–144. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Eaton, J.W.; Balasingam, M.; Steinberg, M.H. Spontaneous oxygen radical generation by sickle erythrocytes. J. Clin. Investig. 1982, 70, 1253–1259. [Google Scholar] [CrossRef]
- Sheng, K.; Shariff, M.; Hebbel, R.P. Comparative oxidation of hemoglobins A and S. Blood 1998, 91, 3467–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebbel, R.P.; Ney, P.A.; Foker, W. Autoxidation, dehydration, and adhesivity may be related abnormalities of sickle erythrocytes. Am. J. Physiol. 1989, 256, C579–C583. [Google Scholar] [CrossRef] [PubMed]
- Barodka, V.M.; Nagababu, E.; Mohanty, J.G.; Nyhan, D.; Berkowitz, D.E.; Rifkind, J.M.; Strouse, J.J. New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Mol. Dis. 2014, 52, 230–235. [Google Scholar] [CrossRef]
- Thamilarasan, M.; Estupinan, R.; Batinic-Haberle, I.; Zennadi, R. Mn porphyrins as a novel treatment targeting sickle cell NOXs to reverse and prevent acute vaso-occlusion in vivo. Blood Adv. 2020, 4, 2372–2386. [Google Scholar] [CrossRef]
- Alayash, A.I. Oxidative pathways in the sickle cell and beyond. Blood Cells Mol. Dis. 2018, 70, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, R.; Vazquez, B.A.; Thiruppathi, M.; Ganesh, B.B.; Ibanez, V.; Cui, S.; Engel, J.D.; Diamond, A.M.; Molokie, R.E.; DeSimone, J.; et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp. Hematol. 2017, 50, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Al-Naama, L.M.; Hassan, M.K.; Mehdi, J.K. Association of erythrocytes antioxidant enzymes and their cofactors with markers of oxidative stress in patients with sickle cell anemia. Qatar Med. J. 2015, 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Gizi, A.; Papassotiriou, I.; Apostolakou, F.; Lazaropoulou, C.; Papastamataki, M.; Kanavaki, I.; Kalotychou, V.; Goussetis, E.; Kattamis, A.; Rombos, I.; et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol. Dis. 2011, 46, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Liu, T.; Sezen, S.F.; Burnett, A.L. Targeting NADPH oxidase decreases oxidative stress in the transgenic sickle cell mouse penis. J. Sex. Med. 2012, 9, 1980–1987. [Google Scholar] [CrossRef] [Green Version]
- Nur, E.; Brandjes, D.P.; Teerlink, T.; Otten, H.M.; Oude Elferink, R.P.; Muskiet, F.; Evers, L.M.; ten Cate, H.; Biemond, B.J.; Duits, A.J.; et al. N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann. Hematol. 2012, 91, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.G.; Belini Junior, E.; Torres Lde, S.; Ricci Junior, O.; Lobo Cde, C.; Bonini-Domingos, C.R.; de Almeida, E.A. Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Mol. Dis. 2011, 47, 23–28. [Google Scholar] [CrossRef]
- Grinberg, L.; Fibach, E.; Amer, J.; Atlas, D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 2005, 38, 136–145. [Google Scholar] [CrossRef]
- May, J.M. Ascorbate function and metabolism in the human erythrocyte. Front. Biosci. 1998, 3, d1–d10. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K.; Shohet, S.B. Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochim. Biophys. Acta 1981, 642, 46–54. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. FEBS 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, R.; Verhoeven, A.J.; Roos, D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014, 67, 377–386. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.U.; Kwon, T.H.; Lee, D.S.; Ha, H.L.; Park, D.S.; Woo, E.J.; Lee, S.H.; Kim, J.M.; Chae, H.B.; et al. Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem. Biophys. Res. Commun. 2012, 426, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Ghebremeskel, K.; Okpala, I.; Lee, A.; Ibegbulam, O.; Crawford, M. Patients with sickle cell disease have reduced blood antioxidant protection. Int. J. Vitam. Nutr. Res. 2008, 78, 139–147. [Google Scholar] [CrossRef]
- Kiefer, C.R.; Snyder, L.M. Oxidation and erythrocyte senescence. Curr. Opin. Hematol. 2000, 7, 113–116. [Google Scholar] [CrossRef]
- Tian, L.; Cai, Q.; Wei, H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic. Biol. Med. 1998, 24, 1477–1484. [Google Scholar] [CrossRef]
- Erwig, L.P.; Henson, P.M. Immunological consequences of apoptotic cell phagocytosis. Am. J. Pathol. 2007, 171, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, I.; Neumann, H. Microglial clearance function in health and disease. Neuroscience 2009, 158, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.E.; Gonzalez, N.; Rios, R.; Merchan, L.; Wuani, H. Phagocytosis in patients with sickle cell disease. J. Clin. Lab. Immunol. 1983, 12, 137–140. [Google Scholar] [PubMed]
- Rifkind, J.M.; Ajmani, R.S.; Heim, J. Impaired hemorheology in the aged associated with oxidative stress. Adv. Exp. Med. Biol. 1997, 428, 7–13. [Google Scholar] [CrossRef]
- Tripette, J.; Alexy, T.; Hardy-Dessources, M.D.; Mougenel, D.; Beltan, E.; Chalabi, T.; Chout, R.; Etienne-Julan, M.; Hue, O.; Meiselman, H.J.; et al. Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica 2009, 94, 1060–1065. [Google Scholar] [CrossRef]
- Aslan, M.; Canatan, D. Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp. Hematol. 2008, 36, 1535–1544. [Google Scholar] [CrossRef]
- Nath, K.A.; Grande, J.P.; Haggard, J.J.; Croatt, A.J.; Katusic, Z.S.; Solovey, A.; Hebbel, R.P. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 2001, 158, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.C.; Hebbel, R.P.; Granger, D.N. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005, 19, 989–991. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ohkubo, N.; Aoto, M.; Maeda, N.; Cicha, I.; Miki, T.; Mitsuda, N. Participation of caspase-3-like protease in oxidation-induced impairment of erythrocyte membrane properties. Biorheology 2007, 44, 179–190. [Google Scholar] [PubMed]
- Nader, E.; Grau, M.; Fort, R.; Collins, B.; Cannas, G.; Gauthier, A.; Walpurgis, K.; Martin, C.; Bloch, W.; Poutrel, S.; et al. Hydroxyurea therapy modulates sickle cell anemia red blood cell physiology: Impact on RBC deformability, oxidative stress, nitrite levels and nitric oxide synthase signalling pathway. Nitric Oxide Biol. Chem. Off. J. Nitric Oxide Soc. 2018, 81, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice-Evans, C.; Omorphos, S.C.; Baysal, E. Sickle cell membranes and oxidative damage. Biochem. J. 1986, 237, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Hierso, R.; Lemonne, N.; Villaescusa, R.; Lalanne-Mistrih, M.L.; Charlot, K.; Etienne-Julan, M.; Tressieres, B.; Lamarre, Y.; Tarer, V.; Garnier, Y.; et al. Exacerbation of oxidative stress during sickle vaso-occlusive crisis is associated with decreased anti-band 3 autoantibodies rate and increased red blood cell-derived microparticle level: A prospective study. Br. J. Haematol. 2017, 176, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Strader, M.B.; Meng, F.; Hicks, W.; Kassa, T.; Tarandovskiy, I.; De Paoli, S.; Simak, J.; Heaven, M.R.; Belcher, J.D.; et al. Hemoglobin oxidation-dependent reactions promote interactions with band 3 and oxidative changes in sickle cell-derived microparticles. JCI Insight 2018, 3, e120451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thein, S.L.; Shet, A.S.; Strader, M.B.; Meng, F.; Heaven, M.; Sirsendu, J.; Alayash, A. Hydroxyurea reverses dysfunctional ubiquitin-proteasomal system in sickle CELL disease and suppresses posttranslational alterations in hemoglobin and CELL membranes. Blood 2019, 134, 4822. [Google Scholar] [CrossRef]
- Fujino, T.; Kojima, M.; Beppu, M.; Kikugawa, K.; Yasuda, H.; Takahashi, K. Identification of the cleavage sites of oxidized protein that are susceptible to oxidized protein hydrolase (OPH) in the primary and tertiary structures of the protein. J. Biochem. 2000, 127, 1087–1093. [Google Scholar] [CrossRef]
- Fujino, T.; Watanabe, K.; Beppu, M.; Kikugawa, K.; Yasuda, H. Identification of oxidized protein hydrolase of human erythrocytes as acylpeptide hydrolase. Biochim. Biophys. Acta 2000, 1478, 102–112. [Google Scholar] [CrossRef]
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Repka, T.; Hebbel, R.P. Hydroxyl radical formation by sickle erythrocyte membranes: Role of pathologic iron deposits and cytoplasmic reducing agents. Blood 1991, 78, 2753–2758. [Google Scholar] [CrossRef] [Green Version]
- Canli, O.; Alankus, Y.B.; Grootjans, S.; Vegi, N.; Hultner, L.; Hoppe, P.S.; Schroeder, T.; Vandenabeele, P.; Bornkamm, G.W.; Greten, F.R. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood 2016, 127, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.K.; Nair, R.C. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br. J. Haematol. 1980, 44, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Baudin-Creuza, V.; Bhattacharyya, A.; Pathak, S.; Delaunay, J.; Kundu, M.; Basu, J. Caspase 3-mediated proteolysis of the N-terminal cytoplasmic domain of the human erythroid anion exchanger 1 (band 3). J. Biol. Chem. 2003, 278, 52551–52558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, M.E.; Giardina, B.; Colucci, D.; Galtieri, A.; Misiti, F. Amyloid-beta peptide affects the oxygen dependence of erythrocyte metabolism: A role for caspase 3. Int. J. Biochem. Cell Biol. 2007, 39, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.L.; Kodippili, G.C.; Simon, K.; Low, P.S. Identification of contact sites between ankyrin and band 3 in the human erythrocyte membrane. Biochemistry 2012, 51, 6838–6846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannu, F.; Arese, P.; Cappellini, M.D.; Fiorelli, G.; Cappadoro, M.; Giribaldi, G.; Turrini, F. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood 1995, 86, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Bordin, L.; Brunati, A.M.; Donella-Deana, A.; Baggio, B.; Toninello, A.; Clari, G. Band 3 is an anchor protein and a target for SHP-2 tyrosine phosphatase in human erythrocytes. Blood 2002, 100, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferru, E.; Giger, K.; Pantaleo, A.; Campanella, E.; Grey, J.; Ritchie, K.; Vono, R.; Turrini, F.; Low, P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 2011, 117, 5998–6006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerman, M.; Pizzey, A.; Hirschman, J.; Cerino, M.; Weil-Weiner, Y.; Ramotar, P.; Eze, A.; Lawrie, A.; Purdy, G.; Mackie, I.; et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br. J. Haematol. 2008, 142, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C.; Silva, A.S.; Goncalves, S.; Martins-Silva, J. Modulation of erythrocyte hemorheological properties by band 3 phosphorylation and dephosphorylation. Clin. Hemorheol. Microcirc. 2007, 36, 183–194. [Google Scholar]
- Carvalho, F.A.; de Almeida, J.P.; Freitas-Santos, T.; Saldanha, C. Modulation of erythrocyte acetylcholinesterase activity and its association with G protein-band 3 interactions. J. Membr. Biol. 2009, 228, 89–97. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Rybicki, A.C.; Heath, R.H.; Lubin, B.H. Protein 4.1 in sickle erythrocytes. Evidence for oxidative damage. J. Biol. Chem. 1987, 262, 15666–15672. [Google Scholar] [CrossRef]
- Shinar, E.; Rachmilewitz, E.A.; Lux, S.E. Differing erythrocyte membrane skeletal protein defects in alpha and beta thalassemia. J. Clin. Investig. 1989, 83, 404–410. [Google Scholar] [CrossRef]
- Zennadi, R.; Whalen, E.J.; Soderblom, E.J.; Alexander, S.C.; Thompson, J.W.; Dubois, L.G.; Moseley, M.A.; Telen, M.J. Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium. Blood 2012, 119, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Pushkaran, S.; Li, L.; An, X.; Zheng, Y.; Mohandas, N.; Joiner, C.H.; Kalfa, T.A. Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: The role of protein kinase C, Rac GTPases, and reactive oxygen species. Blood Cells Mol. Dis. 2010, 45, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Setty, B.N.; Kulkarni, S.; Stuart, M.J. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood 2002, 99, 1564–1571. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.L.; Gibson, D.F.; Tait, J.F. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: Flow-cytometric measurement and clinical associations. Blood 1996, 88, 1873–1880. [Google Scholar] [CrossRef] [Green Version]
- Cytlak, U.M.; Hannemann, A.; Rees, D.C.; Gibson, J.S. Identification of the Ca2+ entry pathway involved in deoxygenation-induced phosphatidylserine exposure in red blood cells from patients with sickle cell disease. Pflugers Arch. Eur. J. Physiol. 2013, 465, 1651–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, D.; Limbrick, A.R.; Thomas, P.; Westerman, M.P. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature 1982, 295, 612–613. [Google Scholar] [CrossRef] [PubMed]
- Samaja, M.; Rubinacci, A.; Motterlini, R.; De Ponti, A.; Portinaro, N. Red cell aging and active calcium transport. Exp. Gerontol. 1990, 25, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Ney, P.A.; Christopher, M.M.; Hebbel, R.P. Synergistic effects of oxidation and deformation on erythrocyte monovalent cation leak. Blood 1990, 75, 1192–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barodka, V.; Mohanty, J.G.; Mustafa, A.K.; Santhanam, L.; Nyhan, A.; Bhunia, A.K.; Sikka, G.; Nyhan, D.; Berkowitz, D.E.; Rifkind, J.M. Nitroprusside inhibits calcium-induced impairment of red blood cell deformability. Transfusion 2014, 54, 434–444. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, T.; Kuypers, F.A. Reactive oxygen species and phosphatidylserine externalization in murine sickle red cells. Br. J. Haematol. 2004, 124, 391–402. [Google Scholar] [CrossRef]
- Das, S.K.; Hinds, J.E.; Hardy, R.E.; Collins, J.C.; Mukherjee, S. Effects of physical stress on peroxide scavengers in normal and sickle cell trait erythrocytes. Free Radic. Biol. Med. 1993, 14, 139–147. [Google Scholar] [CrossRef]
- Reid, M.; Badaloo, A.; Forrester, T.; Jahoor, F. In vivo rates of erythrocyte glutathione synthesis in adults with sickle cell disease. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E73–E79. [Google Scholar] [CrossRef] [Green Version]
- Melo, J.B.; Agostinho, P.; Oliveira, C.R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci. Res. 2003, 45, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Belle, V.S.; Kumbarakeri Rajashekhar, R.; Jogi, S.; Prabhu, R.K. Correlation of red blood cell acetylcholinesterase enzyme activity with various RBC indices. Indian J. Clin. Biochem. 2018, 33, 445–449. [Google Scholar] [CrossRef]
- Aloni, B.; Livne, A. Acetycholinesterase as a probe for erythrocyte-membrane intactness. Biochim. Biophys. Acta 1974, 339, 359–366. [Google Scholar] [CrossRef]
- Eluwa, E.O.; Obidoa, O.; Ogan, A.U.; Onwubiko, H.A. Erythrocyte membrane enzymes in sickle cell anemia. 2. Acetylcholinesterase and ATPase activities. Biochem. Med. Metab. Biol. 1990, 44, 234–237. [Google Scholar] [CrossRef]
- Butikofer, P.; Brodbeck, U.; Ott, P. Modulation of erythrocyte vesiculation by amphiphilic drugs. Biochim. Biophys. Acta 1987, 901, 291–295. [Google Scholar] [CrossRef]
- Saldanha, C.; Santos, N.C.; Martins-Silva, J. A colorimetric process to visualize erythrocyte exovesicles aggregates. Biochem. Mol. Biol. Educ. 2004, 32, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Mankelow, T.J.; Griffiths, R.E.; Trompeter, S.; Flatt, J.F.; Cogan, N.M.; Massey, E.J.; Anstee, D.J. The ins and outs of reticulocyte maturation revisited: The role of autophagy in sickle cell disease. Autophagy 2016, 12, 590–591. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, M.; Ferguson, D.J.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 832–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagadeeswaran, R.; Lenny, H.; Vazquez, B.; Muniz, J.; Schad, A.; Jain, S.; Gowhari, M.; Lavelle, D.; Diamond, A.; Molokie, R.E.; et al. The abnormal presence of mitochondria in circulating red blood cells cause an increased oxygen consumption rate, ROS generation and hemolysis in patients with sickle cell disease. Blood 2017, 130, 2237. [Google Scholar] [CrossRef]
- Kim, J.; Paik, H.D.; Yoon, Y.C.; Park, E. Whey protein inhibits iron overload-induced oxidative stress in rats. J. Nutr. Sci. Vitaminol. 2013, 59, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiffin, R.; Christian, C.; Knecht, E.; Cuervo, A.M. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 2004, 15, 4829–4840. [Google Scholar] [CrossRef] [Green Version]
- Telen, M.J.; Malik, P.; Vercellotti, G.M. Therapeutic strategies for sickle cell disease: Towards a multi-agent approach. Nat. Rev. Drug Disc. 2019, 18, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhou, Y.; Fang, H.; Lin, S.; Wang, P.F.; Xiong, R.P.; Chen, J.; Xiong, X.Y.; Lv, F.L.; Liang, Q.L.; et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann. Neurol. 2014, 75, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Conran, N.; Franco-Penteado, C.F.; Costa, F.F. Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin 2009, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.K.; Fabry, M.E.; Nagel, R.L. Vaso-occlusion by sickle cells: Evidence for selective trapping of dense red cells. Blood 1986, 68, 1162–1166. [Google Scholar] [CrossRef] [Green Version]
- Frenette, P.S. Sickle cell vaso-occlusion: Multistep and multicellular paradigm. Curr. Opin. Hematol. 2002, 9, 101–106. [Google Scholar] [CrossRef]
- Ferrone, F.A. The delay time in sickle cell disease after 40 years: A paradigm assessed. Am. J. Hematol. 2015, 90, 438–445. [Google Scholar] [CrossRef]
- Ferrone, F.A. Polymerization and sickle cell disease: A molecular view. Microcirculation 2004, 11, 115–128. [Google Scholar] [CrossRef]
- Lu, L.; Li, H.; Bian, X.; Li, X.; Karniadakis, G.E. Mesoscopic adaptive resolution scheme toward understanding of interactions between sickle cell fibers. Biophys. J. 2017, 113, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Okpala, I.; Daniel, Y.; Haynes, R.; Odoemene, D.; Goldman, J. Relationship between the clinical manifestations of sickle cell disease and the expression of adhesion molecules on white blood cells. Eur. J. Haematol. 2002, 69, 135–144. [Google Scholar] [CrossRef]
- Montes, R.A.; Eckman, J.R.; Hsu, L.L.; Wick, T.M. Sickle erythrocyte adherence to endothelium at low shear: Role of shear stress in propagation of vaso-occlusion. Am. J. Hematol. 2002, 70, 216–227. [Google Scholar] [CrossRef]
- Hofstra, T.C.; Kalra, V.K.; Meiselman, H.J.; Coates, T.D. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood 1996, 87, 4440–4447. [Google Scholar] [CrossRef] [Green Version]
- Zennadi, R.; Chien, A.; Xu, K.; Batchvarova, M.; Telen, M.J. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood 2008, 112, 3474–3483. [Google Scholar] [CrossRef] [Green Version]
- Turhan, A.; Weiss, L.A.; Mohandas, N.; Coller, B.S.; Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc. Natl. Acad. Sci. USA 2002, 99, 3047–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makis, A.C.; Hatzimichael, E.C.; Bourantas, K.L. The role of cytokines in sickle cell disease. Ann. Hematol. 2000, 79, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Conran, N.; Fattori, A.; Saad, S.T.; Costa, F.F. Increased levels of soluble ICAM-1 in the plasma of sickle cell patients are reversed by hydroxyurea. Am. J. Hematol. 2004, 76, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Solovey, A.A.; Solovey, A.N.; Harkness, J.; Hebbel, R.P. Modulation of endothelial cell activation in sickle cell disease: A pilot study. Blood 2001, 97, 1937–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, M.; Ryan, T.M.; Adler, B.; Townes, T.M.; Parks, D.A.; Thompson, J.A.; Tousson, A.; Gladwin, M.T.; Patel, R.P.; Tarpey, M.M.; et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc. Natl. Acad. Sci. USA 2001, 98, 15215–15220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiu, Y.T.; Udden, M.M.; McIntire, L.V. Perfusion with sickle erythrocytes up-regulates ICAM-1 and VCAM-1 gene expression in cultured human endothelial cells. Blood 2000, 95, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Dworkis, D.A.; Klings, E.S.; Solovieff, N.; Li, G.; Milton, J.N.; Hartley, S.W.; Melista, E.; Parente, J.; Sebastiani, P.; Steinberg, M.H.; et al. Severe sickle cell anemia is associated with increased plasma levels of TNF-R1 and VCAM-1. Am. J. Hematol. 2011, 86, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qari, M.H.; Dier, U.; Mousa, S.A. Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clin. Appl. Thromb. Hemost. Off. J. Int. Acad. Clin. Appl. Thromb. Hemost. 2012, 18, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataga, K.I.; Key, N.S. Hypercoagulability in sickle cell disease: New approaches to an old problem. ASH Educ. Prog. 2007, 2007, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.B., Jr. Platelets, coagulation, and fibrinolysis in sickle cell disease: Their possible role in vascular occlusion. Blood Coagulat. Fibrinol. Int. J. Haemost. Thromb. 1991, 2, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Rusanova, I.; Escames, G.; Cossio, G.; de Borace, R.G.; Moreno, B.; Chahboune, M.; Lopez, L.C.; Diez, T.; Acuna-Castroviejo, D. Oxidative stress status, clinical outcome, and beta-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur. J. Haematol. 2010, 85, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.B.; Franco-Penteado, C.; Saad, S.T.; Costa, F.F.; Conran, N. Sickle cell disease serum induces NADPH enzyme subunit expression and oxidant production in leukocytes. Hematology 2010, 15, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.C.; Granger, D.N. Sickle cell disease: Role of reactive oxygen and nitrogen metabolites. Clin. Exp. Pharmacol. Physiol. 2007, 34, 926–932. [Google Scholar] [CrossRef] [PubMed]
- De Castro, L.M.; Zennadi, R.; Jonassaint, J.C.; Batchvarova, M.; Telen, M.J. Effect of propranolol as antiadhesive therapy in sickle cell disease. Clin. Transl. Sci. 2012, 5, 437–444. [Google Scholar] [CrossRef]
- Zennadi, R.; Hines, P.C.; De Castro, L.M.; Cartron, J.P.; Parise, L.V.; Telen, M.J. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood 2004, 104, 3774–3781. [Google Scholar] [CrossRef] [Green Version]
- Zennadi, R.; Moeller, B.J.; Whalen, E.J.; Batchvarova, M.; Xu, K.; Shan, S.; Delahunty, M.; Dewhirst, M.W.; Telen, M.J. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood 2007, 110, 2708–2717. [Google Scholar] [CrossRef]
- Chiou, E.; Zennadi, R. Galphas proteins activate p72 and p60-c-Src tyrosine kinases to mediate sickle red blood cell adhesion to endothelium via LW-alphavbeta3 and CD44-CD44 interactions. Int. J. Biochem. Cell Biol. 2015, 67, 115–120. [Google Scholar] [CrossRef]
- Brittain, J.E.; Han, J.; Ataga, K.I.; Orringer, E.P.; Parise, L.V. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J. Biol. Chem. 2004, 279, 42393–42402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohandas, N.; Evans, E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J. Clin. Investig. 1985, 76, 1605–1612. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Boogaerts, M.A.; Eaton, J.W.; Steinberg, M.H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N. Engl. J. Med. 1980, 302, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.K.; Chen, D.; Zhan, J. Adhesion of sickle cells to vascular endothelium is critically dependent on changes in density and shape of the cells. Blood 1994, 83, 3006–3017. [Google Scholar] [CrossRef] [Green Version]
- Hebbel, R.P.; Yamada, O.; Moldow, C.F.; Jacob, H.S.; White, J.G.; Eaton, J.W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: Possible mechanism for microvascular occlusion in sickle cell disease. J. Clin. Investig. 1980, 65, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Swerlick, R.A.; Eckman, J.R.; Kumar, A.; Jeitler, M.; Wick, T.M. Alpha 4 beta 1-integrin expression on sickle reticulocytes: Vascular cell adhesion molecule-1-dependent binding to endothelium. Blood 1993, 82, 1891–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, P.C.; Zen, Q.; Burney, S.N.; Shea, D.A.; Ataga, K.I.; Orringer, E.P.; Telen, M.J.; Parise, L.V. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood 2003, 101, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.E.; Mlinar, K.J.; Anderson, C.S.; Orringer, E.P.; Parise, L.V. Activation of sickle red blood cell adhesion via integrin-associated protein/CD47-induced signal transduction. J. Clin. Investig. 2001, 107, 1555–1562. [Google Scholar] [CrossRef] [Green Version]
- Matsui, N.M.; Borsig, L.; Rosen, S.D.; Yaghmai, M.; Varki, A.; Embury, S.H. P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood 2001, 98, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Chaar, V.; Picot, J.; Renaud, O.; Bartolucci, P.; Nzouakou, R.; Bachir, D.; Galacteros, F.; Colin, Y.; Le Van Kim, C.; El Nemer, W. Aggregation of mononuclear and red blood cells through an α4β1-Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica 2010, 95, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Wun, T.; Paglieroni, T.; Field, C.L.; Welborn, J.; Cheung, A.; Walker, N.J.; Tablin, F. Platelet-erythrocyte adhesion in sickle cell disease. J. Investig. Med. Off. Publ. Am. Federat. Clin. Res. 1999, 47, 121–127. [Google Scholar]
- Brittain, J.E.; Knoll, C.M.; Ataga, K.I.; Orringer, E.P.; Parise, L.V. Fibronectin bridges monocytes and reticulocytes via integrin alpha4beta1. Br. J. Haematol. 2008, 141, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.F.; Spring, F.A.; Chasis, J.A.; Anstee, D.J. Erythroid cell adhesion molecules Lutheran and LW in health and disease. Baillieres Best Pract. Res. Clin. Haematol. 1999, 12, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Erythrocytes and platelet adhesion to endothelium are mediated by specialized molecules. Clin. Hemorheol. Microcirc. 2004, 30, 181–184. [Google Scholar] [PubMed]
- Telen, M.J. Erythrocyte adhesion receptors: Blood group antigens and related molecules. Transfus. Med. Rev. 2005, 19, 32–44. [Google Scholar] [CrossRef]
- Mahaseth, H.; Vercellotti, G.M.; Welch, T.E.; Bowlin, P.R.; Sonbol, K.M.; Hsia, C.J.; Ma, L.; Bischof, J.C.; Hebbel, R.P.; Belcher, J.D. Polynitroxyl albumin inhibits inflammation and vasoocclusion in transgenic sickle mice. J. Lab. Clin. Med. 2005, 145, 204–211. [Google Scholar] [CrossRef]
- Kaul, D.K.; Liu, X.D.; Zhang, X.; Ma, L.; Hsia, C.J.; Nagel, R.L. Inhibition of sickle red cell adhesion and vasoocclusion in the microcirculation by antioxidants. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H167–H175. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Zhang, P.; Abdulla, F.; Nguyen, P.; Killeen, T.; Xu, P.; O’Sullivan, G.; Nath, K.A.; et al. Control of oxidative stress and inflammation in sickle cell disease with the Nrf2 activator dimethyl fumarate. Antioxid. Redox Signal. 2017, 26, 748–762. [Google Scholar] [CrossRef]
- Hebbel, R.P. The sickle erythrocyte in double jeopardy: Autoxidation and iron decompartmentalization. Semin. Hematol. 1990, 27, 51–69. [Google Scholar]
- Kuypers, F.A.; Scott, M.D.; Schott, M.A.; Lubin, B.; Chiu, D.T.Y. Use of ektacytometry to determine red-cell susceptibility to oxidative stress. J. Lab. Clin. Med. 1990, 116, 535–545. [Google Scholar]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Wagener, F.A.; Eggert, A.; Boerman, O.C.; Oyen, W.J.; Verhofstad, A.; Abraham, N.G.; Adema, G.; van Kooyk, Y.; de Witte, T.; Figdor, C.G. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood 2001, 98, 1802–1811. [Google Scholar] [CrossRef]
- Wagener, F.A.; Abraham, N.G.; van Kooyk, Y.; de Witte, T.; Figdor, C.G. Heme-induced cell adhesion in the pathogenesis of sickle-cell disease and inflammation. Trends Pharmacol. Sci. 2001, 22, 52–54. [Google Scholar] [CrossRef]
- Belcher, J.D.; Mahaseth, H.; Welch, T.E.; Otterbein, L.E.; Hebbel, R.P.; Vercellotti, G.M. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J. Clin. Investig. 2006, 116, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Beers, E.J.; Yang, Y.; Raghavachari, N.; Tian, X.; Allen, D.T.; Nichols, J.S.; Mendelsohn, L.; Nekhai, S.; Gordeuk, V.R.; Taylor, J.G.t.; et al. Iron, inflammation, and early death in adults with sickle cell disease. Circ. Res. 2015, 116, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helley, D.; Eldor, A.; Girot, R.; Ducrocq, R.; Guillin, M.C.; Bezeaud, A. Increased procoagulant activity of red blood cells from patients with homozygous sickle cell disease and beta-thalassemia. Thromb. Haemost. 1996, 76, 322–327. [Google Scholar]
- Helley, D.; Girot, R.; Guillin, M.C.; Bezeaud, A. Sickle cell disease: Relation between procoagulant activity of red blood cells from different phenotypes and in vivo blood coagulation activation. Br. J. Haematol. 1997, 99, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Van Beers, E.J.; Schaap, M.C.; Berckmans, R.J.; Nieuwland, R.; Sturk, A.; van Doormaal, F.F.; Meijers, J.C.; Biemond, B.J.; CURAMA Study Group. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 2009, 94, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA J. Am. Med. Assoc. 2005, 293, 1653–1662. [Google Scholar] [CrossRef]
- Camus, S.M.; Gausseres, B.; Bonnin, P.; Loufrani, L.; Grimaud, L.; Charue, D.; De Moraes, J.A.; Renard, J.M.; Tedgui, A.; Boulanger, C.M.; et al. Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood 2012, 120, 5050–5058. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Grunenwald, A.; Rajaratnam, H.; Gnemmi, V.; Frimat, M.; Figueres, M.L.; Knockaert, S.; Bouzekri, S.; Charue, D.; Noe, R.; et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 2018, 3, e96910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camus, S.M.; De Moraes, J.A.; Bonnin, P.; Abbyad, P.; Le Jeune, S.; Lionnet, F.; Loufrani, L.; Grimaud, L.; Lambry, J.C.; Charue, D.; et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 2015, 125, 3805–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, P.; Shalev, O.; Hebbel, R.P. The molecular pathobiology of cell membrane iron: The sickle red cell as a model. Free Radic. Biol. Med. 1998, 24, 1040–1048. [Google Scholar] [CrossRef]
- Van Tits, L.J.; van Heerde, W.L.; Landburg, P.P.; Boderie, M.J.; Muskiet, F.A.; Jacobs, N.; Duits, A.J.; Schnog, J.B. Plasma annexin A5 and microparticle phosphatidylserine levels are elevated in sickle cell disease and increase further during painful crisis. Biochem. Biophys. Res. Commun. 2009, 390, 161–164. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Tanaka, Y.; Fidler, I.J.; Chiu, D.T.; Lubin, B.; Schroit, A.J. Increased adherence of sickled and phosphatidylserine-enriched human erythrocytes to cultured human peripheral blood monocytes. J. Clin. Investig. 1985, 75, 1965–1972. [Google Scholar] [CrossRef] [Green Version]
- Ballas, S.K.; Smith, E.D. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood 1992, 79, 2154–2163. [Google Scholar] [CrossRef] [Green Version]
- Meier, E.R.; Byrnes, C.; Lee, Y.T.; Wright, E.C.; Schechter, A.N.; Luban, N.L.; Miller, J.L. Increased reticulocytosis during infancy is associated with increased hospitalizations in sickle cell anemia patients during the first three years of life. PLoS ONE 2013, 8, e70794. [Google Scholar] [CrossRef] [Green Version]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O., 3rd; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2014, 5, 500. [Google Scholar] [CrossRef] [Green Version]
- Neidlinger, N.A.; Larkin, S.K.; Bhagat, A.; Victorino, G.P.; Kuypers, F.A. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J. Biol. Chem. 2006, 281, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Fadok, V.A.; Bratton, D.L.; Rose, D.M.; Pearson, A.; Ezekewitz, R.A.; Henson, P.M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000, 405, 85–90. [Google Scholar] [CrossRef]
- Nader, E.; Romana, M.; Guillot, N.; Fort, R.; Stauffer, E.; Lemonne, N.; Garnier, Y.; Skinner, S.C.; Etienne-Julan, M.; Robert, M.; et al. Association between nitric oxide, oxidative stress, eryptosis, red blood cell microparticles, and vascular function in sickle cell anemia. Front. Immunol. 2020, 11, 551441. [Google Scholar] [CrossRef]
- Setty, B.N.; Kulkarni, S.; Rao, A.K.; Stuart, M.J. Fetal hemoglobin in sickle cell disease: Relationship to erythrocyte phosphatidylserine exposure and coagulation activation. Blood 2000, 96, 1119–1124. [Google Scholar] [CrossRef]
- Woollard, K.J.; Sturgeon, S.; Chin-Dusting, J.P.; Salem, H.H.; Jackson, S.P. Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. J. Biol. Chem. 2009, 284, 13110–13118. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graca-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Adisa, O.A.; Chappa, P.; Tan, F.; Jackson, K.A.; Archer, D.R.; Ofori-Acquah, S.F. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J. Clin. Investig. 2013, 123, 4809–4820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zhang, D.; Fuchs, T.A.; Manwani, D.; Wagner, D.D.; Frenette, P.S. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 2014, 123, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
- Fertakis, A.; Panitsas, G.; Angelopoulos, B. Serum haemopexin concentration in patients with various haemoglobinopathies. Effect of splenectomy. Acta Haematol. 1973, 50, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Orlov, S.N.; Furuya, K.; Grygorczyk, R. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 2014, 124, 2150–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprague, R.S.; Stephenson, A.H.; Ellsworth, M.L. Red not dead: Signaling in and from erythrocytes. Trends Endocrinol. Metab. 2007, 18, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.L.; Linden, J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood 2010, 116, 5010–5020. [Google Scholar] [CrossRef] [Green Version]
- Field, J.J.; Lin, G.; Okam, M.M.; Majerus, E.; Keefer, J.; Onyekwere, O.; Ross, A.; Campigotto, F.; Neuberg, D.; Linden, J.; et al. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood 2013, 121, 3329–3334. [Google Scholar] [CrossRef] [Green Version]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Akohoue, S.A.; Shankar, S.; Milne, G.L.; Morrow, J.; Chen, K.Y.; Ajayi, W.U.; Buchowski, M.S. Energy expenditure, inflammation, and oxidative stress in steady-state adolescents with sickle cell anemia. Pediatr. Res. 2007, 61, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilney, N.L.; Paz, D.; Ames, J.; Gasser, M.; Laskowski, I.; Hancock, W.W. Ischemia-reperfusion injury. Transplant. Proc. 2001, 33, 843–844. [Google Scholar] [CrossRef]
- Nath, K.A.; Grande, J.P.; Croatt, A.J.; Frank, E.; Caplice, N.M.; Hebbel, R.P.; Katusic, Z.S. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am. J. Pathol. 2005, 166, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.C.; Shacks, S.J.; Mitchell, R.A.; Banks, A. Serum interleukin-6 levels in the steady state of sickle cell disease. J. Interferon Cytokine Res. 1995, 15, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.B., Jr.; Haywood, L.J. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J. Natl. Med. Assoc. 1992, 84, 611–615. [Google Scholar] [PubMed]
- Marcal, L.E.; Dias-da-Motta, P.M.; Rehder, J.; Mamoni, R.L.; Blotta, M.H.; Whitney, C.B.; Newburger, P.E.; Costa, F.F.; Saad, S.T.; Condino-Neto, A. Up-regulation of NADPH oxidase components and increased production of interferon-gamma by leukocytes from sickle cell disease patients. Am. J. Hematol. 2008, 83, 41–45. [Google Scholar] [CrossRef]
- Belcher, J.D.; Marker, P.H.; Weber, J.P.; Hebbel, R.P.; Vercellotti, G.M. Activated monocytes in sickle cell disease: Potential role in the activation of vascular endothelium and vaso-occlusion. Blood 2000, 96, 2451–2459. [Google Scholar] [CrossRef]
- Fan, J.; Frey, R.S.; Rahman, A.; Malik, A.B. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J. Biol. Chem. 2002, 277, 3404–3411. [Google Scholar] [CrossRef] [Green Version]
- Paloschi, M.V.; Boeno, C.N.; Lopes, J.A.; Eduardo Dos Santos da Rosa, A.; Pires, W.L.; Pontes, A.S.; da Silva Setubal, S.; Soares, A.M.; Zuliani, J.P. Role of l-amino acid oxidase isolated from Calloselasma rhodostoma venom on neutrophil NADPH oxidase complex activation. Toxicon 2018, 145, 48–55. [Google Scholar] [CrossRef]
- Wun, T. The role of inflammation and leukocytes in the pathogenesis of sickle cell disease—Haemoglobinopathy. Hematology 2001, 5, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wandersee, N.J.; Guo, Y.; Jones, D.W.; Holzhauer, S.L.; Hanson, M.S.; Machogu, E.; Brousseau, D.C.; Hogg, N.; Densmore, J.C.; et al. Sickle cell disease increases high mobility group box 1: A novel mechanism of inflammation. Blood 2014, 124, 3978–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adewoye, A.H.; Klings, E.S.; Farber, H.W.; Palaima, E.; Bausero, M.A.; McMahon, L.; Odhiambo, A.; Surinder, S.; Yoder, M.; Steinberg, M.H.; et al. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am. J. Hematol. 2005, 78, 240–242. [Google Scholar] [CrossRef] [Green Version]
- Idzko, M.; Ferrari, D.; Riegel, A.K.; Eltzschig, H.K. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Tadie, J.M.; Bae, H.B.; Jiang, S.; Park, D.W.; Bell, C.P.; Yang, H.; Pittet, J.F.; Tracey, K.; Thannickal, V.J.; Abraham, E.; et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am. J. Physiol. 2013, 304, L342–L349. [Google Scholar] [CrossRef] [Green Version]
- Sorbara, M.T.; Girardin, S.E. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011, 21, 558–560. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.L.; Marshall, M.A.; Ramos, S.I.; Lannigan, J.A.; Field, J.J.; Strieter, R.M.; Linden, J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood 2009, 114, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavrovsky, Y.; Song, C.S.; Chatterjee, B.; Roy, A.K. Age-dependent increase of heme oxygenase-1 gene expression in the liver mediated by NFkappaB. Mech. Ageing Dev. 2000, 114, 49–60. [Google Scholar] [CrossRef]
- Rattan, V.; Sultana, C.; Shen, Y.; Kalra, V.K. Oxidant stress-induced transendothelial migration of monocytes is linked to phosphorylation of PECAM-1. Am. J. Physiol. 1997, 273, E453–E461. [Google Scholar] [CrossRef] [PubMed]
- Reichard, J.F.; Motz, G.T.; Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007, 35, 7074–7086. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Xi, C.; Thomas, B.; Pace, B.S. Loss of NRF2 function exacerbates the pathophysiology of sickle cell disease in a transgenic mouse model. Blood 2018, 131, 558–562. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Panda, H.; Otsuki, A.; Katsuoka, F.; Saito, R.; Saigusa, D.; Uruno, A.; Yamamoto, M. Nrf2 activation in myeloid cells and endothelial cells differentially mitigates sickle cell disease pathology in mice. Blood Adv. 2019, 3, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Bivalacqua, T.J.; Musicki, B.; Hsu, L.L.; Berkowitz, D.E.; Champion, H.C.; Burnett, A.L. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS ONE 2013, 8, e68028. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.C.; Hebbel, R.P.; Lefer, D.J.; Granger, D.N. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic. Biol. Med. 2006, 40, 1443–1453. [Google Scholar] [CrossRef]
- Chou, T.C.; Yen, M.H.; Li, C.Y.; Ding, Y.A. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 1998, 31, 643–648. [Google Scholar] [CrossRef] [Green Version]
- El-Hattab, A.W.; Emrick, L.T.; Craigen, W.J.; Scaglia, F. Citrulline and arginine utility in treating nitric oxide deficiency in mitochondrial disorders. Mol. Genet. Metab. 2012, 107, 247–252. [Google Scholar] [CrossRef]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, G.J.; Martyr, S.; Blackwelder, W.C.; Nichols, J.S.; Coles, W.A.; Hunter, L.A.; Brennan, M.L.; Hazen, S.L.; Gladwin, M.T. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br. J. Haematol. 2005, 130, 943–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurantsin-Mills, J.; Ofosu, F.A.; Safa, T.K.; Siegel, R.S.; Lessin, L.S. Plasma factor VII and thrombin-antithrombin III levels indicate increased tissue factor activity in sickle cell patients. Br. J. Haematol. 1992, 81, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Westwick, J.; Watson-Williams, E.J.; Krishnamurthi, S.; Marks, G.; Ellis, V.; Scully, M.F.; White, J.M.; Kakkar, V.V. Platelet activation during steady state sickle cell disease. J. Med. 1983, 14, 17–36. [Google Scholar] [PubMed]
- Ataga, K.I.; Orringer, E.P. Hypercoagulability in sickle cell disease: A curious paradox. Am. J. Med. 2003, 115, 721–728. [Google Scholar] [CrossRef]
- Goel, M.S.; Diamond, S.L. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood 2002, 100, 3797–3803. [Google Scholar] [CrossRef]
- Barr, J.D.; Chauhan, A.K.; Schaeffer, G.V.; Hansen, J.K.; Motto, D.G. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 2013, 121, 3733–3741. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Role of red blood cells in haemostasis and thrombosis. ISBT Sci. Ser. 2017, 12, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, R.; Barshtein, G.; Mardi, T.; Deutch, V.; Elkayam, O.; Yedgar, S.; Berliner, S. A synergistic effect of albumin and fibrinogen on immunoglobulin-induced red blood cell aggregation. Am. J Physiol. 2003, 285, H2663–H2669. [Google Scholar] [CrossRef] [Green Version]
- Spring, F.A.; Parsons, S.F.; Ortlepp, S.; Olsson, M.L.; Sessions, R.; Brady, R.L.; Anstee, D.J. Intercellular adhesion molecule-4 binds alpha(4)beta(1) and alpha(V)-family integrins through novel integrin-binding mechanisms. Blood 2001, 98, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Hermand, P.; Gane, P.; Huet, M.; Jallu, V.; Kaplan, C.; Sonneborn, H.H.; Cartron, J.P.; Bailly, P. Red cell ICAM-4 is a novel ligand for platelet-activated alpha IIbbeta 3 integrin. J. Biol. Chem. 2003, 278, 4892–4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F.A.; Connell, S.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Tavares, A.; Ariens, R.A.; Santos, N.C. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 2010, 4, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Austin, H.; Key, N.S.; Benson, J.M.; Lally, C.; Dowling, N.F.; Whitsett, C.; Hooper, W.C. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood 2007, 110, 908–912. [Google Scholar] [CrossRef]
- Schafer, A.I. Bleeding and thrombosis in the myeloproliferative disorders. Blood 1984, 64, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, K.A., Jr.; Ou, J.; Ou, Z.; Shi, Y.; Franciosi, J.P.; Signorino, P.; Kaul, S.; Ackland-Berglund, C.; Witte, K.; Holzhauer, S.; et al. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L705–L714. [Google Scholar] [CrossRef]
- Holland, J.A.; Meyer, J.W.; Chang, M.M.; O’Donnell, R.W.; Johnson, D.K.; Ziegler, L.M. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium 1998, 6, 113–121. [Google Scholar] [CrossRef]
- Kovalski, N.N.; de Lamirande, E.; Gagnon, C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil. Steril. 1992, 58, 809–816. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Thornley-Brown, D.; Freeman, B.A. Reactive species in sickle cell disease. Ann. N. Y. Acad. Sci. 2000, 899, 375–391. [Google Scholar] [CrossRef]
- Rank, B.H.; Carlsson, J.; Hebbel, R.P. Abnormal redox status of membrane-protein thiols in sickle erythrocytes. J. Clin. Investig. 1985, 75, 1531–1537. [Google Scholar] [CrossRef]
- Akinola, N.O.; Stevens, S.M.; Franklin, I.M.; Nash, G.B.; Stuart, J. Rheological changes in the prodromal and established phases of sickle cell vaso-occlusive crisis. Br. J. Haematol. 1992, 81, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Detterich, J.; Alexy, T.; Rabai, M.; Wenby, R.; Dongelyan, A.; Coates, T.; Wood, J.; Meiselman, H. Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion 2013, 53, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Gayen Betal, S.; Setty, B.N. Phosphatidylserine-positive erythrocytes bind to immobilized and soluble thrombospondin-1 via its heparin-binding domain. Transl. Res. J. Lab. Clin. Med. 2008, 152, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, F.; Ammollo, C.T.; Esmon, N.L.; Esmon, C.T. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J. Thromb. Haemost. 2014, 12, 1697–1702. [Google Scholar] [CrossRef] [Green Version]
- Wautier, M.P.; Heron, E.; Picot, J.; Colin, Y.; Hermine, O.; Wautier, J.L. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J. Thromb. Haemost. 2011, 9, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xie, R.; Yu, C.; Wang, Q.; Shi, F.; Yao, C.; Xie, R.; Zhou, J.; Gilbert, G.E.; Shi, J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb. Haemost. 2012, 107, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelihan, M.F.; Zachary, V.; Orfeo, T.; Mann, K.G. Prothrombin activation in blood coagulation: The erythrocyte contribution to thrombin generation. Blood 2012, 120, 3837–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonomini, M.; Sirolli, V.; Merciaro, G.; Antidormi, T.; Di Liberato, L.; Brummer, U.; Papponetti, M.; Cappelli, P.; Di Gregorio, P.; Arduini, A. Red blood cells may contribute to hypercoagulability in uraemia via enhanced surface exposure of phosphatidylserine. Nephrol. Dial. Transplant. 2005, 20, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, E.; Rees, D.C.; Gibson, J.S. Role of calcium in phosphatidylserine externalisation in red blood cells from sickle cell patients. Anemia 2011, 2011, 379894. [Google Scholar] [CrossRef] [Green Version]
- Kamp, D.; Sieberg, T.; Haest, C.W. Inhibition and stimulation of phospholipid scrambling activity. Consequences for lipid asymmetry, echinocytosis, and microvesiculation of erythrocytes. Biochemistry 2001, 40, 9438–9446. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, A.V.; Zvyagilskaya, R.A.; Labas, Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry 2003, 68, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Bookchin, R.M.; Lew, V.L.; Roth, E.F., Jr. Elevated red cell calcium: Innocent bystander or kiss of death? Prog. Clin. Biol. Res. 1985, 195, 369–380. [Google Scholar]
- Clark, M.R. Senescence of red blood cells: Progress and problems. Physiol. Rev. 1988, 68, 503–554. [Google Scholar] [CrossRef]
- Romero, P.J.; Romero, E.A. The role of calcium metabolism in human red blood cell ageing: A proposal. Blood Cells Mol. Dis. 1999, 25, 9–19. [Google Scholar] [CrossRef]
- Makhro, A.; Hanggi, P.; Goede, J.S.; Wang, J.; Bruggemann, A.; Gassmann, M.; Schmugge, M.; Kaestner, L.; Speer, O.; Bogdanova, A. N-methyl-D-aspartate receptors in human erythroid precursor cells and in circulating red blood cells contribute to the intracellular calcium regulation. Am. J. Physiol. Cell Physiol. 2013, 305, C1123–C1138. [Google Scholar] [CrossRef] [Green Version]
- Friederichs, E.; Meiselman, H.J. Effects of calcium permeabilization on RBC rheologic behavior. Biorheology 1994, 31, 207–215. [Google Scholar] [CrossRef]
- Steffen, P.; Jung, A.; Nguyen, D.B.; Muller, T.; Bernhardt, I.; Kaestner, L.; Wagner, C. Stimulation of human red blood cells leads to Ca2+-mediated intercellular adhesion. Cell Calcium 2011, 50, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaestner, L.; Steffen, P.; Nguyen, D.B.; Wang, J.; Wagner-Britz, L.; Jung, A.; Wagner, C.; Bernhardt, I. Lysophosphatidic acid induced red blood cell aggregation in vitro. Bioelectrochemistry 2012, 87, 89–95. [Google Scholar] [CrossRef]
- Aleman, M.M.; Byrnes, J.R.; Wang, J.G.; Tran, R.; Lam, W.A.; Di Paola, J.; Mackman, N.; Degen, J.L.; Flick, M.J.; Wolberg, A.S. Factor XIII activity mediates red blood cell retention in venous thrombi. J. Clin. Investig. 2014, 124, 3590–3600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atici, A.G.; Kayhan, S.; Aydin, D.; Yilmaz, Y.A. Plasma viscosity levels in pulmonary thromboembolism. Clin. Hemorheol. Microcirc. 2013, 55, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, M.; Codeluppi, L.; Poggi, M.; Baraldi, P. Abnormal blood viscosity and erythrocyte deformability in retinal vein occlusion. Am. J. Ophthalmol. 1983, 96, 399–400. [Google Scholar] [CrossRef]
- Xu, D.; Kaliviotis, E.; Munjiza, A.; Avital, E.; Ji, C.; Williams, J. Large scale simulation of red blood cell aggregation in shear flows. J. Biomech. 2013, 46, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Sheinman, G.; Yedgar, S.; Eldor, A.; Roth, A.; Berliner, A.S.; Barshtein, G. Thrombolytic therapy reduces red blood cell aggregation in plasma without affecting intrinsic aggregability. Thromb. Res. 2002, 105, 487–492. [Google Scholar] [CrossRef]
- Von Bruhl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Kollnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Pawloski, J.R.; Hess, D.T.; Stamler, J.S. Export by red blood cells of nitric oxide bioactivity. Nature 2001, 409, 622–626. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Yalcin, O.; Ozdem, S.; Armstrong, J.K.; Meiselman, H.J. Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H222–H229. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Freeman, B.A. Redox-dependent impairment of vascular function in sickle cell disease. Free Radic. Biol. Med. 2007, 43, 1469–1483. [Google Scholar] [CrossRef] [Green Version]
- Gutsaeva, D.R.; Montero-Huerta, P.; Parkerson, J.B.; Yerigenahally, S.D.; Ikuta, T.; Head, C.A. Molecular mechanisms underlying synergistic adhesion of sickle red blood cells by hypoxia and low nitric oxide bioavailability. Blood 2014, 123, 1917–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chigaev, A.; Smagley, Y.; Sklar, L.A. Nitric oxide/cGMP pathway signaling actively down-regulates alpha4beta1-integrin affinity: An unexpected mechanism for inducing cell de-adhesion. BMC Immunol. 2011, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Gromotowicz-Poplawska, A.; Kloza, M.; Aleksiejczuk, M.; Marcinczyk, N.; Szemraj, J.; Kozlowska, H.; Chabielska, E. Nitric oxide as a modulator in platelet- and endothelium-dependent antithrombotic effect of eplerenone in diabetic rats. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Space, S.L.; Lane, P.A.; Pickett, C.K.; Weil, J.V. Nitric oxide attenuates normal and sickle red blood cell adherence to pulmonary endothelium. Am. J. Hematol. 2000, 63, 200–204. [Google Scholar] [CrossRef]
- Tran, P.L.; Pietropaolo, M.G.; Valerio, L.; Brengle, W.; Wong, R.K.; Kazui, T.; Khalpey, Z.I.; Redaelli, A.; Sheriff, J.; Bluestein, D.; et al. Hemolysate-mediated platelet aggregation: An additional risk mechanism contributing to thrombosis of continuous flow ventricular assist devices. Perfusion 2016, 31, 401–408. [Google Scholar] [CrossRef]
- Radomski, M.W.; Moncada, S. The biological and pharmacological role of nitric oxide in platelet function. Adv. Exp. Med. Biol. 1993, 344, 251–264. [Google Scholar] [CrossRef]
- Pakbaz, Z.; Wun, T. Role of the hemostatic system on sickle cell disease pathophysiology and potential therapeutics. Hematol. Oncol. Clin. N. Am. 2014, 28, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noubouossie, D.; Key, N.S.; Ataga, K.I. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016, 30, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokarev, A.A.; Butylin, A.A.; Ataullakhanov, F.I. Platelet adhesion from shear blood flow is controlled by near-wall rebounding collisions with erythrocytes. Biophys. J. 2011, 100, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Joist, J.H.; Bauman, J.E.; Sutera, S.P. Platelet adhesion and aggregation in pulsatile shear flow: Effects of red blood cells. Thromb. Res. 1998, 92, S47–S52. [Google Scholar] [CrossRef]
- Klatt, C.; Kruger, I.; Zey, S.; Krott, K.J.; Spelleken, M.; Gowert, N.S.; Oberhuber, A.; Pfaff, L.; Luckstadt, W.; Jurk, K.; et al. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J. Clin. Investig. 2018, 128, 3906–3925. [Google Scholar] [CrossRef] [PubMed]
- Solum, N.O. Procoagulant expression in platelets and defects leading to clinical disorders. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2841–2846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, J.; Santos, M.T.; Aznar, J.; Martinez, M.; Moscardo, A.; Pinon, M.; Broekman, M.J.; Marcus, A.J. Platelet-erythrocyte interactions enhance alpha(IIb)beta(3) integrin receptor activation and P-selectin expression during platelet recruitment: Down-regulation by aspirin ex vivo. Blood 2002, 99, 3978–3984. [Google Scholar] [CrossRef] [Green Version]
- Reimers, R.C.; Sutera, S.P.; Joist, J.H. Potentiation by red blood cells of shear-induced platelet aggregation: Relative importance of chemical and physical mechanisms. Blood 1984, 64, 1200–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomer, A.; Harker, L.A.; Kasey, S.; Eckman, J.R. Thrombogenesis in sickle cell disease. J. Lab. Clin. Med. 2001, 137, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Villagra, J.; Shiva, S.; Hunter, L.A.; Machado, R.F.; Gladwin, M.T.; Kato, G.J. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood 2007, 110, 2166–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamant, M.; Tushuizen, M.E.; Sturk, A.; Nieuwland, R. Cellular microparticles: New players in the field of vascular disease? Eur. J. Clin. Investig. 2004, 34, 392–401. [Google Scholar] [CrossRef]
- Rubin, O.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.D.; Angelillo-Scherrer, A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Schifferli, J.A. Microvesicles are messengers. Semin. Immunopathol. 2011, 33, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Willekens, F.L.; Roerdinkholder-Stoelwinder, B.; Groenen-Dopp, Y.A.; Bos, H.J.; Bosman, G.J.; van den Bos, A.G.; Verkleij, A.J.; Werre, J.M. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood 2003, 101, 747–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willekens, F.L.; Werre, J.M.; Groenen-Dopp, Y.A.; Roerdinkholder-Stoelwinder, B.; de Pauw, B.; Bosman, G.J. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef]
- Bosman, G.J. Erythrocyte aging in sickle cell disease. Cell. Mol. Biol. 2004, 50, 81–86. [Google Scholar]
- Setty, B.N.; Rao, A.K.; Stuart, M.J. Thrombophilia in sickle cell disease: The red cell connection. Blood 2001, 98, 3228–3233. [Google Scholar] [CrossRef] [Green Version]
- Horne, M.K., 3rd; Cullinane, A.M.; Merryman, P.K.; Hoddeson, E.K. The effect of red blood cells on thrombin generation. Br. J. Haematol. 2006, 133, 403–408. [Google Scholar] [CrossRef]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renne, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. 2012, 10, 1355–1362. [Google Scholar] [CrossRef]
- Iwata, H.; Kaibara, M. Activation of factor IX by erythrocyte membranes causes intrinsic coagulation. Blood Coagulat. Fibrinol. Int. J. Haemost. Thromb. 2002, 13, 489–496. [Google Scholar] [CrossRef]
- Stuart, J.; Stone, P.C.; Akinola, N.O.; Gallimore, J.R.; Pepys, M.B. Monitoring the acute phase response to vaso-occlusive crisis in sickle cell disease. J. Clin. Pathol. 1994, 47, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vordermeier, S.; Singh, S.; Biggerstaff, J.; Harrison, P.; Grech, H.; Pearson, T.C.; Dumonde, D.C.; Brown, K.A. Red blood cells from patients with sickle cell disease exhibit an increased adherence to cultured endothelium pretreated with tumour necrosis factor (TNF). Br. J. Haematol. 1992, 81, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Uadia, P.O.; Gadzama, A.A. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med. 2010, 9, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, S.; Vitorino de Almeida, V.; Calado, A.; Rosario, H.S.; Saldanha, C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim. Biophys. Acta 2012, 1818, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, F.P.; Lublin, D.M.; Telen, M.J.; Veile, R.A.; Miller, Y.E.; Donis-Keller, H.; Brown, E.J. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. J. Biol. Chem. 1994, 269, 1567–1570. [Google Scholar] [CrossRef]
- Joneckis, C.C.; Shock, D.D.; Cunningham, M.L.; Orringer, E.P.; Parise, L.V. Glycoprotein IV-independent adhesion of sickle red blood cells to immobilized thrombospondin under flow conditions. Blood 1996, 87, 4862–4870. [Google Scholar] [CrossRef] [Green Version]
- Tracz, M.J.; Juncos, J.P.; Grande, J.P.; Croatt, A.J.; Ackerman, A.W.; Katusic, Z.S.; Nath, K.A. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am. J. Pathol. 2008, 173, 1882–1890. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, S.; Weltermann, A.; Fritsche, R.; Marsik, C.; Wagner, O.; Kyrle, P.A.; Eichinger, S. Genetic variation in heme oxygenase 1 (HMOX1) and the risk of recurrent venous thromboembolism. J. Vasc. Surg. 2008, 47, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Day, S.M.; Reeve, J.L.; Pedersen, B.; Farris, D.M.; Myers, D.D.; Im, M.; Wakefield, T.W.; Mackman, N.; Fay, W.P. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood 2005, 105, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Iacoviello, L.; Kolpakov, V.; Salvatore, L.; Amore, C.; Pintucci, G.; de Gaetano, G.; Donati, M.B. Human endothelial cell damage by neutrophil-derived cathepsin G. Role of cytoskeleton rearrangement and matrix-bound plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2037–2046. [Google Scholar] [CrossRef]
- Saha, P.; Humphries, J.; Modarai, B.; Mattock, K.; Waltham, M.; Evans, C.E.; Ahmad, A.; Patel, A.S.; Premaratne, S.; Lyons, O.T.; et al. Leukocytes and the natural history of deep vein thrombosis: Current concepts and future directions. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [Green Version]
- Bahl, N.; Winarsih, I.; Tucker-Kellogg, L.; Ding, J.L. Extracellular haemoglobin upregulates and binds to tissue factor on macrophages: Implications for coagulation and oxidative stress. Thromb. Haemost. 2014, 111, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkura, N.; Hiraishi, S.; Itabe, H.; Hamuro, T.; Kamikubo, Y.; Takano, T.; Matsuda, J.; Horie, S. Oxidized phospholipids in oxidized low-density lipoprotein reduce the activity of tissue factor pathway inhibitor through association with its carboxy-terminal region. Antioxid. Redox Signal. 2004, 6, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Nalian, A.; Iakhiaev, A.V. Possible mechanisms contributing to oxidative inactivation of activated protein C: Molecular dynamics study. Thromb. Haemost. 2008, 100, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.B.; Morser, J.; Clarke, J.H.; Blasko, E.; McLean, K.; Kuhn, I.; Chang, R.J.; Lin, J.H.; Vilander, L.; Andrews, W.H.; et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J. Clin. Investig. 1992, 90, 2565–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upchurch Jr, G.R.; Ramdev, N.; Walsh, M.T.; Loscalzo, J. Prothrombotic consequences of the oxidation of fibrinogen and their inhibition by aspirin. J. Thromb. Thromb. 1998, 5, 9–14. [Google Scholar] [CrossRef]
- De Cristofaro, R.; Landolfi, R. Oxidation of human alpha-thrombin by the myeloperoxidase-H2O2-chloride system: Structural and functional effects. Thromb. Haemost. 2000, 83, 253–261. [Google Scholar] [PubMed]
- Van Patten, S.M.; Hanson, E.; Bernasconi, R.; Zhang, K.; Manavalan, P.; Cole, E.S.; McPherson, J.M.; Edmunds, T. Oxidation of methionine residues in antithrombin. Effects on biological activity and heparin binding. J. Biol. Chem. 1999, 274, 10268–10276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ay, C.; Jungbauer, L.V.; Sailer, T.; Tengler, T.; Koder, S.; Kaider, A.; Panzer, S.; Quehenberger, P.; Pabinger, I.; Mannhalter, C. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin. Chem. 2007, 53, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Klyubin, I.V.; Kirpichnikova, K.M.; Gamaley, I.A. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur. J. Cell Biol. 1996, 70, 347–351. [Google Scholar]
- Kenawy, H.I.; Boral, I.; Bevington, A. Complement-coagulation cross-talk: A potential mediator of the physiological activation of complement by low pH. Front. Immunol. 2015, 6, 215. [Google Scholar] [CrossRef] [Green Version]
- Frimat, M.; Tabarin, F.; Dimitrov, J.D.; Poitou, C.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 2013, 122, 282–292. [Google Scholar] [CrossRef]
- Wiedmer, T.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 1986, 68, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Wiedmer, T.; Esmon, C.T.; Sims, P.J. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J. Biol. Chem. 1986, 261, 14587–14592. [Google Scholar] [CrossRef]
- Ikeda, K.; Nagasawa, K.; Horiuchi, T.; Tsuru, T.; Nishizaka, H.; Niho, Y. C5a induces tissue factor activity on endothelial cells. Thromb. Haemost. 1997, 77, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, G.M.; Dalmasso, A.P.; Schaid, T.R., Jr.; Nguyen, J.; Chen, C.; Ericson, M.E.; Abdulla, F.; Killeen, T.; Lindorfer, M.A.; Taylor, R.P.; et al. Critical role of C5a in sickle cell disease. Am. J. Hematol. 2019, 94, 327–337. [Google Scholar] [CrossRef]
- Chudwin, D.S.; Papierniak, C.; Lint, T.F.; Korenblit, A.D. Activation of the alternative complement pathway by red blood cells from patients with sickle cell disease. Clin. Immunol. Immunopathol. 1994, 71, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.; Terry, H.S.; Kleinert, D.; Laurence, J. The role of complement activation in thrombosis and hemolytic anemias. Transfus. Apher. Sci. 2016, 54, 191–198. [Google Scholar] [CrossRef]
- Merle, N.S.; Paule, R.; Leon, J.; Daugan, M.; Robe-Rybkine, T.; Poillerat, V.; Torset, C.; Fremeaux-Bacchi, V.; Dimitrov, J.D.; Roumenina, L.T. P-selectin drives complement attack on endothelium during intravascular hemolysis in TLR-4/heme-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 6280–6285. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.; Little, J.A.; Pecker, L.H. Advances in the Treatment of Sickle Cell Disease. Mayo Clin. Proc. 2018, 93, 1810–1824. [Google Scholar] [CrossRef] [Green Version]
- Zerez, C.R.; Lachant, N.A.; Lee, S.J.; Tanaka, K.R. Decreased erythrocyte nicotinamide adenine dinucleotide redox potential and abnormal pyridine nucleotide content in sickle cell disease. Blood 1988, 71, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.R.; Suh, J.H.; Hagar, W.; Larkin, S.; Bland, D.A.; Steinberg, M.H.; Vichinsky, E.P.; Shigenaga, M.; Ames, B.; Kuypers, F.A.; et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 2008, 111, 402–410. [Google Scholar] [CrossRef]
- Cui, M.H.; Suzuka, S.M.; Branch, N.A.; Ambadipudi, K.; Thangaswamy, S.; Acharya, S.A.; Billett, H.H.; Branch, C.A. Brain neurochemical and hemodynamic findings in the NY1DD mouse model of mild sickle cell disease. NMR Biomed. 2017, 30, 437–443. [Google Scholar] [CrossRef]
- Niihara, Y.; Matsui, N.M.; Shen, Y.M.; Akiyama, D.A.; Johnson, C.S.; Sunga, M.A.; Magpayo, J.; Embury, S.H.; Kalra, V.K.; Cho, S.H.; et al. L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disord. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.E.; Hart, E.; Kirkham, F.J.; Stotesbury, H. L-Glutamine in sickle cell disease. Drugs Today 2020, 56, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.G.H.; Belini Junior, E.; de Almeida, E.A.; Bonini-Domingos, C.R. Oxidative stress in sickle cell disease: An overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free Radic. Biol. Med. 2013, 65, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Ghoti, H.; Rachmilewitz, E.; Koren, A.; Levin, C.; Fibach, E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br. J. Haematol. 2006, 132, 108–113. [Google Scholar] [CrossRef]
- Pace, B.S.; Shartava, A.; Pack-Mabien, A.; Mulekar, M.; Ardia, A.; Goodman, S.R. Effects of N-acetylcysteine on dense cell formation in sickle cell disease. Am. J. Hematol. 2003, 73, 26–32. [Google Scholar] [CrossRef]
- Lal, A.; Atamna, W.; Killilea, D.W.; Suh, J.H.; Ames, B.N. Lipoic acid and acetyl-carnitine reverse iron-induced oxidative stress in human fibroblasts. Redox Rep. 2008, 13, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn porphyrin-based redox-active drugs: Differential effects as cancer therapeutics and protectors of normal tissue against oxidative injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, M.C.; Briehl, M.M.; Batinic-Haberle, I.; Tome, M.E. Manganese (III) meso-tetrakis N-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radic. Biol. Med. 2015, 83, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, M.C.; Briehl, M.M.; Crapo, J.D.; Batinic-Haberle, I.; Tome, M.E. Manganese porphyrin, MnTE-2-PyP5+, Acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic. Biol. Med. 2012, 52, 1272–1284. [Google Scholar] [CrossRef] [Green Version]
- Tovmasyan, A.; Sampaio, R.S.; Boss, M.K.; Bueno-Janice, J.C.; Bader, B.H.; Thomas, M.; Reboucas, J.S.; Orr, M.; Chandler, J.D.; Go, Y.M.; et al. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015, 89, 1231–1247. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Yang, W.; Fukuda, S.; Tse, H.M.; Paschen, W.; Johnson, K.; Batinic-Haberle, I.; Crapo, J.D.; Pearlstein, R.D.; Piganelli, J.; et al. Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radic. Biol. Med. 2009, 47, 917–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, H.; Spasojevic, I.; Tse, H.M.; Jung, J.Y.; Hong, J.; Zhang, Z.; Piganelli, J.D.; Batinic-Haberle, I.; Warner, D.S. Neuroprotective efficacy from a lipophilic redox-modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: Rodent models of ischemic stroke and subarachnoid hemorrhage. J. Pharmacol. Exp. Ther. 2011, 338, 906–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.K.; Tovmasyan, A.; Batinic-Haberle, I.; Devi, G.R. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radic. Biol. Med. 2014, 68, 302–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.W.; Choi, C.; Lee, G.H.; Son, A.; Kim, S.H.; Park, H.C.; Batinic-Haberle, I.; Park, W. Mechanism of the Antitumor and Radiosensitizing Effects of a Manganese Porphyrin, MnHex-2-PyP. Antioxid. Redox Signal. 2017, 27, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Bueno-Janice, J.C.; Jaramillo, M.C.; Sampaio, R.S.; Reboucas, J.S.; Kyui, N.; Benov, L.; Deng, B.; Huang, T.T.; Tome, M.E.; et al. Radiation-mediated tumor growth inhibition is significantly enhanced with redox-active compounds that cycle with ascorbate. Antioxid. Redox Signal. 2018, 29, 1196–1214. [Google Scholar] [CrossRef]
- Pazhanisamy, S.K.; Li, H.; Wang, Y.; Batinic-Haberle, I.; Zhou, D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis 2011, 26, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Carroll, D.W.; You, Y.; Chaiswing, L.; Wen, R.; Batinic-Haberle, I.; Bondada, S.; Liang, Y.; St Clair, D.K. A novel redox regulator, MnTnBuOE-2-PyP(5+), enhances normal hematopoietic stem/progenitor cell function. Redox Biol. 2017, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bruni, A.; Pepper, A.R.; Pawlick, R.L.; Gala-Lopez, B.; Gamble, A.; Kin, T.; Malcolm, A.J.; Jones, C.; Piganelli, J.D.; Crapo, J.D.; et al. BMX-001, a novel redox-active metalloporphyrin, improves islet function and engraftment in a murine transplant model. Am. J. Transplant. 2018, 18, 1879–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, A.; Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Abualhassan, N.; Crapo, J.D.; Piganelli, J.D.; Shapiro, A.M. A novel redox-active metalloporphyrin reduces reactive oxygen species and inflammatory markers but does not improve marginal mass engraftment in a murine donation after circulatory death islet transplantation model. Islets 2016, 8, e1190058. [Google Scholar] [CrossRef] [PubMed] [Green Version]




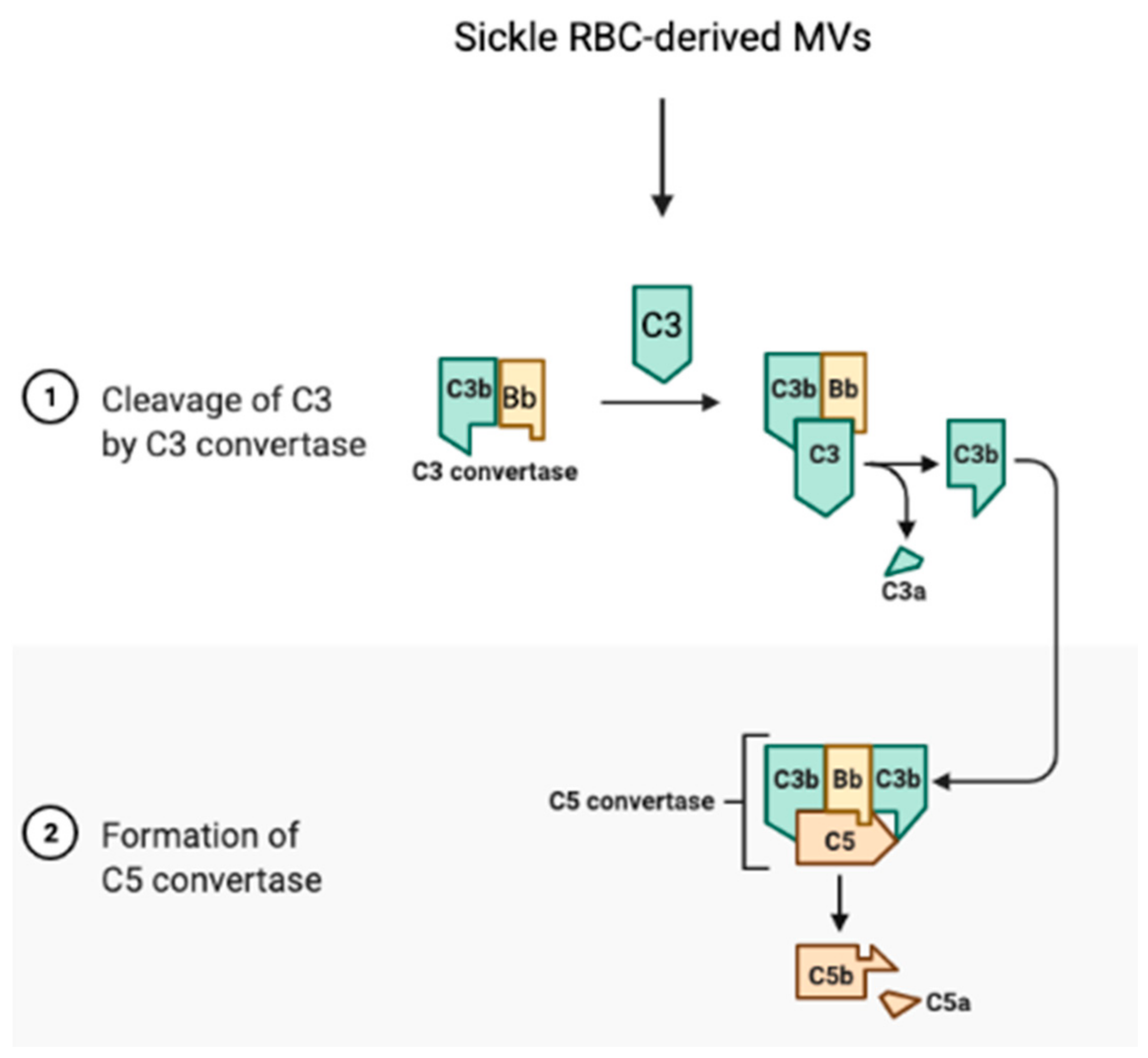
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zennadi, R. The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications. Antioxidants 2021, 10, 1608. https://doi.org/10.3390/antiox10101608
Wang Q, Zennadi R. The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications. Antioxidants. 2021; 10(10):1608. https://doi.org/10.3390/antiox10101608
Chicago/Turabian StyleWang, Qinhong, and Rahima Zennadi. 2021. "The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications" Antioxidants 10, no. 10: 1608. https://doi.org/10.3390/antiox10101608
APA StyleWang, Q., & Zennadi, R. (2021). The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications. Antioxidants, 10(10), 1608. https://doi.org/10.3390/antiox10101608





