Oxidative Stress and Inflammatory Mediators in Exhaled Breath Condensate of Patients with Pulmonary Tuberculosis. A Pilot Study with a Biomarker Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. EBC Sample Collection
2.3. Serum Collection
2.4. Redox State Biomarker Detection
2.4.1. Glutathione (GSH)
2.4.2. Malondialdehyde (MDA)
2.4.3. 8-Isoprostane
2.5. Nucleosomes’ Quantitation
2.6. Eicosanoids Detection
2.7. Cytokines Detection
2.8. Statistics
3. Results
3.1. Characteristics of the Study Group
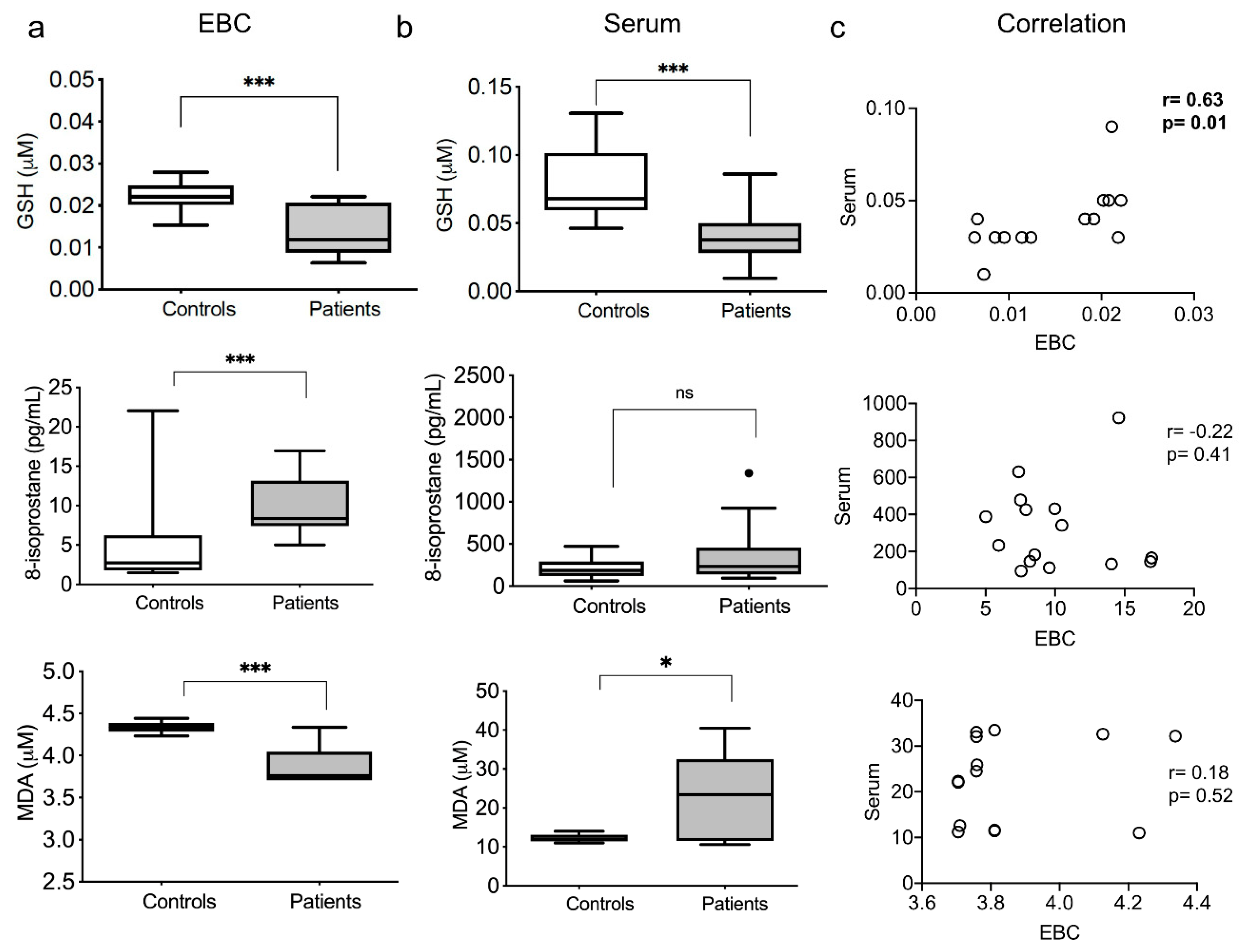
3.2. Redox Exhaled and Systemic Markers
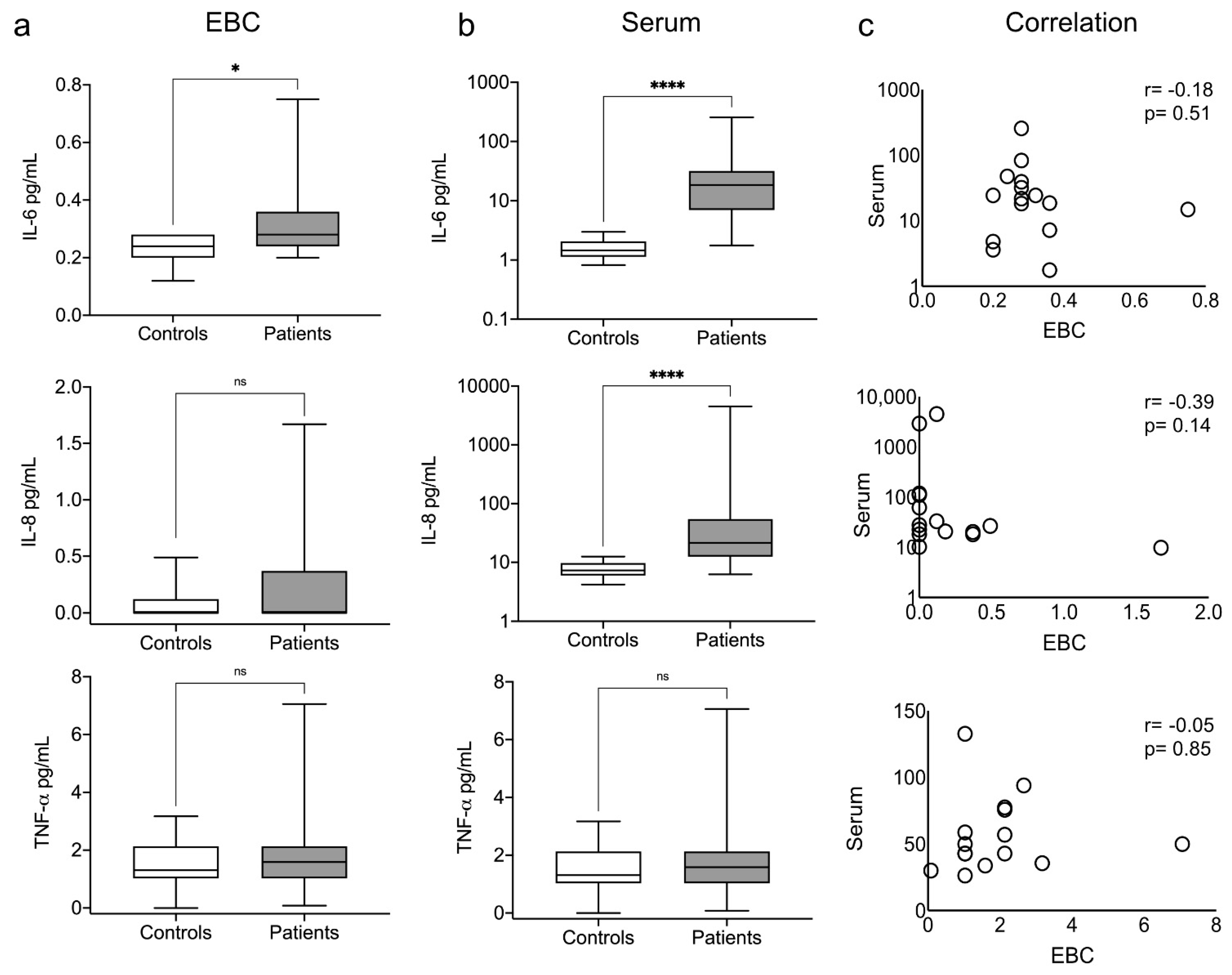
3.3. Exhaled and Systemic Cytokines
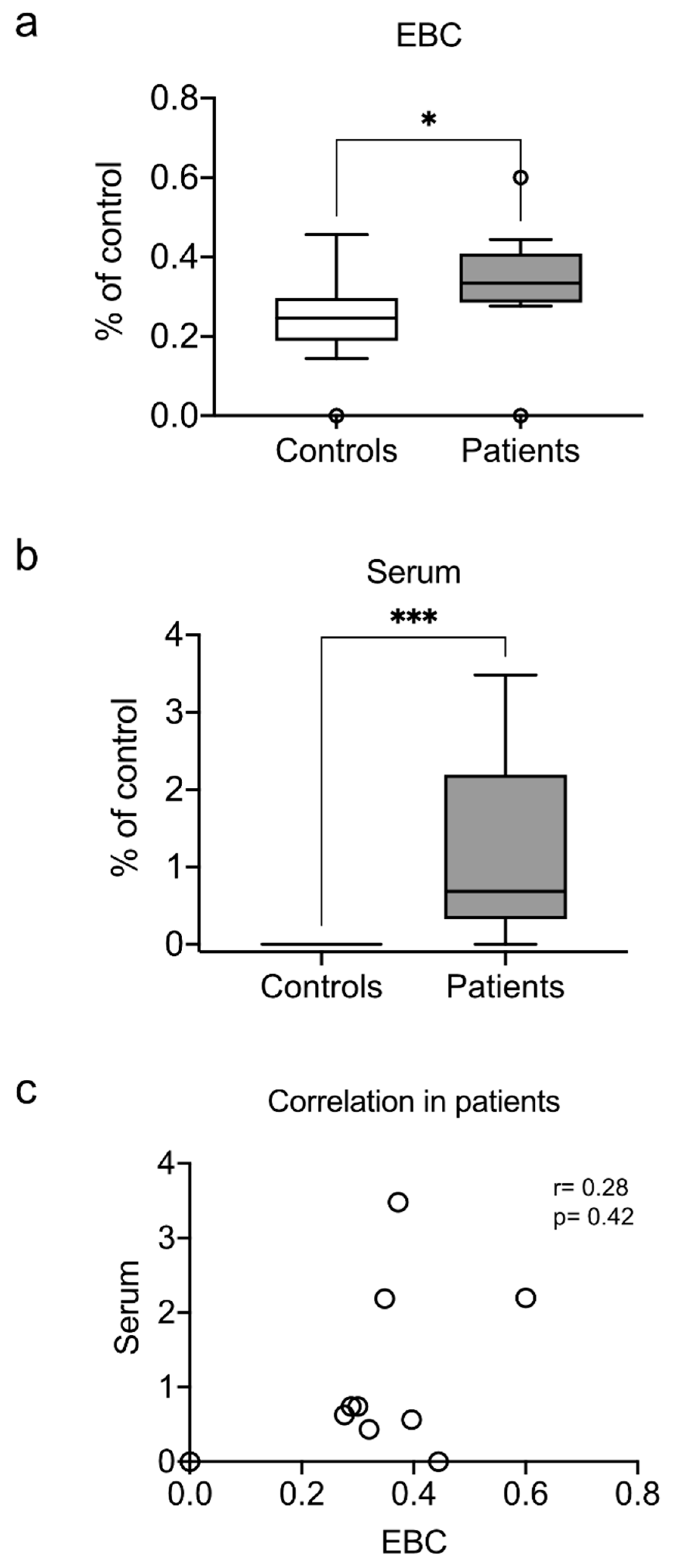
3.4. Exhaled and Systemic Nucleosomes
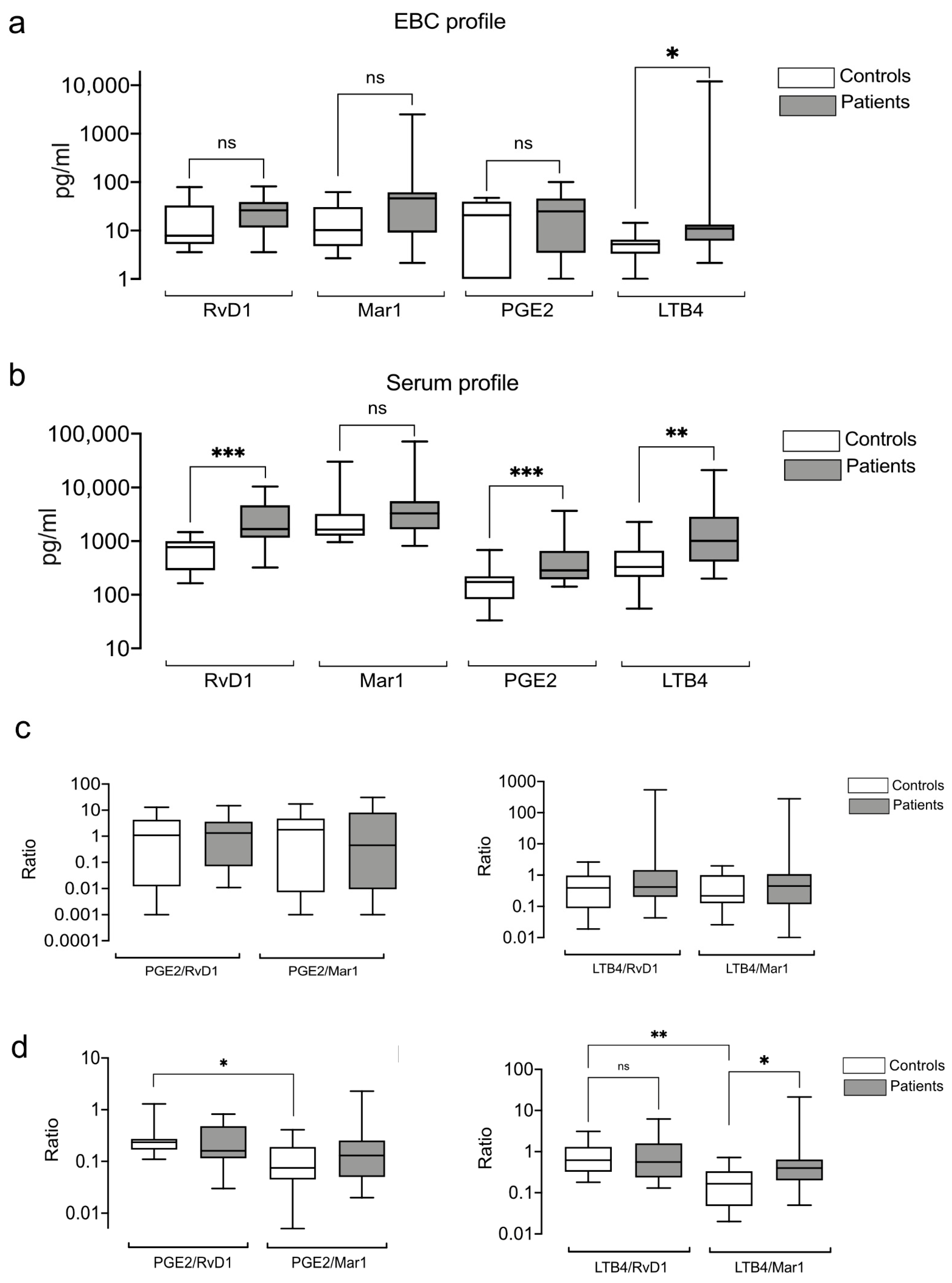
3.5. Exhaled and Systemic Eicosanoids
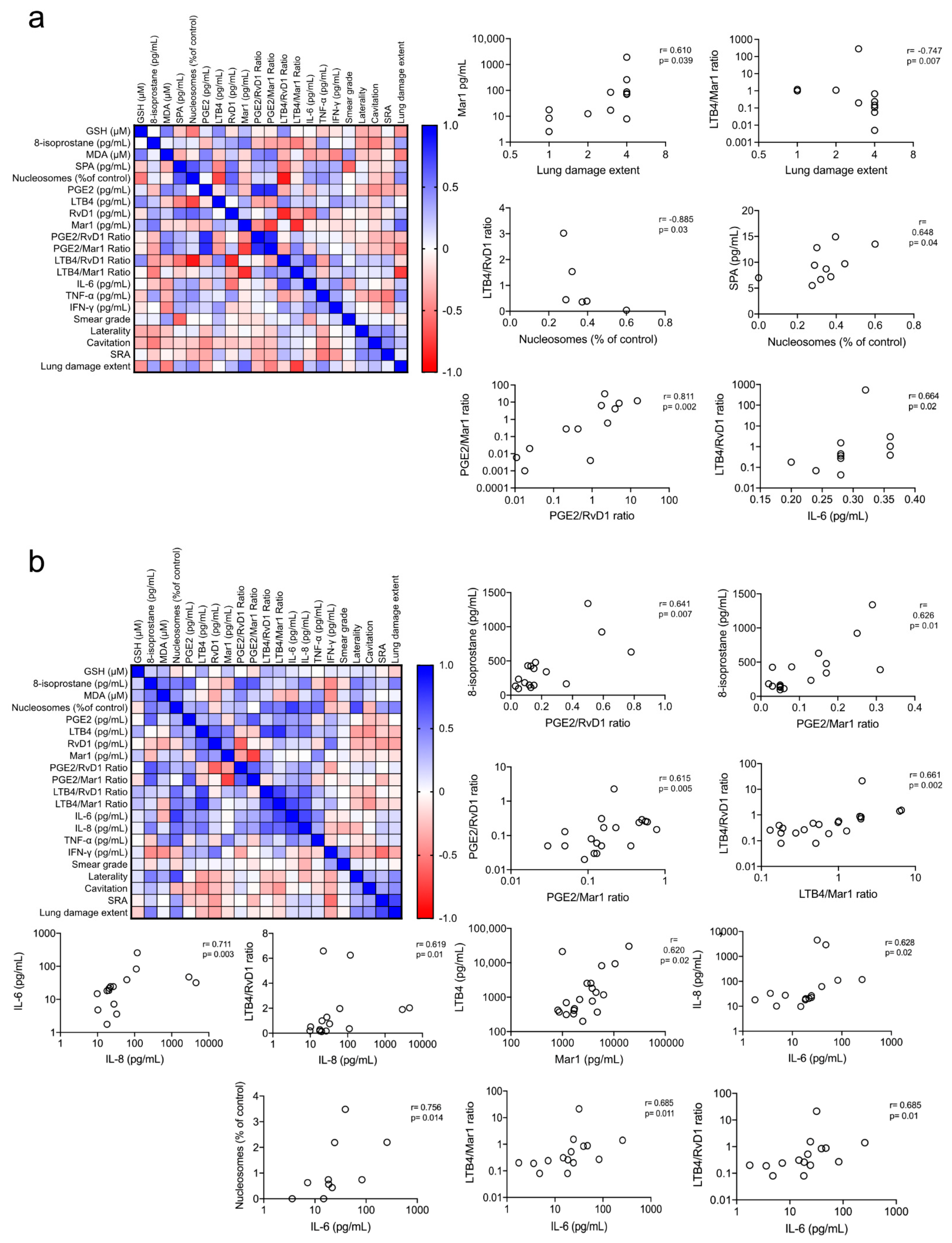
3.6. Redox and Pro-Inflammatory Markers Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ufimtseva, E.G.; Eremeeva, N.I.; Umpeleva, T.V.; Vakhrusheva, D.V.; Skornyakov, S.N. Mycobacterium tuberculosis load in host cells and the antibacterial activity of alveolar macrophages are linked and differentially regulated in various lung lesions of patients with pulmonary tuberculosis. Int. J. Mol. Sci. 2021, 22, 3452. [Google Scholar] [CrossRef] [PubMed]
- Belton, M.; Brilha, S.; Manavaki, R.; Mauri, F.; Nijran, K.; Hong, Y.T.; Patel, N.H.; Dembek, M.; Tezera, L.; Green, J.; et al. Hypoxia and tissue destruction in pulmonary TB. Thorax 2016, 71, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Kumar, N.P.; Wei, W.; et al. Host-directed therapy of tuberculosis based on interleukin-1 and type i interferon crosstalk. Nature 2014, 511, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Shivakoti, R.; Dalli, J.; Kadam, D.; Gaikwad, S.; Barthwal, M.; Colas, R.A.; Mazzacuva, F.; Lokhande, R.; Dharmshale, S.; Bharadwaj, R.; et al. Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes. Prostaglandins Other Lipid Mediat. 2020, 147, 106398. [Google Scholar] [CrossRef] [PubMed]
- Sandhaus, S.; Swick, A.G. Specialized proresolving mediators in infection and lung injury. BioFactors 2021, 47, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.I.A.; Jackson, M.J.; Hind, C.R.K. Circulating markers of free radical activity in patients with pulmonary tuberculosis. Tuberc. Respir. Dis. 1994, 75, 132–137. [Google Scholar] [CrossRef]
- Vidhya, R.; Rathnakumar, K.; Balu, V.; Pugalendi, K.V. Oxidative stress, antioxidant status and lipid profile in pulmonary tuberculosis patients before and after anti-tubercular therapy. Indian J. Tuberc. 2019, 66, 375–381. [Google Scholar] [CrossRef]
- Lyakh, L.; Trinchieri, G.; Provezza, L.; Carra, G.; Gerosa, F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 2008, 226, 112–131. [Google Scholar] [CrossRef]
- Wang, X.; Barnes, P.F.; Huang, F.; Alvarez, I.B.; Neuenschwander, P.F.; Sherman, D.R.; Samten, B. Early Secreted Antigenic Target of 6-kDa Protein of Mycobacterium tuberculosis Primes Dendritic Cells To Stimulate Th17 and Inhibit Th1 Immune Responses. J. Immunol. Res. 2012, 189, 3092–3103. [Google Scholar] [CrossRef] [Green Version]
- Joshi, L.; Ponnana, M.; Sivangala, R.; Chelluri, L.K.; Nallari, P.; Penmetsa, S.; Valluri, V.; Gaddam, S. Evaluation of TNF-α, il-10 and il-6 cytokine production and their correlation with genotype variants amongst tuberculosis patients and their household contacts. PLoS ONE 2015, 10, e0137727. [Google Scholar] [CrossRef] [Green Version]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Barber, K.D.D.; Sher, A. Cytokine and lipid mediator networks in tuberculosis. Immunol. Rev. 2015, 264, 264–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.P.; Moideen, K.; Banurekha, V.V.; Nair, D.; Babu, S. Plasma Proinflammatory Cytokines Are Markers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. Open Forum Infect. Dis. 2019, 6, ofz257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Adult pulmonary tuberculosis as a pathological manifestation of hyperactive antimycobacterial immune response. J. Transl. Med. 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nienaber, A.; Hayford, F.E.A.; Variava, E.; Martinson, N.; Malan, L. The Manipulation of the Lipid Mediator Metabolism as Adjunct Host-Directed Therapy in Tuberculosis. Front. Immunol. 2021, 12, 623941. [Google Scholar] [CrossRef] [PubMed]
- Bafica, A.; Scanga, C.A.; Serhan, C.; Machado, F.; White, S.; Sher, A.; Aliberti, J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J. Clin. Investig. 2005, 115, 1601–1606. [Google Scholar] [CrossRef]
- Kaushal, D. Eicosanoids, prostaglandins, and the progression of tuberculosis. J. Infect. Dis. 2012, 206, 1803–1805. [Google Scholar] [CrossRef] [Green Version]
- Moloney, E.D.; Mumby, S.E.; Gajdocsi, R.; Cranshaw, J.H.; Kharitonov, S.A.; Quinlan, G.J.; Griffiths, M.J. Exhaled Breath Condensate Detects Markers of Pulmonary Inflammation after Cardiothoracic Surgery. Am. J. Respir. Crit. Care Med. 2004, 169, 64–69. [Google Scholar] [CrossRef]
- Báez-saldaña, R.; López-arteaga, Y.; Bizarrón-muro, A.; Ferreira-guerrero, E.; Ferreyra-reyes, L.; Delgado-sánchez, G.; Cruz-hervert, L.P.; Mongua-, N. A Novel Scoring System to Measure Radiographic Abnormalities and Related Spirometric Values in Cured Pulmonary Tuberculosis. PLoS ONE 2013, 8, e78926. [Google Scholar] [CrossRef]
- Mutti, A.; Corradi, M.; Goldoni, M.; Vettori, M.V.; Bernard, A.; Apostoli, P. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest 2006, 129, 1288–1297. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Peel, A.M.; Crossman-Barnes, C.J.; Tang, J.; Fowler, S.J.; Davies, G.A.; Wilson, A.M.; Loke, Y.K. Biomarkers in adult asthma: A systematic review of 8-isoprostane in exhaled breath condensate. J. Breath Res. 2017, 11, 016011. [Google Scholar] [CrossRef] [Green Version]
- Zhong, D.; Wu, C.; Bai, J.; Zhao, J.; Xu, D.; Li, M.; Wang, Q.; Wang, F.; Zeng, X. Aberrant expression of cell-free nucleosomes in dermatomyositis/polymyositis. Dermatol. Ther. 2020, 33, e14460. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Roberts, L.J. The isoprostanes: Unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997, 36, 1–21. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twaddell, S.H.; Baines, K.J.; Grainge, C.; Gibson, P.G. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest 2019, 156, 774–782. [Google Scholar] [CrossRef]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Steinbacher, P.; Winterberg, N.; Bathke, C.A.; Klappacher, M.; Studnicka, M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schechter, M.C.; Buac, K.; Adekambi, T.; Cagle, S.; Celli, J.; Ray, S.M.; Mehta, C.C.; Rada, B.; Rengarajan, J. Neutrophil extracellular trap (NET) levels in human plasma are associated with active TB. PLoS ONE 2017, 12, e0182587. [Google Scholar] [CrossRef] [Green Version]
- Shastri, M.D.; Shukla, S.D.; Chong, W.C.; Dua, K.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Eri, R.; O’Toole, R.F. Role of oxidative stress in the pathology and management of human tuberculosis. Oxid. Med. Cell. Longev. 2018, 2018, 7695364. [Google Scholar] [CrossRef] [Green Version]
- Meca, A.D.; Turcu-Stiolica, A.; Stanciulescu, E.C.; Andrei, A.M.; Nitu, F.M.; Banita, I.M.; Matei, M.; Pisoschi, C.G. Variations of serum oxidative stress biomarkers under first-line antituberculosis treatment: A pilot study. J. Pers. Med. 2021, 11, 112. [Google Scholar] [CrossRef]
- Venketaraman, V.; Millman, A.; Salman, M.; Swaminathan, S.; Goetz, M.; Lardizabal, A.; Hom, D.; Connell, N.D. Glutathione levels and immune responses in tuberculosis patients. Microb. Pathog. 2008, 44, 255–261. [Google Scholar] [CrossRef]
- Suresh, D.R.; Annam, V. Lipid peroxidation and total antioxidant capacity in health and disease—Pathophysiology and markers: An overview. Int. J. Public Health 2013, 2, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Zhang, W.; Zhu, M.; Xu, J.; Deng, G.; Wang, D. Assessment of ventilator-associated pneumonia by combining 8-isoprostane and nitric oxide levels in exhaled breath condensate with the clinical pulmonary infection score. Int. J. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun. 2000, 68, 3322–3326. [Google Scholar] [CrossRef] [Green Version]
- Dlugovitzky, D.; Rateni, L.; Torres-Morales, A.; Ruiz-Silva, J.; Piñesky, R.; Canosa, B.; Molteni, O.; Bottasso, O. Levels of interleukin-8 in tuberculous pleurisy and the profile of immunocompetent cells in pleural and peripheral compartments. Immunol. Lett. 1997, 55, 35–39. [Google Scholar] [CrossRef]
- Sadek, M.I.; Sada, E.; Toossi, Z.; Schwander, S.K.; Rich, E.A. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 1998, 19, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Friedland, J.S.; Hartley, J.C.; Hartley, C.G.C.; Shattock, R.J.; Griffin, G.E. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin. Exp. Immunol. 1995, 100, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hua, H.; Zhou, L.; Zou, X. Association between interleukin-8 −251A/T polymorphism and the risk of tuberculosis: A meta-analysis. Int. J. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Law, K.; Weiden, M.; Harkin, T.; Tchou-Wong, K.; Chi, C.; Rom, W.N. Increased release of interleukin-1β, interleukin-6, and tumor necrosis factor-α by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 1996, 153, 799–804. [Google Scholar] [CrossRef]
- Dorhoi, A.; Kaufmann, S.H.E. Tumor Necrosis Factor Alpha in Mycobacterial Infection; Academic Press: Cambridge, MA, USA, 2014; Volume 26, pp. 203–209. [Google Scholar]
- Ong, C.W.M.; Elkington, P.T.; Brilha, S.; Ugarte-Gil, C.; Tome-Esteban, M.T.; Tezera, L.B.; Pabisiak, P.J.; Moores, R.C.; Sathyamoorthy, T.; Patel, V.; et al. Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis. PLoS Pathog. 2015, 11, e1004917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford-Hutchinson, A.W.; Bray, M.A.; Doig, M.V.; Shipley, M.E.; Smith, M.J.H. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980, 286, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Nore, K.G.; Jørgensen, M.J.; Dyrhol-Riise, A.M.; Jenum, S.; Tonby, K. Elevated Levels of Anti-Inflammatory Eicosanoids and Monocyte Heterogeneity in Mycobacterium tuberculosis Infection and Disease. Front. Immunol. 2020, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Corhay, J.L.; Moermans, C.; Henket, M.; Dang, D.N.; Duysinx, B.; Louis, R. Increased of exhaled breath condensate neutrophil chemotaxis in acute exacerbation of COPD. Respir. Res. 2014, 15, 115. [Google Scholar] [CrossRef] [Green Version]
| TB Patients (N = 20) Total (%) or Median (Range) | Healthy Controls (N = 12) Total (%) or Median (Range) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age, year | 47.5 (18–64) | 50 (31–62) | 0.751 |
| Sex, male (%) | 13 (65) | 8 (66.6) | 0.999 |
| BMI | 21.8 (12.7–39.7) | 23.8 (21.3–27.5) | 0.078 |
| Comorbidities | |||
| Diabetes (%) | 13 (65) | 0 (0) | 0.999 |
| Hypertension (%) | 2 (10) | 0 (0) | 0.999 |
| Smoking (%) | 2 (10) | 1 (8.3) | 0.999 |
| Tuberculosis | |||
| Unilateral | 6 (30) | - | |
| Bilateral | 14 (70) | - | |
| Smear grade | |||
| 0+ | 4 (20) | - | |
| 1+ | 10 (50) | - | |
| 2+ | 1 (5) | - | |
| 3+ | 5 (25) | - | |
| Score of radiographical abnormalities * | 10 (2–18) | - | |
| Leukocytes | |||
| WBC count, ×109/L | 8.6 (4.1–15.2) | 6.6 (3.6–8) | <0.001 |
| Neutrophil count, ×109/L | 6.3 (2.2–13) | 3.8 (1.2–4.3) | <0.001 |
| Lymphocyte count, ×109/L | 1.1 (0.5–2.4) | 2.1 (1.6–2.8) | <0.001 |
| Monocyte count, ×109/L | 0.8 (0.1–1.5) | 0.5 (0.3–0.7) | <0.001 |
| Platelet count, ×109/L | 335 (13.3–662) | 252 (205–373) | 0.122 |
| TB Patients (N = 20) | Healthy Controls (N = 12) | p Value | |
|---|---|---|---|
| Fasting, h | 12 (2–14) | 12 (10–16) | 0.540 |
| Total volume, L/10 min | 1050 (260–1600) | 1350 (480–2300) | 0.041 |
| Room temperature, °C | 22.7 (19.5–29.9) | 22.5 (21.3–22.8) | 0.263 |
| Humidity, % | 43 (20–73) | 43.5 (35–50) | 0.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Beltrán, S.; Carreto-Binaghi, L.E.; Carranza, C.; Torres, M.; Gonzalez, Y.; Muñoz-Torrico, M.; Juárez, E. Oxidative Stress and Inflammatory Mediators in Exhaled Breath Condensate of Patients with Pulmonary Tuberculosis. A Pilot Study with a Biomarker Perspective. Antioxidants 2021, 10, 1572. https://doi.org/10.3390/antiox10101572
Guzmán-Beltrán S, Carreto-Binaghi LE, Carranza C, Torres M, Gonzalez Y, Muñoz-Torrico M, Juárez E. Oxidative Stress and Inflammatory Mediators in Exhaled Breath Condensate of Patients with Pulmonary Tuberculosis. A Pilot Study with a Biomarker Perspective. Antioxidants. 2021; 10(10):1572. https://doi.org/10.3390/antiox10101572
Chicago/Turabian StyleGuzmán-Beltrán, Silvia, Laura Elena Carreto-Binaghi, Claudia Carranza, Martha Torres, Yolanda Gonzalez, Marcela Muñoz-Torrico, and Esmeralda Juárez. 2021. "Oxidative Stress and Inflammatory Mediators in Exhaled Breath Condensate of Patients with Pulmonary Tuberculosis. A Pilot Study with a Biomarker Perspective" Antioxidants 10, no. 10: 1572. https://doi.org/10.3390/antiox10101572







