Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Sample Treatment
2.3. UHPLC-PDA-ESI-OT-MS Instrument
2.4. Antioxidant Activity Assays
2.4.1. DPPH Test
2.4.2. ABTS Method
2.5. Polyphenol (Folin-Ciocalteau)
2.6. Determination of Proximal Composition
2.7. Mineral Analysis
2.8. Total Carotene Content
2.9. Induction of Hypercholesterolemia
2.10. Statistical Analysis
3. Results and Discussion
3.1. Nutritional and Physicochemical Properties of 5 Cocona Ecotypes
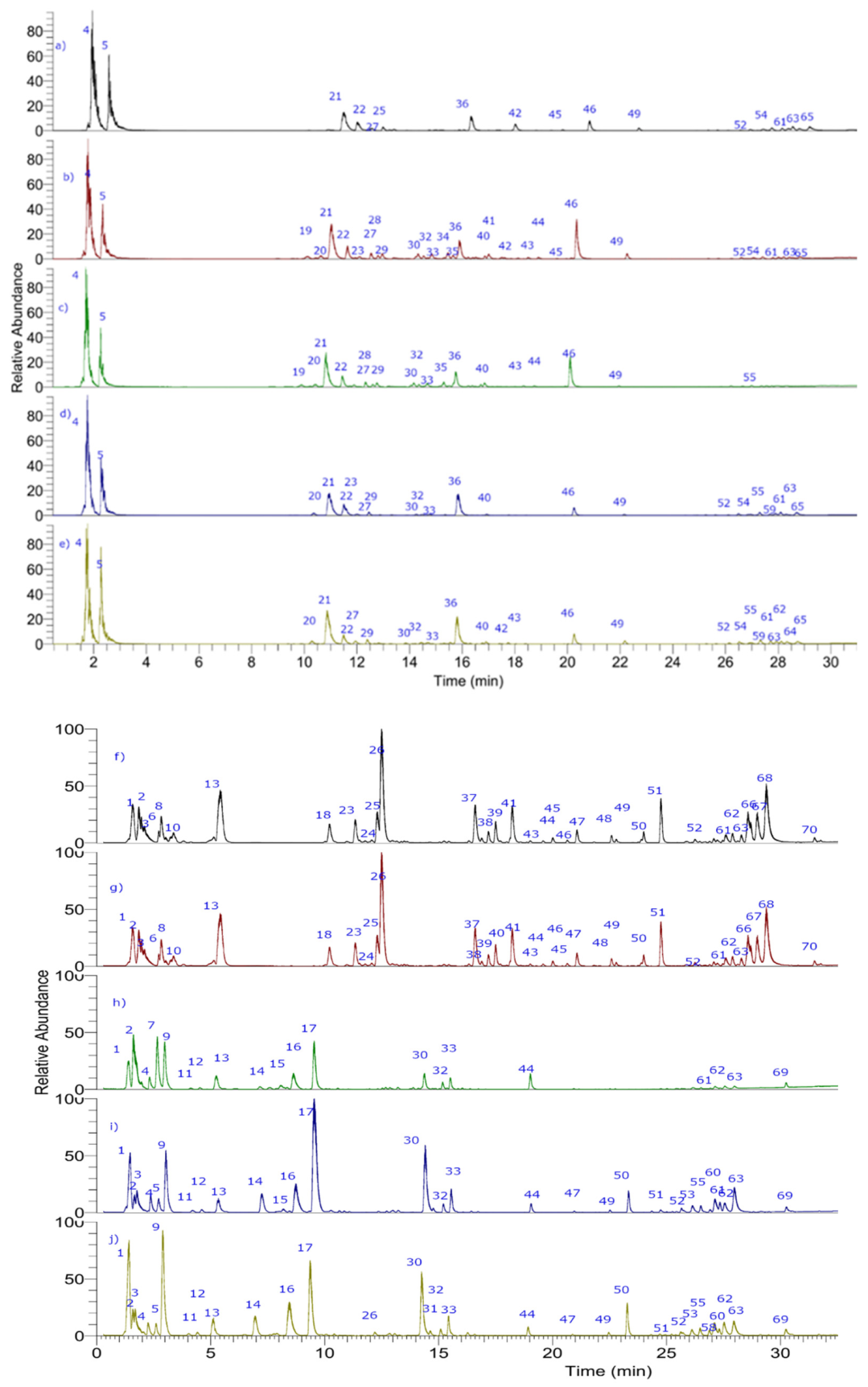
3.2. Metabolite Profiling using UHPLC-PDA-ESI-OT-MS
3.2.1. Spermidines
3.2.2. Amines or Aminoacids
3.2.3. Fatty Acids and Derivatives
3.2.4. Phenolic Acids
3.2.5. Flavonoids
3.2.6. Terpenes
3.2.7. Cyanidins
3.2.8. Citric Acid
3.2.9. Carotenoids
3.3. Antioxidant Activity, Total Carotenes and Total Polyphenols Content
3.4. Antihyperlipidemic Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J. Agric. Food Chem. 2013, 61, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- Mascato, D.R.D.L.H.; Monteiro, J.B.; Passarinho, M.M.; Galeno, D.M.L.; Cruz, R.J.; Ortiz, C.; Morales, L.; Lima, E.S.; Carvalho, R.P. Evaluation of Antioxidant Capacity of Solanum sessiliflorum (Cubiu) Extract: An in Vitro Assay. J. Nutr. Metab. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Muñoz, D.P.; Cardoso da Silva, L.; Neves de Oliveira, L.A.; Teixeira Godoy, H.; Kurozawa, L.E. 5-caffeoylquinic acid retention in spray drying of cocona, an Amazonian fruit, using hydrolyzed collagen and maltodextrin as encapsulating agents. Dry. Technol. 2020, 39, 1854–1868. [Google Scholar] [CrossRef]
- Faria, J.V.; Valido, I.H.; Paz, W.H.P.; da Silva, F.M.A.; de Souza, A.D.L.; Acho, L.R.D.; Lima, E.S.; Boleti, A.P.A.; Marinho, J.V.N.; Salvador, M.J.; et al. Comparative evaluation of chemical composition and biological activities of tropical fruits consumed in Manaus, central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836. [Google Scholar] [CrossRef]
- Dos Santos Montagner, G.F.F.; Barbisan, F.; Ledur, P.C.; Bolignon, A.; De Rosso Motta, J.; Ribeiro, E.E.; De Souza Praia, R.; Azzolin, V.F.; Cadoná, F.C.; Machado, A.K.; et al. In Vitro Biological Properties of Solanum sessiliflorum (Dunal), an Amazonian Fruit. J. Med. Food 2020, 23, 978–987. [Google Scholar] [CrossRef]
- Barrientos, R.; Fernández-Galleguillos, C.; Pastene, E.; Simirgiotis, M.; Romero-Parra, J.; Ahmed, S.; Echeverría, J. Metabolomic Analysis, Fast Isolation of Phenolic Compounds, and Evaluation of Biological Activities of the Bark from Weinmannia trichosperma Cav. (Cunoniaceae). Front. Pharmacol. 2020, 11, 780. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Manrique, S.; Piñeiro, M.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. Uhplc-esi-ot-ms phenolics profiling, free radical scavenging, antibacterial and nematicidal activities of “yellow-brown resins” from larrea spp. Antioxidants 2021, 10, 185. [Google Scholar] [CrossRef]
- Jiménez-González, A.; Quispe, C.; Bórquez, J.; Sepúlveda, B.; Riveros, F.; Areche, C.; Nagles, E.; García-Beltrán, O.; Simirgiotis, M.J. UHPLC-ESI-ORBITRAP-MS analysis of the native Mapuche medicinal plant palo negro (Leptocarpha rivularis DC.—Asteraceae) and evaluation of its antioxidant and cholinesterase inhibitory properties. J. Enzyme Inhib. Med. Chem. 2018, 33, 936–944. [Google Scholar] [CrossRef]
- Cobos, M.; Pérez, S.; Braga, J.; Vargas-Arana, G.; Flores, L.; Paredes, J.D.; Maddox, J.D.; Marapara, J.L.; Castro, J.C. Nutritional evaluation and human health-promoting potential of compounds biosynthesized by native microalgae from the Peruvian Amazon. World J. Microbiol. Biotechnol. 2020, 36, 1–14. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International—18th Edition, Revision 3. Available online: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-18th-edition-revision-3?product_id=1678986 (accessed on 12 July 2021).
- Asaolu, M.F.; Asaolu, S.S. Proximate and mineral compositions of cooked and uncooked Solanum melongena. Int. J. Food Sci. Nutr. 2002, 53, 103–107. [Google Scholar] [CrossRef]
- Barreto, G.P.M.; Benassi, M.T.; Mercadante, A.Z. Bioactive compounds from several tropical fruits and correlation by multivariate analysis to free radical scavenger activity. J. Braz. Chem. Soc. 2009, 20, 1856–1861. [Google Scholar] [CrossRef]
- Venkadeswaran, K.; Muralidharan, A.R.; Annadurai, T.; Ruban, V.V.; Sundararajan, M.; Anandhi, R.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and antioxidative potential of an extract of the plant, piper betle, and its active constituent, eugenol, in triton WR-1339-Induced hypercholesterolemia in experimental rats. Evid. -Based Complement. Altern. Med. 2014, 2014, 478973. [Google Scholar] [CrossRef] [Green Version]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzyme Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Narváez-Cuenca, C.E.; Vincken, J.P.; Zheng, C.; Gruppen, H. Diversity of (dihydro) hydroxycinnamic acid conjugates in Colombian potato tubers. Food Chem. 2013, 139, 1087–1097. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef]
- Guerrero-Castillo, P.; Reyes, S.; Robles, J.; Simirgiotis, M.J.; Sepulveda, B.; Fernandez-Burgos, R.; Areche, C. Biological activity and chemical characterization of Pouteria lucuma seeds: A possible use of an agricultural waste. Waste Manag. 2019, 88, 319–327. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Do Amaral, B.S.; da Silva, L.R.G.; Valverde, A.L.; de Sousa, L.R.F.; Severino, R.P.; de Souza, D.H.F.; Cass, Q.B. Phosphoenolpyruvate carboxykinase from T. cruzi magnetic beads affinity-based screening assays on crude plant extracts from Brazilian Cerrado. J. Pharm. Biomed. Anal. 2021, 193, 113710. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Angolini, C.F.F.; Adani, H.B.; Usberti, F.C.S.; Paulino, B.N.; Clerici, M.T.P.S.; Neri-numa, I.A.; Moro, T.d.M.A.; Eberlin, M.N.; Pastore, G.M. Impact of ripening on the health-promoting components from fruta-do-lobo (Solanum lycocarpum St. Hill). Food Res. Int. 2021, 139, 109910. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Areche, C.; Sepúlveda, B. Phenolic compounds in chilean mistletoe (quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties. Molecules 2016, 21, 245. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, R.E.; Ahmed, S.; Cortés, C.; Fernández-Galleguillos, C.; Romero-Parra, J.; Simirgiotis, M.J.; Echeverría, J. Chemical Fingerprinting and Biological Evaluation of the Endemic Chilean Fruit Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) by UHPLC-PDA-Orbitrap-Mass Spectrometry. Molecules 2020, 25, 3750. [Google Scholar] [CrossRef]
- Yin, J.; Heo, J.H.; Hwang, Y.J.; Le, T.T.; Lee, M.W. Inhibitory activities of phenolic compounds isolated from adina rubella leaves against 5α-reductase associated with benign prostatic hypertrophy. Molecules 2016, 21, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- Nishibe, S. Bioactive Phenolic Compounds for Cancer Prevention from Herbal Medicines. Food Factors Cancer Prev. 1997, 276–279. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Huang, J.-C.; Kuo, P.-L.; Chen, C.-Y. Bioactive Constituents from Michelia champaca. Nat. Prod. Commun. 2011, 6, 1251–1252. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Iwashina, T.; Kitajima, J.; Shiuchi, T.; Itou, Y. Chalcones and other flavonoids from Asarum sensu lato (Aristolochiaceae). Biochem. Syst. Ecol. 2005, 33, 571–584. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. Molecules HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Khan, A.; Bresnick, A.; Cahill, S.; Girvin, M.; Almo, S.; Quinn, R. Advantages of Molecular Weight Identification during Native MS Screening. Planta Med. 2018, 84, 1201–1212. [Google Scholar] [CrossRef]
- Mubashir, N.; Fatima, R.; Naeem, S. Identification of Novel Phyto-chemicals from Ocimum basilicum for the Treatment of Parkinson’s Disease using In Silico Approach. Curr. Comput. Aided. Drug Des. 2020, 16, 420–434. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.J.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Quercetin 3-O-malonylglucoside in the leaves of mulberry (Morus alba) is a functional analog of ghrelin. J. Food Biochem. 2020, 44, e13379. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure–antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Patra, J.K.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.J.; Koyanagi, J.; Ohmori, K.; Uesato, S.; Tsuchido, T.; Saito, S. Preparations of heterospirostanols and their pharmacological activities. Eur. J. Med. Chem. 2002, 37, 659–669. [Google Scholar] [CrossRef]
- Kołota, A.; Głabska, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. Analysis of association between intake of red wine polyphenols and oxidative stress parameters in the liver of growing male rats. Appl. Sci. 2020, 10, 6389. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of BV-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152. [Google Scholar] [CrossRef]
- Harnafi, H.; Bouanani, N.e.H.; Aziz, M.; Serghini Caid, H.; Ghalim, N.; Amrani, S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: A comparison with fenofibrate. J. Ethnopharmacol. 2007, 109, 156–160. [Google Scholar] [CrossRef]
- Khanna, A.K.; Rizvi, F.; Chander, R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J. Ethnopharmacol. 2002, 82, 19–22. [Google Scholar] [CrossRef]
- Sundaram, R.; Ayyakkannu, P.; Muthu, K.; parveen Nazar, S.; Palanivelu, S.; Panchanatham, S. Acyclic Isoprenoid Attenuates Lipid Anomalies and Inflammatory Changes in Hypercholesterolemic Rats. Indian J. Clin. Biochem. 2019, 34, 395–406. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant Foods Hum. Nutr. 2007, 62, 19–24. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef]
- Jahromi, M.A.F.; Ray, A.B.; Chansouria, J.P.N. Antihyperlipidemic effect of flavonoids from pterocarpus marsupium. J. Nat. Prod. 1993, 56, 989–994. [Google Scholar] [CrossRef]
- Shabana, M.M.; El-Sherei, M.M.; Moussa, M.Y.; Sleem, A.A.; Abdallah, H.M. Flavonoid constituents of Carduncellus mareoticus (Del.) Hanelt and their biological activities. Nat. Prod. Commun. 2008, 3, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Tacherfiout, M.; Petrov, P.D.; Mattonai, M.; Ribechini, E.; Ribot, J.; Bonet, M.L.; Khettal, B. Antihyperlipidemic effect of a Rhamnus alaternus leaf extract in Triton-induced hyperlipidemic rats and human HepG2 cells. Biomed. Pharmacother. 2018, 101, 501–509. [Google Scholar] [CrossRef]
- Maia, J.R.P.; Schwertz, M.C.; Sousa, R.F.S.; Aguiar, J.P.L.; Lima, E.S. Efeito hipolipemiante da suplementação dietética com a farinha do cubiu (solanum sessiliforum dunal) em ratos hipercolesterolêmicos. Rev. Bras. Plantas Med. 2015, 17, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Pardo, M.A. Efecto de Solanum sessiliflorum dunal sobre el metabolismo lipídico y de la glucosa. Cienc. Investig. 2004, 7, 43–48. [Google Scholar] [CrossRef]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008, 67, 42–47. [Google Scholar] [CrossRef]

| Ecotypes | Humidity | Ashes | Total Lipids | Crude protein | Crude fiber | Carbohydrates |
|---|---|---|---|---|---|---|
| NMA1 | 91.85 ± 0.09 a | 0.75 ± 0.01 a | 0.65 ± 0.00 a | 1.08 ± 0.04 a | 1.68 ± 0.04 a | 3.99 |
| CD1 | 86.64 ± 0.36 b | 1.24 ± 0.06 b | 0.88 ± 0.00 b | 1.93 ± 0.05 b | 5.03 ± 0.15 b | 4.28 |
| CTR | 92.82 ± 0.03 c | 0.71 ± 0.03 a | 0.45 ± 0.01 c | 1.09 ± 0.04 a | 1.03 ± 0.05 c | 3.9 |
| SRN9 | 86.67 ± 0.12 b | 0.94 ± 0.02 c | 0.93 ± 0.01 d | 2.72 ± 0.04 c | 4.76 ± 0.17 b | 3.98 |
| UNT2 | 93.52 ± 0.08 d | 0.79 ± 0.02 a | 0.19 ± 0.00 e | 1.64 ± 0.06 d | 0.76 ± 0.03 c | 3.1 |
| Ecotypes | Fe | Zn | Mn | Cu | Mg | K | Na | Ca |
|---|---|---|---|---|---|---|---|---|
| NMA1 | 71.17 ± 2.69 a | 26.33 ± 0.95 a | 8.18 ± 0.30 a | 12.27 ± 0.52 a | 42.54 ± 0.93 a | 846.47 ± 19.85 a | 4.09 ± 0.13 a | 28.63 ± 0.86 a |
| CD1 | 70.07 ± 2.65 a | 67.05 ± 1.56 b | 34.35 ± 1.16 b | 41.22 ± 0.73 b | 164.88 ± 6.55 b | 2382.24 ± 29.95 b | 6.87 ± 0.13 b | 70.07 ± 1.62 b |
| CTR | 40.70 ± 1.82 b | 17.83 ± 0.84 c | 7.85 ± 0.15 ac | 11.42 ± 0.42 ac | 38.56 ± 0.69 ac | 638.10 ± 6.28 c | 3.57 ± 0.09 c | 17.85 ± 0.46 c |
| SRN9 | 58.09 ± 1.15 c | 45.93 ± 2.19 d | 9.46 ± 0.08 d | 14.86 ± 0.65 d | 91.87 ± 1.54 d | 2004.88 ± 33.34 d | 6.76 ± 0.18 b | 58.09 ± 1.05 d |
| UNT2 | 52.02 ± 1.17 d | 18.85 ± 0.90 c | 8.45 ± 0.21 acd | 10.40 ± 0.30 c | 41.60 ± 0.84 ac | 570.83 ± 12.13 e | 3.25 ± 0.04 c | 41.60 ± 0.72 e |
| Peak# | Retention Time (min.) | UV Max | Tentative Identification | Molecular Formula | Theoretical Mass (m/z) | Measured Mass [M-H]− or [M+H]+ (m/z) | Accuracy (ppm) | MSn Ions | Ecotype |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.25 | - | Spermine | C10H26N4 | 203.22302 | 203.2229 | −2.448 | 129.1385, 112.1122, 84.0812, 73.0813 | a–e |
| 2 | 1.33 | - | Spermidine | C7H19N3 | 146.16517 | 146.1651 | −1.96 | 129.1385, 112.1122, 84.0812, 73.0813 | a–e |
| 3 | 1.35 | - | Histamine | C5H9N3 | 112.08692 | 112.0872 | 0.79 | 95.0606, 83.0608, 68.0500, 55.0549 | a–e |
| 4 | 1.69 | - | Citric acid | C6H8O7 | 191.01944 | 191.01863 | 4.24 | 129.1385 | c–e |
| 5 | 1.75 | - | Isocitric acid | C6H8O7 | 191.01944 | 191.01947 | 3.25 | 111.00794 | d–e |
| 6 | 1.97 | - | Asparagine | C4H8N2O3 | 131.0449 | 131.0454 | 3.81 | 114.0187, 113.0337, 95.0251, 88.0394 70.0288 | a–b |
| 7 | 2.29 | - | Arginine | C6H14N4O2 | 175.1181 | 175.1188 | 3.99 | 158.0920, 140.0702, 130.0972, 116.0706 97.0661 70.0656 | c |
| 8 | 2.43 | - | Pyroglutamic acid | C5H7NO3 | 130.0493 | 130.0499 | 4.61 | 102.0251, 84.0448, 56.0551, | a–b |
| 9 | 3.01 | - | Nicotinamide | C6H6N2O | 123.0550 | 123.0553 | 2.43 | 106.0289, 96.0446, 80.0499, 53.0391 | c–e |
| 10 | 3.15 | - | N-phenyl ethyl amide | C8H10N | 120.08810 | 120.08080 | 2.42 | 85.02876 | a–b |
| 11 | 4.1 | - | N-Fructosyl isoleucine | C12H23NO7 | 294.1541 | 294.1544 | 1.01 | 276.1436, 258.1332, 230.1383, 212.1278 161.0681, 144.1017, 132.1017, 86.0968 | c–e |
| 12 | 4.32 | - | Norleucine | C6H13NO2 | 132.1016 | 132.1019 | 2.27 | 105.0696, 86.0968, 69.0704 | c–e |
| 13 | 5.21 | - | Tyrosine | C9H11NO3 | 188.0210 | 188.0211 | 0.53 | 165.0554, 147.0438, 136.0755, 123.0441 105.0337 | a–e |
| 14 | 7.03 | - | Adenosine | C10H13N5O4 | 268.1037 | 268.1039 | 0.74 | 178.0730, 136.0616, 57.0341 | c–e |
| 15 | 8.12 | - | Phenylacetaldehyde | C8H8O | 121.0642 | 121.0649 | 5.48 | 103.0544, 93.0702, 91.0546, 77.0387 53.0392 | c–e |
| 16 | 8.53 | - | Guanosine | C10H13N5O5 | 284.0983 | 284.0988 | 1.75 | 152.0564, 133.0494, 121.0647, 95.0609 | c–e |
| 17 | 9.62 | - | Phenylalanine | C9H11NO2 | 166.0859 | 166.0861 | 1.20 | 149.0594, 131.0491, 120.0808, 103.0543 93.0703 | c–e |
| 18 | 10.23 | - | Aminobutyl benzamide | C11H16N2O | 193.1332 | 193.1336 | 2.07 | 176.1067, 134.0599, 105.0337, 72.0813 | a–b |
| 19 | 10.34 | 290–335 | Chlorogenic acid | C8H14O4− | 353.0863 | 353.0882 | 4.21 | 191.05574, 707.18678 | b–c |
| 20 | 10.5 | 208 | Quinic acid | C7H11O6− | 191.0550 | 191.0557 | 2.34 | 135.04477, 85.02844 | b–e |
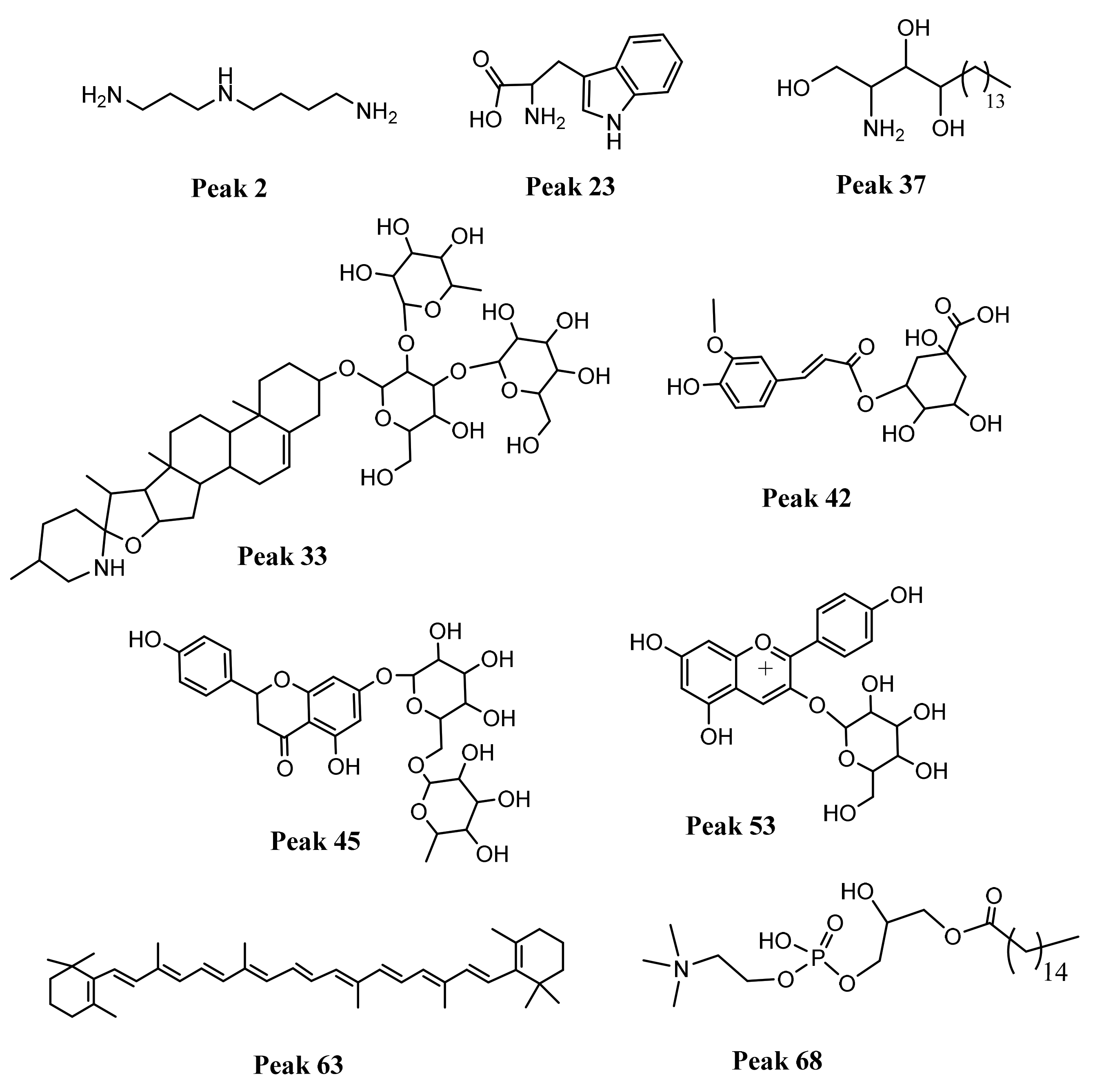
| 21 | 10.88 | 235 | 3-O-diglucosyl-4-methoxy-3-hydroxybenzoic acid | C20H27O14− | 491.1395 | 491.1412 | 3.46 | a–d | |
| 22 | 11.25 | - | Pantothenic acid | C9H17NO5 | 220.1173 | 220.1179 | 2.72 | 202.1069, 184.0965, 160.0965, 142.0860 124.0756 | a–d |
| 23 | 11.43 | - | Tryptophan | C11H12N2O2 | 205.0961 | 205.0968 | 3.41 | 188.0701, 170.0596, 159.0914, 146.0597 132.0804 118.0650 | a–b |
| 24 | 11.75 | - | Tryptophol | C10H11NO | 144.0802 [M- H2O+H] | 144.0807 | 3.47 | 128.0491, 117.0699, 103.0506, 91.0547 | a–b |
| 25 | 11.97 | 330 | N-Caffeoyl-N-(dihydrocaffeoyl)spermidine | C25H33N3O6 | 472.2439 | 472.2441 | 0.42 | 455.2163, 310.2118, 293.1852, 222.1120 163.0386, 72.0813 | a |
| 26 | 12.03 | 330 | N-Caffeoyl-N-(dihydrocaffeoyl)spermidine | C26H37N3O6 | 488.2751 | 488.2756 | 1.02 | 471.2478, 324.2273, 293.1844, 236.1275 222.1119, 165.0542 | a–b |
| 27 | 12.02 | 325 | 3-O-Diglucosyl-4-methoxy-3-hydroxybenzoic acid | C20H27O14− | 491.13953 | 491.14124 | 3.46 | a–d | |
| 28 | 12.24 | 254–354 | Rutin | C27H30O16 | 609.14702 | 609.14709 | 0.11 | 463.0920, 343.0465, 301.0254, 300.0280, 271.0252 178.9982, 151.0031 | b–c |
| 29 | 12.46 | 240 | Apiosyl-(1→6)-glucosyl 4-hydroxybenzoate | C18H24O12 | 431.0980 | 431.0983 | 0.46 | 431.1196, 299.0768, 281.0679, 137.0237 93.0336 | b–d |
| 30 | 14.03 | 280 | Naringenin-5,7-di-O-D-glucopyranoside | C27H31O15− | 595.16575 | 595.16772 | 3.32 | 271.06152, 153.01845, 147.04482, 119.05661 | c–e |
| 31 | 14.21 | 280 | Genistein 5-O-glucoside | C21H20O10 | 431.0980 | 431.0984 433.1123 | 0.46 | 414.3355, 271.0595, 269.0390, 253.0485, 215.0698 146.0598, 127.0389, 85.0288 | d–e |
| 32 | 15.02 | 254–354 | Isoquercitrin | C21H20O12 | 463.1022 | 463.1027 | 1.08 | 300.0280, 271.0251, 255.0301, 178.9982 151.0032 | c–e |
| 33 | 15.21 | - | Spirosol-5-en-3-ol, 3-O-[Rhamnosyl-(1→2)- glucosyl-(1→3)]-galactoside | C45H73NO16 | 884.4982 | 884.4987 928.4909 [M+FA-H] | 0.56 | 722.4060, 576.3906, 414.3356 | b–e |
| 34 | 15.24 | 329 | 1-O-Sinapoyl-glucoside | C17H22O10 | 385.1142 | 385.1147 | 1.29 | 247.0612, 223.0611, 205.0504, 190.0269 164.0704, 119.0342, | b |
| 35 | 15.36 | 280 | Protocatechuic acid 5-O-[apiofuranosyl-(1→6)-glucopyranoside] | C19H26O13 | 461.1301 | 461.1302 | 0.21 | 329.0872, 167.0344, 152.0108, 123.0443 108.0208 | b–c |
| 36 | 15.57 | 325 | 4-O-(3′-O-Glucopyranosyl)-caffeoyl quinic acid | C22H28O14 | 515.1401 | 515.1407 | 1.16 | 395.0990, 353.0876, 191.0557, 179.0344 161.0238, 135.0444 | a–e |
| 37 | 15.87 | - | Phytosphingosine | C18H39NO3 | 318.2990 | 318.2995 | 1.57 | 300.2890, 282.2785, 270.2785, 60.0450 | a, b, d, e |
| 38 | 16.23 | 280 | N,N″-Bis[3-(4-hydroxy-3-methoxyphenyl)propanoyl] spermidine | C27H39N3O6 | 502.2902 | 502.2907 | 0.99 | 485.2633, 307.1996, 236.1275, 179.0698 137.0594 | a–b |
| 39 | 16.73 | 325 | N,N,N-tris(dihydrocaffeoyl) spermidine | C34H43O9N3 | 638.3059 | 638.3062 | 0.46 | 474.2588, 456.2484, 293.1852, 222.1120 165.0543, 123.0439 | a–b |
| 40 | 17.23 | - | Spirosol-5-en-3-ol, O-[Rhamnosyl-(1→2)-[xylosyl- (1→2)-rhamnosyl-(1→4)]-galactoside | C50H81NO19 | 1000.5450 | 1000.5456 | 0.59 | 868.4970, 722.4737, 576.3879, 414.3358 | a–b |
| 41 | 17.55 | - | Cholest-5-ene-3,16,22,26-tetrol, 3-O-[Rhamnosyl- (1→4)-[rhamnosyl-(1→2)]glucoside], 26-O- glucoside | C51H86O22 | 1051.5660 | 1051.5665 | 0.47 | 1049.5541, 903.4961, 757.4382, 595.3851 433.3324 | a–b |
| 42 | 17.67 | 330 | 3-O-Feruloylquinic acid | C17H20O9 | 367.1032 | 367.1039 | 1.90 | 191.0558, 173.0451, 134.0366, 111.0443 93.0336, | a–b |
| 43 | 18.05 | 329 | 2-O-Sinapoyl-glucoside | C17H22O10 | 385.1141 | 385.1147 | 1.55 | 247.0612, 223.0611, 205.0504, 190.0269 164.0704, 119.0342 | a–b |
| 44 | 19.01 | 330 | Syringaresinol 4-gentiobioside | C34H46O18 | 787.2676 | 787.2678 [M+FA-H] | 0.25 | 417.1560, 402.1323, 387.1069, 371.1494 356.1233, 181.0502, 166.0266 | a–e |
| 45 | 19.53 | 280 | Naringenin 7- O-rutinoside | C27H31O14− | 579.17083 | 579.17291 | 3.59 | 271.06152, 151.00319 | a–b |
| 46 | 19.72 | 254–354 | Quercetin 3-galactoside | C12H19O5− | 243.12270 | 463.08893 | 3.94 | 350.20898, 301.02795, 151.00310 | a–e |
| 47 | 20.45 | - | Spirosol-5-en-3-ol, 3-O-[Rhamnosyl-(1→2)-[rhamnosyl-(1→4)]-glucoside] | C45H73NO15 | 868.5031 | 868.5035 | 0.46 | 722.4411, 576.3845, 414.3357 | a, b, d, e |
| 48 | 22.32 | 280 | Biochanin A 7-O-rutinoside | C28H32O14 | 593.1852 | 593.1859 | 1.18 | 447.1269, 327.0856, 285.0750, 153.0191 | a, b |
| 49 | 22.51 | 280 | Genistin | C21H20O10 | 431.0982 | 433.1123 431.0984 | 0.69 | 414.3355, 271.0595, 253.0485, 215.0698 146.0598, 127.0389, 85.0288, | a–e |
| 50 | 23.35 | 255–340 | 3,5-Dihydroxy-4′,7-dimethoxyflavone 3-O-[Rhamnosyl-(1→2)-glucoside (Pectolarin) | C29H34O15 | 623.1961 | 623.1963 | 0.32 | 477.1342, 315.0815, 300.0622, 284.0679 | a, b, d, e |
| 51 | 24.93 | 281 | Naringenin-7-O-glucoside | C21H22O10 | 435.1279 | 435.1282 433.1117 | 0.95 | 313.0506, 271.0617, 193.0138, 151.0032 119.0405 | d–e |
| 52 | 25.5 | 520 | Pelargonidin 3-O-sophoroside | C27H31O15 | 595.1652 | 595.1656 | 0.67 | 433.1080, 271.0595, 215.0695, 163.0596 127.0389 | a–b |
| 53 | 26.1 | 520 | Pelargonidin 3-O-glucoside | C21H20O10 | 433.1121 | 433.1126 | 1.15 | 311.0556, 269.0460, 163.0031 | a–b |
| 54 | 26.5 | 325 | Methyl chlorogenate | C17H20O9 | 367.1031 | 367.1035 | 1.08 | 191.0556, 179.0344, 161.0237, 135.0443 93.0335 | a–d |
| 55 | 26.7 | - | Peak 55, 1-Hexadecanoyl-sn-glycero-3-Phosphoethanolamine | C21H44NO7P | 454.2920 | 454.2923 452.2786 | 0.66 | 255.2630, 214.0482, 214.0284, 140.0111 | c–e |
| 56 | 27.0 | 280 | Naringenin-5-O-glucoside | C21H22O10 | 433.1132 | 433.1138 | 1.38 | 313.0551, 271.0613, 151.0030, 119.0493 93.00335 | a–e |
| 57 | 27.1 | 280 | Eriodictyol-7-O-glucoside | C21H22O10 | 449.1080 | 449.1083 | 0.66 | 287.0567, 205.0144, 175.0033, 151.0032 135.0445, 125.0237 | a–e |
| 58 | 27.2 | - | 1-Hexadecanoyl-sn-glycero-3-phospho-(1′-myo-inositol) | C25H49O12P | 573.3030 | 573.3034 571.2089 | 0.69 | 391.2256, 333.0594, 315.0487, 255.2329 241.0118, 152.9951 | a–e |
| 59 | 27.3 | 330 | Syringaresinol-glucoside | C28H36O13 | 579.2080 | 579.2084 | 0.89 | 417.1557, 402.1320, 387.1085, 223.0616 181.0501, 166.0265 | c |
| 60 | 27.5 | - | 1-(9Z-Octadecenoyl)-sn-glycero-3-phospho-(1′-myo-inositol) | C27H51O12P | 599.3189 | 599.3192 597.3046 | 0.50 | 333.0594, 315.0488, 281.2487, 259.0223 241.0118 | d–e |
| 61 | 27.7 | 254–354 | Quercetin 3-O-malonylglucoside | C24H22O15 | 579.2080 | 579.2084 | 0.69 | 463.0888, 300.0280, 271.0252, 255.0301 178.9981, 151.0032 | a–e |
| 62 | 27.9 | 450 | Lutein | C40H56O2 | 568.4280 | 568.4282 | 124.08689, 145.0845, 105.08564, 335.12485 | a–e | |
| 63 | 28.0 | 450 | β-carotene | C40H56 | 536.4379 | 536.4382 | 337.09189, 476.17609 | a–e | |
| 64 | 28.1 | 280 | Naringenin | C15H12O5 | 271.06010 | 271.06155 | 5.36 | 153.01832, 147.04453, 119.05632 | e |
| 65 | 28.3 | 280 | Phloretin | C15H14O5 | 349.18569 | 349.18723 | 4.03 | 229.0871, 179.0347, 167.0347, 125.0237 | e |
| 66 | 28.4 | 254–354 | Cirsimarin | C23H24O11 | 475.12668 | 475.12524 | 4.36 | 315.07642 | c–e |
| 67 | 28.9 | - | 1-(9Z-Octadecenoyl)-sn-glycero-3-phosphoethanolamine | C23H46NO7P | 480.3080 | 480.3085 478.2937 | 0.83 | 281.2486, 214.0482, 196.0386, 152.9950 140.0110 | a–b |
| 68 | 29.2 | - | 1-hexadecanoyl-sn-glycero-3-phosphocholine | C24H50NO7P | 496.3391 | 496.3396 | 1.00 | 313.2128, 184.0721, 124.9998, 104.1072 86.0968 | a–b |
| 69 | 29.7 | 215 | 1-(9Z-Octadecenoyl)-sn-glycero-3-phosphocholine | C26H52NO7P | 566.3419 | 566.3422 [M+FA-H] 522.3555 | 0.56 | 504.3450, 445.2715, 339.2892, 240.1001 199.0370, 187.0732, 124.9988 | c–e |
| 70 | 30.2 | 210 | 1-Oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine | C42H82NO8P | 760.5832 | 760.5839 758.542 | 0.92 | 184.0730, 125.9997, 104.1071, 86.0964 | a–b |
| Ecotypes | DPPH (µmol Trolox/g) | ABTS (µmol Trolox/g) | Total Phenolics (mg GAE/g) | Total Carotenoids (μg β-carotene)/g) |
|---|---|---|---|---|
| NMA1 | 19.88 ± 0.34 a | 19.70 ± 0.81 a | 32.68 ± 1.33 a | 101.22 ± 4.47 a |
| CD1 | 18.37 ± 0.24 b | 25.67 ± 0.28 b | 28.03 ± 0.90 b | 122.65 ± 4.24 b |
| CTR | 21.92 ± 0.53 c | 21.98 ± 0.90 ac | 35.79 ± 0.84 ac | 85.13 ± 1.81 c |
| SRN9 | 18.21 ± 0.24 bd | 23.97 ± 1.12 bc | 27.86 ± 0.81 b | 92.12 ± 3.64 ac |
| UNT2 | 19.15 ± 0.84 abd | 20.25 ± 0.79 ac | 34.26 ± 1.32 ac | 58.81 ± 0.46 ab |
| Groups | Cholesterol | Triglyceride | HDL | LDL |
|---|---|---|---|---|
| Group I (control) | 78.9 ± 5.90 | 72.28 ± 3.98 | 50.97 ± 4.02 | 27.97 ± 5.26 |
| Group II hypercholesterolemic, saline treated | 378.17 ± 6.27 a | 461.65 ± 8.82 a | 42.68 ± 3.14 | 225.64 ± 12.16 a |
| Group III hypercholesterolemic, atorvastatin treated | 118.41 ± 10.05 b | 93.90 ± 11.23 b | 60.13 ± 5.08 b | 50.79 ± 4.46 b |
| Group IV hypercholesterolemic, NMA1 pulp treated | 302.76 ± 17.87 a | 268.90 ± 7.92 a | 47.44 ± 5.06 | 145.33 ± 9.56 a |
| Group V hypercholesterolemic, CD1 pulp treated | 287.28 ± 10.03 a | 259.63 ± 13.24 a | 48.12 ± 8.07 | 139.06 ± 8.07 a |
| Group VI hypercholesterolemic, CTR pulp treated | 130.09 ± 8.55 b | 108.51 ± 10.04 b | 57.30 ± 5.72 b | 65.41 ± 7.68 b |
| Group VII hypercholesterolemic, SRN9 pulp treated | 126.74 ± 6.63 b | 102.11 ± 9.47 b | 58.16 ± 6.64 b | 61.05 ± 4.00 b |
| Group VIII hypercholesterolemic, UNT2 pulp treated | 338.81 ± 15.95 ac | 299.86 ± 17.81 ac | 45.56 ± 7.46 c | 165.85 ± 7.42 ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon. Antioxidants 2021, 10, 1566. https://doi.org/10.3390/antiox10101566
Vargas-Arana G, Merino-Zegarra C, Riquelme-Penaherrera M, Nonato-Ramirez L, Delgado-Wong H, Pertino MW, Parra C, Simirgiotis MJ. Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon. Antioxidants. 2021; 10(10):1566. https://doi.org/10.3390/antiox10101566
Chicago/Turabian StyleVargas-Arana, Gabriel, Claudia Merino-Zegarra, Marcos Riquelme-Penaherrera, Luis Nonato-Ramirez, Henry Delgado-Wong, Mariano Walter Pertino, Claudio Parra, and Mario J. Simirgiotis. 2021. "Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon" Antioxidants 10, no. 10: 1566. https://doi.org/10.3390/antiox10101566
APA StyleVargas-Arana, G., Merino-Zegarra, C., Riquelme-Penaherrera, M., Nonato-Ramirez, L., Delgado-Wong, H., Pertino, M. W., Parra, C., & Simirgiotis, M. J. (2021). Antihyperlipidemic and Antioxidant Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Cocona Fruits (Solanum sessiliflorum Dunal) from the Peruvian Amazon. Antioxidants, 10(10), 1566. https://doi.org/10.3390/antiox10101566










