An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Dopamine Oxidation Assessment
2.3. Detection of Reactive Oxygen Species
2.4. Detection of Hydroxyl Radicals
2.5. Detection of DNA Damage
2.6. NBT/Glycinate Redox-Cycling Staining of Quinoprotein
2.7. Dopamine Retention Detected by High Performance Liquid Chromatography (HPLC)
2.8. Determination of Ceruloplasmin Ferrous Oxidase Activity
3. Results and Discussion
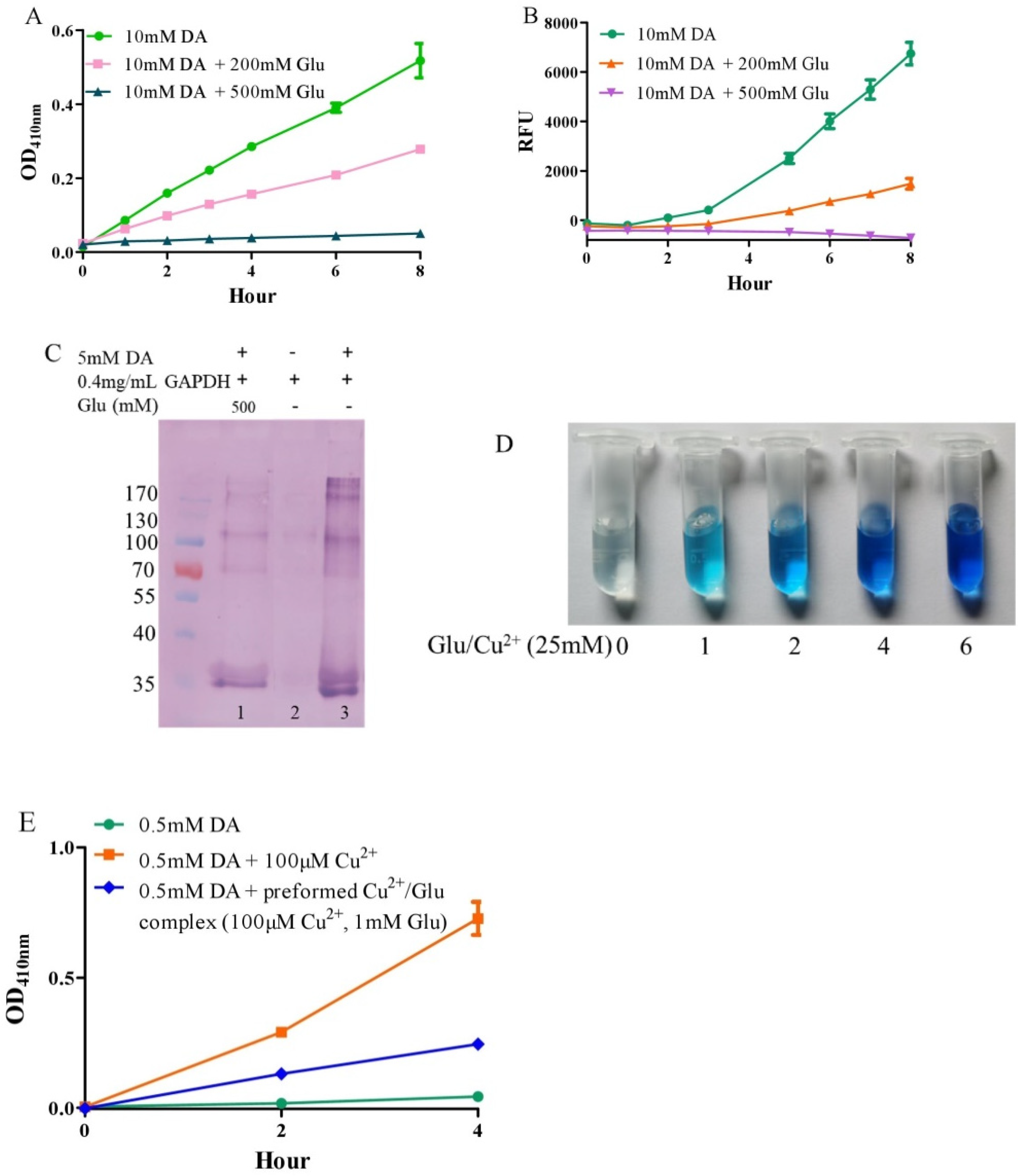
3.1. Autoxidation of Dopamine and the Protective Role of Glutamate
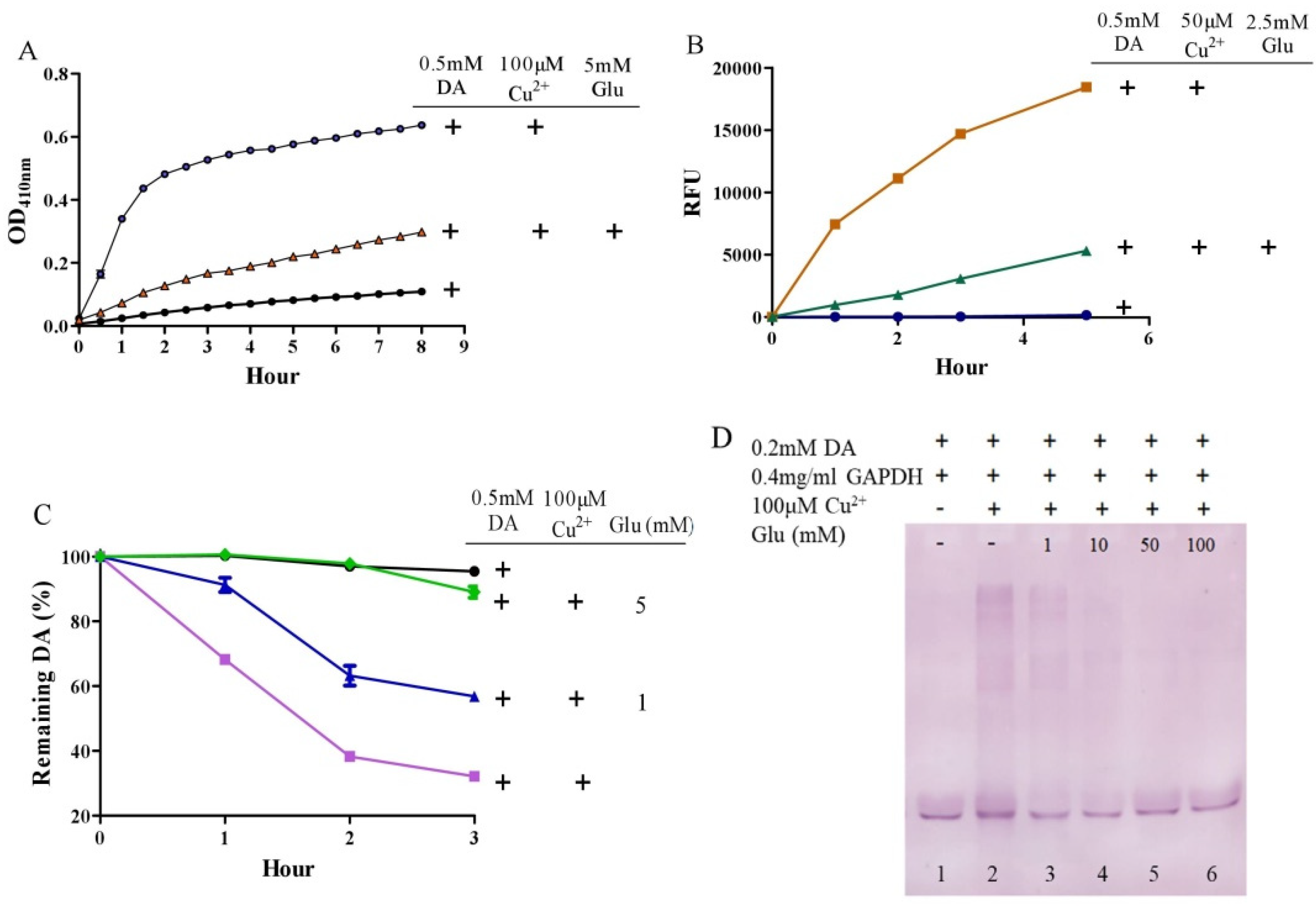
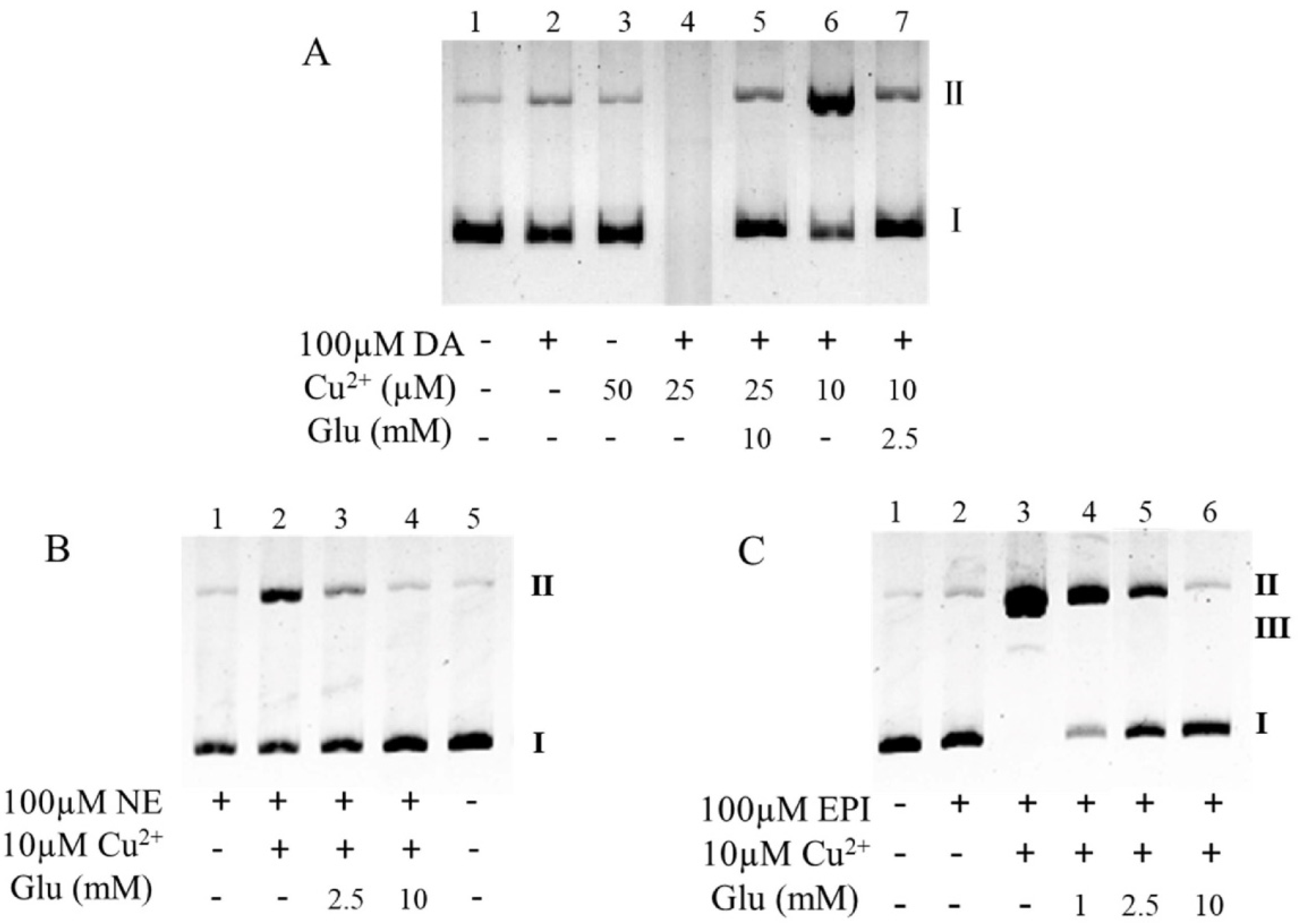
3.2. Glutamate Protects against Copper-Facilitated Dopamine Oxidation
3.3. Glutamate Protects against Ceruloplasmin-Facilitated Dopamine Oxidation and Dopamine Oxidation-Caused Modification of Ceruloplamin
3.4. Glutamate Inhibits Copper/Catecholamine-Induced DNA Damage
3.5. Glutamate Protects against the Toxicity of 3, 4-Dihydroxy Phenylacetaldehyde
3.6. Considerations of Molar Ratio of Glutamate to Dopamine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. ROS initiated oxidation of dopamine under oxidative stress conditions in aqueous and lipidic environments. J. Phys. Chem. B 2011, 115, 12234–12246. [Google Scholar] [CrossRef]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Iversen, S.D.; Iversen, L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007, 30, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, M.; Bonci, A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron 2015, 86, 1145–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.S. Catecholamines 101. Clin. Auton. Res. 2010, 20, 331–352. [Google Scholar] [CrossRef]
- Lai, C.T.; Yu, P.H. Dopamine- and L-beta-3,4-dihydroxyphenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem. Pharmacol. 1997, 53, 363–372. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Felix, C.C.; Sealy, R.C. Semiquinone anion radicals of catechol(amine)s, catechol estrogens, and their metal ion complexes. Environ. Health Perspect. 1985, 64, 185–198. [Google Scholar] [CrossRef]
- Miller, J.W.; Selhub, J.; Joseph, J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med. 1996, 21, 241–249. [Google Scholar] [CrossRef]
- Jodko-Piórecka, K.; Litwinienko, G. Antioxidant activity of dopamine and L-DOPA in lipid micelles and their cooperation with an analogue of α-tocopherol. Free Radic. Biol. Med. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein-Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Hasegawa, T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahn, S.; Cohen, G. The oxidant stress hypothesis in Parkinson’s disease: Evidence supporting it. Ann. Neurol. 1992, 32, 804–812. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Riederer, P. Is Parkinson’s disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta Neurol. Scand. Suppl. 1989, 126, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Roth, G.S. The roles of dopamine oxidative stress and dopamine receptor signaling in aging and age-related neurodegeneration. Antioxid. Redox Signal. 2000, 2, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Banerjee, K.; Munshi, S.; Sen, O.; Pramanik, V.; Roy Mukherjee, T.; Chakrabarti, S. Dopamine Cytotoxicity Involves Both Oxidative and Nonoxidative Pathways in SH-SY5Y Cells: Potential Role of Alpha-Synuclein Overexpression and Proteasomal Inhibition in the Etiopathogenesis of Parkinson’s Disease. Parkinsons Dis. 2014, 2014, 878935. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef]
- Li, H.-T.; Lin, D.-H.; Luo, X.-Y.; Zhang, F.; Ji, L.-N.; Du, H.-N.; Song, G.-Q.; Hu, J.; Zhou, J.-W.; Hu, H.-Y. Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J. 2005, 272, 3661–3672. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.B.; Le, W.; Smith, R.G.; Appel, S.H. Antibodies from patients with Parkinson’s disease react with protein modified by dopamine oxidation. J. Neurosci. Res. 1998, 53, 551–558. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef] [PubMed]
- Asthana, A.; Bollapalli, M.; Tangirala, R.; Bakthisaran, R.; Mohan Rao, C. Hsp27 suppresses the Cu(2+)-induced amyloidogenicity, redox activity, and cytotoxicity of α-synuclein by metal ion stripping. Free Radic. Biol. Med. 2014, 72, 176–190. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P.; Deeble, D.J. Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic. Biol. Med. 1992, 13, 391–405. [Google Scholar] [CrossRef]
- Nishino, Y.; Ando, M.; Makino, R.; Ueda, K.; Okamoto, Y.; Kojima, N. Different mechanisms between copper and iron in catecholamines-mediated oxidative DNA damage and disruption of gene expression in vitro. Neurotox. Res. 2011, 20, 84–92. [Google Scholar] [CrossRef]
- Spencer, W.A.; Jeyabalan, J.; Kichambre, S.; Gupta, R.C. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: Role of reactive oxygen species. Free Radic. Biol. Med. 2011, 50, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.; Mao, L.; Qu, N.; Wang, Y.-F.; Gao, H.-Y.; Li, F.; Qin, L.; Shao, J.; Huang, C.-H.; Xu, D.; et al. Mechanism of synergistic DNA damage induced by the hydroquinone metabolite of brominated phenolic environmental pollutants and Cu(II): Formation of DNA-Cu complex and site-specific production of hydroxyl radicals. Free Radic. Biol. Med. 2017, 104, 54–63. [Google Scholar] [CrossRef]
- Hudspith, M.J. Glutamate: A role in normal brain function, anaesthesia, analgesia and CNS injury. Br. J. Anaesth. 1997, 78, 731–747. [Google Scholar] [CrossRef] [Green Version]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Djuricić, B. Glutamate in brain: Transmitter and poison. Glas. Srp. Akad. Nauka. Med. 2002, 47, 55–76. [Google Scholar]
- Simonyi, A.; Schachtman, T.R.; Christoffersen, G.R.J. The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect. 2005, 18, 353–361. [Google Scholar] [CrossRef]
- Egbenya, D.L.; Hussain, S.; Lai, Y.-C.; Xia, J.; Anderson, A.E.; Davanger, S. Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol. Cell. Neurosci. 2018, 92, 93–103. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. JAD 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Plaitakis, A.; Shashidharan, P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: Implications for the pathogenesis of Parkinson’s disease. J. Neurol. 2000, 247 (Suppl. 2), II25–II35. [Google Scholar] [CrossRef]
- Featherstone, D.E.; Shippy, S.A. Regulation of synaptic transmission by ambient extracellular glutamate. Neuroscientist 2008, 14, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Miner, L.L.; Sora, I.; Ujike, H.; Revay, R.S.; Kostic, V.; Jackson-Lewis, V.; Przedborski, S.; Uhl, G.R. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl. Acad. Sci. USA 1997, 94, 9938–9943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Callahan, B.T.; McCann, U.D.; Ricaurte, G.A. Evidence against an essential role of endogenous brain dopamine in methamphetamine-induced dopaminergic neurotoxicity. J. Neurochem. 2001, 77, 1338–1347. [Google Scholar] [CrossRef]
- Ponzio, F.; Achilli, G.; Calderini, G.; Ferretti, P.; Perego, C.; Toffano, G.; Algeri, S. Depletion and recovery of neuronal monoamine storage in rats of different ages treated with reserpine. Neurobiol. Aging 1984, 5, 101–104. [Google Scholar] [CrossRef]
- Yu, G.; Liu, H.; Zhou, W.; Zhu, X.; Yu, C.; Wang, N.; Zhang, Y.; Ma, J.; Zhao, Y.; Xu, Y.; et al. In vivo protein targets for increased quinoprotein adduct formation in aged substantia nigra. Exp. Neurol. 2015, 271, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Diaz-Corrales, F.J.; Fukuda, M.; Kitaichi, K.; Miyoshi, K.; Ogawa, N. Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone-formation-related molecules. FASEB J. 2006, 20, 571–573. [Google Scholar] [CrossRef]
- Umek, N.; Geršak, B.; Vintar, N.; Šoštarič, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic pH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zecca, L.; Zucca, F.A.; Wilms, H.; Sulzer, D. Neuromelanin of the substantia nigra: A neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003, 26, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Hastings, T.G.; Lewis, D.A.; Zigmond, M.J. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc. Natl. Acad. Sci. USA 1996, 93, 1956–1961. [Google Scholar] [CrossRef] [Green Version]
- Hastings, T.G.; Zigmond, M.J. Identification of catechol-protein conjugates in neostriatal slices incubated with [3H]dopamine: Impact of ascorbic acid and glutathione. J. Neurochem. 1994, 63, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Yu, G.; Yuan, C.; Ma, J. Quinoprotein adducts accumulate in the substantia nigra of aged rats and correlate with dopamine-induced toxicity in SH-SY5Y cells. Neurochem. Res. 2011, 36, 2169–2175. [Google Scholar] [CrossRef]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Pham, A.N.; Waite, T.D. Cu(II)-catalyzed oxidation of dopamine in aqueous solutions: Mechanism and kinetics. J. Inorg. Biochem. 2014, 137, 74–84. [Google Scholar] [CrossRef]
- Ryan, T.P.; Miller, D.M.; Aust, S.D. The role of metals in the enzymatic and nonenzymatic oxidation of epinephrine. J. Biochem. Toxicol. 1993, 8, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Rico-Yuson, C.A.; Hornyak, G.L.; Bora, T. Cyanide-free environment-friendly alternative to copper electroplating for zinc die-cast alloys. Environ. Sci. Pollut. Res. Int. 2021, 28, 38065–38073. [Google Scholar] [CrossRef]
- Niu, J.; Guo, D.; Zhang, W.; Tang, J.; Tang, G.; Yang, J.; Wang, W.; Huo, H.; Jiang, N.; Cao, Y. Preparation and characterization of nanosilica copper (II) complexes of amino acids. J. Hazard. Mater. 2018, 358, 207–215. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Stöckel, J.; Safar, J.; Wallace, A.C.; Cohen, F.E.; Prusiner, S.B. Prion protein selectively binds copper (II) ions. Biochemistry 1998, 37, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Pall, H.S.; Williams, A.C.; Blake, D.R.; Lunec, J.; Gutteridge, J.M.; Hall, M.; Taylor, A. Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet 1987, 2, 238–241. [Google Scholar] [CrossRef]
- Boll, M.-C.; Alcaraz-Zubeldia, M.; Montes, S.; Rios, C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem. Res. 2008, 33, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Dean, R.T.; Davies, M.J. Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catechol(amine)s. Toxicology 2002, 177, 23–37. [Google Scholar] [CrossRef]
- Lévay, G.; Ye, Q.; Bodell, W.J. Formation of DNA adducts and oxidative base damage by copper mediated oxidation of dopamine and 6-hydroxydopamine. Exp. Neurol. 1997, 146, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.H.; Dooley, D.M.; Gray, H.B. Coordination environment and fluoride binding of type 2 copper in the blue copper oxidase ceruloplasmin. Proc. Natl. Acad. Sci. USA 1978, 75, 4078–4081. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.N.; David, S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J. Biol. Chem. 1997, 272, 20185–20190. [Google Scholar] [CrossRef]
- Patel, B.N.; Dunn, R.J.; Jeong, S.Y.; Zhu, Q.; Julien, J.-P.; David, S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002, 22, 6578–6586. [Google Scholar] [CrossRef] [Green Version]
- Gutteridge, J.M. Inhibition of the Fenton reaction by the protein caeruloplasmin and other copper complexes. Assessment of ferroxidase and radical scavenging activities. Chem.-Biol. Interact. 1985, 56, 113–120. [Google Scholar] [CrossRef]
- De Domenico, I.; Ward, D.M.; di Patti, M.C.B.; Jeong, S.Y.; David, S.; Musci, G.; Kaplan, J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007, 26, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Gitlin, J.D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J. Biol. Chem. 1991, 266, 5128–5134. [Google Scholar] [CrossRef]
- Nittis, T.; Gitlin, J.D. The copper-iron connection: Hereditary aceruloplasminemia. Semin. Hematol. 2002, 39, 282–289. [Google Scholar] [CrossRef]
- Løvstad, R.A. Activating effect of copper ions on the interaction of ceruloplasmin with catecholamines. Gen. Pharmacol. 1979, 10, 147–151. [Google Scholar] [CrossRef]
- Rosei, M.A.; Foppoli, C.; Wang, X.T.; Coccia, R.; Mateescu, M.A. Production of melanins by ceruloplasmin. Pigment Cell Res. 1998, 11, 98–102. [Google Scholar] [CrossRef]
- Vashchenko, G.; MacGillivray, R.T.A. Multi-copper oxidases and human iron metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef] [Green Version]
- Curzon, G. The effects of some ions and chelating agents on the oxidase activity of caeruloplasmin. Biochem. J. 1960, 77, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Bharucha, K.J.; Friedman, J.K.; Vincent, A.S.; Ross, E.D. Lower serum ceruloplasmin levels correlate with younger age of onset in Parkinson’s disease. J. Neurol. 2008, 255, 1957–1962. [Google Scholar] [CrossRef]
- Jin, L.; Wang, J.; Zhao, L.; Jin, H.; Fei, G.; Zhang, Y.; Zeng, M.; Zhong, C. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain. 2011, 134, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Wang, J.; Jin, H.; Fei, G.; Zhang, Y.; Chen, W.; Zhao, L.; Zhao, N.; Sun, X.; Zeng, M.; et al. Nigral iron deposition occurs across motor phenotypes of Parkinson’s disease. Eur. J. Neurol. 2012, 19, 969–976. [Google Scholar] [CrossRef]
- Olivieri, S.; Conti, A.; Iannaccone, S.; Cannistraci, C.V.; Campanella, A.; Barbariga, M.; Codazzi, F.; Pelizzoni, I.; Magnani, G.; Pesca, M.; et al. Ceruloplasmin oxidation, a feature of Parkinson’s disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J. Neurosci. 2011, 31, 18568–18577. [Google Scholar] [CrossRef] [Green Version]
- Ayton, S.; Lei, P.; Duce, J.A.; Wong, B.X.W.; Sedjahtera, A.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013, 73, 554–559. [Google Scholar] [CrossRef]
- Barbariga, M.; Curnis, F.; Andolfo, A.; Zanardi, A.; Lazzaro, M.; Conti, A.; Magnani, G.; Volontè, M.A.; Ferrari, L.; Comi, G.; et al. Ceruloplasmin functional changes in Parkinson’s disease-cerebrospinal fluid. Mol. Neurodegener. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, K.; Sharma, A.; Talukder, G. Effects of copper on mammalian cell components. Chem.-Biol. Interact. 1989, 69, 1–16. [Google Scholar] [CrossRef]
- Hasebe, Y.; Toda, S.; Aoki, K.; Tonobe, H.; Uchiyama, S. Specific and amplified current responses to histidine and histamine using immobilized copper-monoamine oxidase membrane electrode, based on novel ascorbate oxidase activity induced by exogenous ligands. Anal. Biochem. 1997, 251, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Sharabi, Y. N-Acetylcysteine Prevents the Increase in Spontaneous Oxidation of Dopamine During Monoamine Oxidase Inhibition in PC12 Cells. Neurochem. Res. 2017, 42, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Follmer, C.; Coelho-Cerqueira, E.; Yatabe-Franco, D.Y.; Araujo, G.D.T.; Pinheiro, A.S.; Domont, G.B.; Eliezer, D. Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of α-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL). J. Biol. Chem. 2015, 290, 27660–27679. [Google Scholar] [CrossRef] [Green Version]
- Burke, W.J. 3,4-dihydroxyphenylacetaldehyde: A potential target for neuroprotective therapy in Parkinson’s disease. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 143–148. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Isonaka, R.; Sharabi, Y.; Goldstein, D.S. 3,4-Dihydroxyphenylacetaldehyde Is More Efficient than Dopamine in Oligomerizing and Quinonizing -Synuclein. J. Pharmacol. Exp. Ther. 2020, 372, 157–165. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wu, X.; Yang, C.S.; Zhang, J. An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation. Antioxidants 2021, 10, 1564. https://doi.org/10.3390/antiox10101564
Wang W, Wu X, Yang CS, Zhang J. An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation. Antioxidants. 2021; 10(10):1564. https://doi.org/10.3390/antiox10101564
Chicago/Turabian StyleWang, Wenping, Ximing Wu, Chung S. Yang, and Jinsong Zhang. 2021. "An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation" Antioxidants 10, no. 10: 1564. https://doi.org/10.3390/antiox10101564
APA StyleWang, W., Wu, X., Yang, C. S., & Zhang, J. (2021). An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation. Antioxidants, 10(10), 1564. https://doi.org/10.3390/antiox10101564






