The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation
Abstract
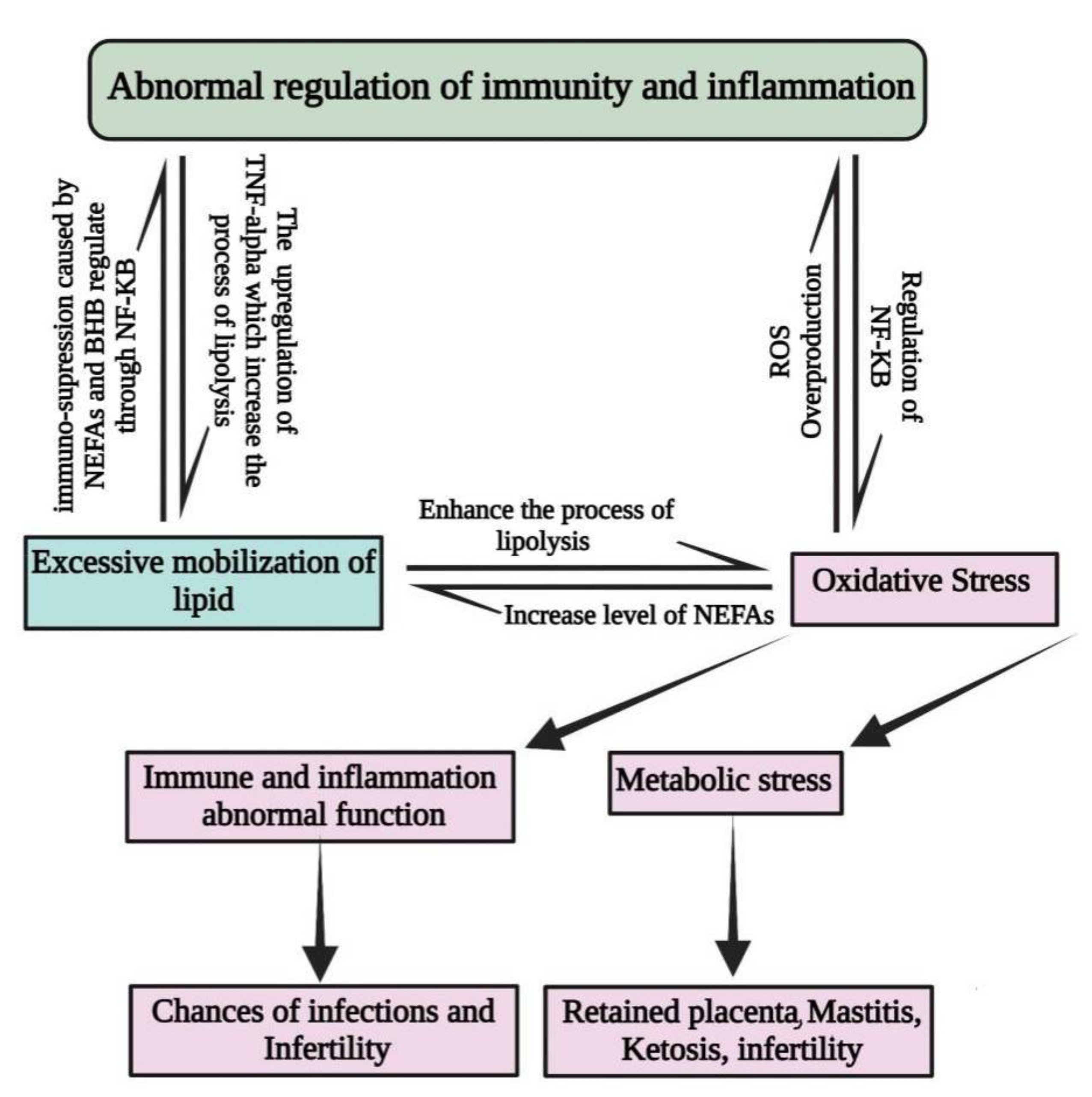
:1. Introduction
2. Factors That Predispose Periparturient Dairy Cattle to Oxidative Stress
3. Antioxidant Properties of Selenium and Their Role in Dairy Cattle Health
3.1. Selenium Role in Cattle Health
3.1.1. Selenium Role in Mastitis Control
3.1.2. Effect of Selenium on Reproduction of Animals
3.1.3. Role of Selenium in Ketosis and Fatty Liver Control
4. Antioxidant Properties of Vitamin E and Their Role in Dairy Cattle Health
Vitamin E Role in Cattle Health
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sordillo, L.M. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Prod. Sci. 2005, 98, 89–99. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Brzezinska-Slebodzinska, E.; Miller, J.K.; Quigley, J.D.; Moore, J.R.; Madsen, F.C. Antioxidant status of dairy cows sup-plemented prepartum with vitamin E and selenium. J. Diary Sci. 1994, 77, 3087–3095. [Google Scholar] [CrossRef]
- Wullepit, N.; Raes, K.; Beerda, B.; Veerkamp, R.F.; Fremaut, D.; Smet, S. Influence of management and genetic merit for milk yield on the oxidative status of plasma in heifers. Livest. Sci. 2009, 123, 276–282. [Google Scholar] [CrossRef]
- Mikulková, K.; Kadek, R.; Filípek, J. Evaluation of oxidant/antioxidant status, metabolic profile and milk production in cows with metritis. Ir. Vet. J. 2020, 73, 1–11. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.; Singh, O.; Pandey, V.; Verma, P. Oxidative Stress and Antioxidant Status during Transition Period in Dairy Cows. Asian-Australas. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Jankowiak, D. Changes of reduction-oxidation balance in pregnant ruminants. Postepy Biochem. 2009, 55, 323–328. [Google Scholar]
- Markiewicz, H.; Gehrke, M.; Malinowski, E.; Kaczmarowski, M. Evaluating the antioxidant potential in the blood of tran-sition cows. Medycyna Weterynaryjna 2005, 61, 1382–1384. [Google Scholar]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory respons-es in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.M.J.; Mangual, J.C.; Gandy, L.; Sordillo, M. 15-F2t-Isoprostane concentrations and oxidant status in lac-tating dairy cattle with acute coliform mastitis. J. Vet. Intern. Med. 2016, 30, 339–347. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kankofer, M. Placental Release/Retention in Cows and its Relation to Peroxidative Damage of Macromolecules. Reprod. Domest. Anim. 2002, 37, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of Body Condition Score on Relationship Between Metabolic Status and Oxidative Stress in Periparturient Dairy Cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; López, M.-A.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Hoedemaker, M.; Prange, D.; Gundelach, Y. Body condition change ante- and postpartum, health and reproductive per-formance in German Holstein cows. Reprod. Domest. Anim. 2010, 44, 167–173. [Google Scholar] [CrossRef]
- Kizil, O.; Akar, Y.; Yuksel, M.; Saat, N. Oxidative stress in cows with acute puerperal metritis. Revue Med. Vet. 2010, 161, 353–357. [Google Scholar]
- Mattioli, A.G.; Diana, E.R.; Esteban, T.; Sebastián, J.P.; Santiago, J.R.; Antonio, H.H.M.; Luis, E.F. Effects of Parenteral Supplementation with Minerals and Vitamins on Oxidative Stress and Humoral Immune Response of Weaning Calves. Animals 2020, 10, 1298. [Google Scholar] [CrossRef]
- Ottaviano, F.G.; Tang, S.-S.; Handy, D.E.; Loscalzo, J. Regulation of the extracellular antioxidant selenoprotein plasma glutathione peroxidase (GPx-3) in mammalian cells. Mol. Cell. Biochem. 2009, 327, 111–126. [Google Scholar] [CrossRef]
- Kegley, E.B.; Ball, J.J.; Beck, P. Impact of mineral and vitamin status on beef cattle immune function and health. J. Anim. Sci. 2016, 94, 59. [Google Scholar] [CrossRef]
- Bordignon, R.; Volpato, A.; Glombowsky, P.; Souza, C.F.; Baldissera, M.D.; Secco, R.; Pereira, W.A.B.; Leal, M.L.R.; Vedovatto, M.; Silva, A.S. Nutraceutical effect of vitamins and minerals on performance and immune and antioxidant systems in dairy calves during the nutritional transition period in summer. J. Therm. Biol. 2019, 84, 451–459. [Google Scholar] [CrossRef]
- Teixeira, A.G.V.; Lima, F.S.; Bicalho, M.L.S.; Kussler, A.; Lima, S.F.; Felippe, M.J.; Bicalho, R.C. Effect of an injectable trace mineral supplement containing selenium, copper, zinc, and manganese on immunity, health, and growth of dairy calves. J. Dairy Sci. 2014, 97, 4216–4226. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Alves-Nores, V.; Hernandez, J.; Muiño, R.; Benedito, J.L.; Castillo, C. Effect of parenteral antioxidant supple-mentation during the dry period on postpartum glucose tolerance in dairy cows. J. Vet. Intern. Med. 2016, 30, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Cusack, P.; McMeniman, N.; Rabiee, A.; Lean, I. Assessment of the eects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis. Prev. Vet. Med. 2009, 88, 229–246. [Google Scholar] [CrossRef]
- Volpato, A.; Da Silva, A.S.; Crecencio, R.B.; Tomasi, T.; Fortuoso, B.F.; Ribeiro, M.P.; Morsch, V.M.M. A prophylactic protocol to stimulate the immune response also control infectious disease and, consequently, minimizes diarrhea in newborn heifers. Microb. Pathog. 2018, 121, 262–268. [Google Scholar] [CrossRef]
- Villar, D.; Arthur, J.R.; Gonzalez, J.M. Selenium status in cattle: Interpretation of laboratory results. Bov. Pract. 2002, 36, 73–80. [Google Scholar]
- Flohé, J.R.A.L.; Flohé, L.; Andreesen, J.R.; Brigelius, R.-F.; Maiorino, M.; Ursini, F. Selenium, the Element of the Moon, in Life on Earth. IUBMB Life 2000, 49, 411–420. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Gladyshev, V.N. Selenium in biology and human health: Controversies and perspectives. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D.L., Ed.; Springer: Boston, MA, USA, 2001; pp. 313–317. [Google Scholar]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Hogan, J.S.; Weiss, W.P.; Smith, K.L. Role of Vitamin E and Selenium in Host Defense Against Mastitis. J. Dairy Sci. 1993, 76, 2795–2803. [Google Scholar] [CrossRef]
- Smith, K.L.; Hogan, J.S.; Weiss, W.P. Dietary vitamin E and selenium affect mastitis and milk quality. J. Anim. Sci. 1997, 75, 1659–1665. [Google Scholar] [CrossRef]
- Weiss, W.P.; Hogan, J.S.; Todhunter, D.A.; Smith, K.L. Effect of Vitamin E Supplementation in Diets with a Low Concentration of Selenium on Mammary Gland Health of Dairy Cows. J. Dairy Sci. 1997, 80, 1728–1737. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Collier, R.J.; Bauman, D.E. A 100-Year Review: Regulation of nutrient partitioning to support lactation. J. Dairy Sci. 2017, 100, 10353–10366. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Abuelo, A.; Joaquín, H.; José, L.B.; Cristina, C. Redox Biology in Transition Periods of Dairy Cattle: Role in the Health of Periparturient and Neonatal Animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disor-ders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 21–29. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.E.; LeBlanc, S.J.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.; Overton, M.W.; Duffield, T.F. The association of serum metabolites in the transition period with milk production and early-lactation reproductive perfor-mance. J. Dairy Sci. 2012, 95, 1301–1309. [Google Scholar] [CrossRef]
- Konvicná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev. 2005, 24, 55–99. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Gabai, G. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Hernández, J.; López-Alonso, M.; Miranda, M.; Benedito Luís, J.L. Values of plasma lipid hydroperoxides and total antioxidant status in healthy dairy cows: Preliminary observations. Arch. Anim. Breed. 2003, 46, 227–233. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Singh, S.P.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J. Dairy Sci. 2013, 96, 165–180. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Dewhurst, R.J.; Friggens, N.C. On the relationship between lactational performance and health: Is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livest. Prod. Sci. 2003, 83, 277–308. [Google Scholar] [CrossRef]
- Van Engelen, E.; De Groot, M.W.; Breeveld-Dwarkasing, V.N.A.; Everts, M.E.; Van Der Weyden, G.C.; Taverne, M.A.M.; Rutten, V.P. Cervical Ripening and Parturition in Cows are Driven by a Cascade of Pro-Inflammatory Cytokines. Reprod. Domest. Anim. 2009, 44, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Farney, J.K.; Mamedova, L.K.; Coetzee, J.F.; KuKanich, B.; Sordillo, L.M.; Stoakes, S.K.; Minton, J.E.; Hollis, L.C.; Bradford, B.J. Anti-inflammatory salicylate treatment alters the metabolic adaptations to lactation in dairy cattle. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R110–R117. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of Mammalian Selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W. Micronutrients and immune function in cattle. Proc. Nutr. Soc. 2000, 59, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Kasper, K.; Traber, M.G.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of su-pranutritional organic selenium supplementation on postpartum blood micronutrients, antioxidants, metabolites, and in-flammation biomarkers in selenium-replete dairy cows. Biol. Trace Elem. Res. 2014, 161, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, A.; Sanchez, J.; Stryhn, H.; Montgomery, J.B.; Barkema, H.W.; Wichtel, J.J. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009, 92, 324–342. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Jankowiak, D.; Tomza-Marciniak, A.; Pilarczyk, R.; Sablik, P.; Drozd, R.; Tylkowska, A.; Skólmowska, M. Selenium Concentration and Glutathione Peroxidase (GSH-Px) Activity in Serum of Cows at Different Stages of Lactation. Biol. Trace Elem. Res. 2012, 147, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Diyabalanage, S.; Dangolla, A.; Mallawa, C.; Rajapakse, S.; Chandrajith, R. Bioavailability of selenium (Se) in cattle pop-ulation in Sri Lanka based on qualitative determination of glutathione peroxidase (GSH-Px) activities. Environ. Geochem. Health 2020, 42, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Khol-Parisini, A.; Schafft, H.; Lahrssen-Wiederholt, M.; Hulan, H.W.; Dinse, D.; Zentek, J. The role of dietary selenium in bovine mammary gland health and immune function. Anim. Health Res. Rev. 2009, 10, 21–34. [Google Scholar] [CrossRef]
- McGrath, J. Accelerated pre-weaning growth rates in dairy calves: Do antioxidants have a place? Anim. Prod. Sci. 2016, 56, 1275–1284. [Google Scholar] [CrossRef]
- Han, S.-J.; Lee, B.C.; Yim, S.H.; Gladyshev, V.N.; Lee, S.-R. Characterization of Mammalian Selenoprotein O: A Redox-Active Mitochondrial Protein. PLoS ONE 2014, 9, e95518. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Darke, A.K.; Penney, K.L.; Tangen, C.M.; Goodman, P.J.; Lee, G.-S.M.; Sun, T.; Peisch, S.; Tinianow, A.M.; Rae, J.M.; et al. Selenium- or Vitamin E–Related Gene Variants, Interaction with Supplementation, and Risk of High-Grade Prostate Cancer in SELECT. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.L.; Lancia, J.K.; Mathur, A.; Smith, M.L. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006, 26, 899–904. [Google Scholar] [PubMed]
- Ahmadi, K.; Roshan-Milani, S.; Asgharzadeh, F.; Pourjabali, M.; Fard, A.A. In Vitro and In Vivo Pretreatment with Selenium Mitigates Tetrahydrocannabinol-Induced Testicular Cell Apoptosis: The Role of AKT and p53 Pathways. Biol. Trace Elem. Res. 2021, 199, 2278–2287. [Google Scholar] [CrossRef]
- Lynch, S.J.; Horgan, K.A.; White, B.; Walls, D. Selenium source impacts protection of porcine jejunal epithelial cells from cadmium-induced DNA damage, with maximum protection exhibited with yeast-derived selenium compounds. Biol. Trace Elem. Res. 2017, 176, 311–320. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to ther-apeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.G.; Liu, H.K.; Qi, G.Y.; Wang, Q.; Sun, X.R.; Chen, B.Q.; Liu, J.R. γ-Tocotrienol inhibits angiogene-sis of human umbilical vein endothelial cell induced by cancer cell. J. Nutr. Biochem. 2011, 22, 1127–1136. [Google Scholar] [CrossRef]
- Koyama, H.; Omura, K.; Ejima, A.; Kasanuma, Y.; Watanabe, C.; Satoh, H. Separation of selenium-containing proteins in human and mouse plasma using tandem high-performance liquid chromatography columns coupled with inductively coupled plasma-mass spectrometry. Anal. Biochem. 1999, 267, 84–91. [Google Scholar] [CrossRef]
- Conrad, M.; Schneider, M.; Seiler, A.; Bornkamm, G.W. Physiological role of phospholipid hydroperoxide glutathione pe-roxidase in mammals. Biol. Chem. 2007, 388, 1019–1025. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Freitas, F.P.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422. [Google Scholar] [CrossRef]
- Panee, J.; Stoytcheva, Z.R.; Liu, W.; Berry, M.J. Selenoprotein H Is a Redox-sensing High Mobility Group Family DNA-binding Protein That Up-regulates Genes Involved in Glutathione Synthesis and Phase II Detoxification. J. Biol. Chem. 2007, 282, 23759–23765. [Google Scholar] [CrossRef]
- Novoselov, S.V.; Kryukov, G.V.; Xu, X.-M.; Carlson, B.A.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein H Is a Nucleolar Thioredoxin-like Protein with a Unique Expression Pattern. J. Biol. Chem. 2007, 282, 11960–11968. [Google Scholar] [CrossRef]
- Boukhzar, L.; Hamieh, A.; Cartier, D.; Tanguy, Y.; Alsharif, I.; Castex, M.; Arabo, A.; El Hajji, S.; Bonnet, J.-J.; Errami, M.; et al. Selenoprotein T Exerts an Essential Oxidoreductase Activity That Protects Dopaminergic Neurons in Mouse Models of Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 557–574. [Google Scholar] [CrossRef]
- Jeon, Y.H. Vitamin E, an Antioxidant, as a Possible Therapeutic Agent for Treating Pain. Korean J. Pain 2013, 26, 314–315. [Google Scholar] [CrossRef]
- Xu, X.-M.; Carlson, B.A.; Irons, R.; Mix, H.; Zhong, N.; Gladyshev, V.N.; Hatfield, D.L. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem. J. 2007, 404, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Marta, K.; Sylvie, S.; Carlos, F.; Branislav, R.; Thembinkosi, D.M.; Jiri, S.; Mojmir, B. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Jozwik, A.; Krzyzewski, J.; Strzalkowska, N.; Polawska, E.; Bagnicka, E.; Wierzbicka, A.; Niemczuk, K.; Lipinska, P.; Horbanczuk, J.O. Relations between the oxidative status, mastitis, milk quality and disorders of reproductive functions in dairy cows—A review. Anim. Sci. Pap. Rep. 2012, 30, 297–307. [Google Scholar]
- Gabai, G.; De Luca, E.; Miotto, G.; Zin, G.; Stefani, A.; Da Dalt, L.; Barberio, A.; Celi, P. Protein Oxidation Biomarkers and Uterine Health in Dairy Cows during the Postpartum Period. Antioxidants 2019, 8, 21. [Google Scholar] [CrossRef]
- Glombowsky, P.; Bottari, N.B.; Klauck, V.; Fávero, J.F.; Soldá, N.M.; Baldissera, M.D.; Perin, G.; Morsch, V.M.; Schetinger, M.R.C.; Stefani, L.M.; et al. Oxidative stress in dairy cows seropositives for Neospora caninum. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.D.; Da Silva, A.S.; Baldissera, M.D.; Schwertz, C.I.; Bottari, N.B.; Carmo, G.M.; Machado, G.; Lucca, N.J.; Henker, L.C.; Piva, M.M.; et al. Oxidative stress in dairy cows naturally infected with the lungworm Dictyocaulus viviparus (Nematoda: Trichostrongyloidea). J. Helminthol. 2017, 91, 462–469. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female repro-duction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Talukder, S.; Kerrisk, K.L.; Gabai, G.; Celi, P. Role of oxidant–antioxidant balance in reproduction of domestic animals. Anim. Prod. Sci. 2017, 57, 1588. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted repro-duction: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.L.; Karcher, E.L.; Rezamend, P.; Gandy, J.C.; VandeHaar, M.J.; Capuco, A.V.; Sorgidillo, L.M. Evaluation of an-tioxidant and proinflammatory gene expression in bovine mammary tissue during the periparturient period. J. Dairy Sci. 2009, 92, 589–598. [Google Scholar] [CrossRef]
- Miranda, S.; Wang, Y.J.; Purdie, N.G.; Osborne, V.R.; Coomber, B.L.; Cant, J.P. Selenomethionine stimulates expression of glutathione peroxidase 1 and 3 and growth of bovine mammary epithelial cells in primary culture. J. Dairy Sci. 2009, 92, 2670–2683. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jia, D.; He, R.; Lian, S.; Wang, J.; Wu, R. Association Between Serum Selenium Level and Subclinical Mastitis in Dairy Cattle. Biol. Trace Elem. Res. 2021, 199, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.G.; Bobe, G.; Pirelli, W.; Mosher, J. Organic and inorganic selenium: III. Ewe and progeny performance. J. Anim. Sci. 2012, 90, 4536–4543. [Google Scholar]
- Gong, J.; Ni, L.; Wang, D.; Shi, B.; Yan, S. Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows. Livest. Sci. 2014, 170, 84–90. [Google Scholar] [CrossRef]
- Gong, J.; Xiao, M. Selenium and antioxidant status in dairy cows at different stages of lactation. Biol. Trace Elem. Res. 2016, 171, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.; Liu, W.; Bu, D.P.; Liu, S.J.; Zhang, K.Z. Hydroxy-selenomethionine: A novel organic selenium source that improves antioxidant status and selenium concentrations in milk and plasma of mid-lactation dairy cows. J. Dairy Sci. 2017, 100, 9602–9610. [Google Scholar] [CrossRef] [PubMed]
- Juniper, D.T.; Rymer, C.; Briens, M. Bioefficacy of hydroxy-selenomethionine as a selenium supplement in pregnant dairy heifers and on the selenium status of their calves. J. Dairy Sci. 2019, 102, 7000–7010. [Google Scholar] [CrossRef]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Vet. Res. 2018, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, R. The influences of dietary selenium and vitamin E intakes on milk somatic cell counts and mastitis in cows. Vet. Res. Commun. 1999, 23, 481–499. [Google Scholar] [CrossRef]
- Wei, M.-J.; Wang, Z.-N.; Yang, Y.; Zhang, S.-J.; Tang, H.; Li, H.; Bi, C.-L. Selenium Attenuates, S. aureus-Induced Inflammation by Regulation TLR2 Signaling Pathway and NLRP3 Inflammasome in RAW 264.7 Macrophages. Biol. Trace Elem. Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Guo, Y.F.; Li, C.Y.; Qiu, C.W.; Guo, M.Y. Selenium influences mmu-miR-155 to inhibit inflammation in Staphylococcus aureus-induced mastitis in mice. Food Funct. 2019, 10, 6543–6555. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, S.; Guo, Y.; Hao, M.; Chen, Y.; Wang, Y.; Yang, M.; Chen, J.; Guo, M. Selenium attenuates Staphylococcus aureus mastitis in mice by inhibiting the activation of the NALP3 Inflammasome and NF-κB/MAPK pathway. Biol. Trace Elem. Res. 2019, 191, 159–166. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, H.W.; Cheng, W.-H. Beneficial and paradoxical roles of selenium at nutritional levels of intake in healthspan and longevity. Free Radic. Biol. Med. 2018, 127, 3–13. [Google Scholar] [CrossRef]
- Kim, S.H.; Johnson, V.J.; Shin, T.Y.; Sharma, R.P. Selenium attenuates lipopolysaccharide-induced oxidative stress re-sponses through modulation of p38 MAPK and NF-kappa B signaling pathways. Exp. Biol. Med. 2004, 229, 203–213. [Google Scholar] [CrossRef]
- Liu, K.; Ding, T.; Fang, L.; Cui, L.; Li, J.; Meng, X.; Zhu, G.; Qian, C.; Wang, H.; Li, J. Organic Selenium Ameliorates Staphylococcus aureus-Induced Mastitis in Rats by Inhibiting the Activation of NF-κB and MAPK Signaling Path-ways. Front. Vet. Sci. 2020, 7, 443. [Google Scholar] [CrossRef]
- Bi, C.L.; Wang, H.; Wang, Y.J.; Sun, J.; Dong, J.S.; Meng, X.; Li, J.J. Selenium inhibits Staphylococcus aureus induced in-flammation by suppressing the activation of the NF-κB and MAPK signaling pathways in RAW264.7 macrophages. Eur. J. Pharmacol. 2016, 780, 159–165. [Google Scholar] [CrossRef]
- Vunta, H.; Belda, B.J.; Arner, R.J.; Reddy, C.C.; Heuvel, J.P.V.; Prabhu, K.S. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2010, 52, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Petzer, I.M.; Ferreira, G.M. Injectable organic and inorganic selenium in dairy cows–Effects on milk, blood and somatic cell count levels. Onderstepoort J. Vet. Res. 2019, 86, 1–8. [Google Scholar]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, K. Role of Antioxidants in the Treatment of Male Infertility. Surg. Med. Open Access J. 2018, 1. [Google Scholar] [CrossRef]
- Kommisrud, E.; Østerås, O.; Vatn, T. Blood Selenium Associated with Health and Fertility in Norwegian Dairy Herds. Acta Vet. Scand. 2005, 46, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Giadinis, N.D.; Loukopoulos, P.; Petridou, E.J.; Panousis, N.; Konstantoudaki, K.; Filioussis, G.; Tsousis, G.; Brozos, C.; Koutsoumpas, A.T.; Chaintoutis, S.C.; et al. Abortions in three beef cattle herds attributed to selenium deficiency. Pak. Vet. J. 2016, 36, 145–148. [Google Scholar]
- Kamada, H. Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 347–354. [Google Scholar] [CrossRef]
- Uematsu, M.; Kitahara, G.; Sameshima, H.; Osawa, T. Serum selenium and liposoluble vitamins in Japanese Black cows that had stillborn calves. J. Vet. Med. Sci. 2016, 78, 1501–1504. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 66–77. [Google Scholar]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006; pp. 487–587. [Google Scholar]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and Reproduction: Boars and Semen Quality—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef]
- Badade, Z.G.; More, K.; Narshetty, J. Oxidative stress adversely affects spermatogenesis in male infertility. Biomed. Res. 2011, 22, 323–328. [Google Scholar]
- Brouwers, J.F.; Gadella, B.M. In Situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Duru, N.K.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Chandra, G.; Aggarwal, A.; Singh, A.; Singh, A.K.; Kumar, M.; Kushwaha, R.; Singh, Y.K. Oxidative stress in sperm bi-ology—A review. Agric. Rev. 2012, 33, 54–61. [Google Scholar]
- Tavilani, H.; Goodarzi, M.T.; Vaisi-Raygani, A.; Salimi, S.; Hassanzadeh, T. Activity of antioxidant enzymes in seminal plasma and their relationship with lipid peroxidation of spermatozoa. Int. Braz. J. Urol. 2008, 34, 485–491. [Google Scholar] [CrossRef]
- Shang, X.J.; Li, K.; Ye, Z.Q.; Chen, Y.G.; Yu, X.; Huang, Y.F. Analysis of lipid peroxidative levels in seminal plasma of in-fertile men by high-performance liquid chromatography. Arch. Androl. 2004, 50, 411–416. [Google Scholar] [CrossRef]
- Sahinduran, S.; Sezer, K.; Büyükoğlu, T.; Albay, M.K.; Karakurum, M.C. Evaluation of Some Haematological and Biochemical Parameters Before and After Treatment in Cows with Ketosis and Comparison of Different Treatment Methods. J. Anim. Vet. Adv. 2010, 9, 266–271. [Google Scholar] [CrossRef]
- Katoh, N. Relevance apolipoproteins in the development of fatty liver and fatty liver-related peripartum disease in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef]
- Folnozic, I.; Turk, R.; Đuričić, D.; Vince, S.; Pleadin, J.; Flegar-Meštrić, Z.; Valpotić, H.; Dobranic, T.; Gracner, D.; Samardzija, M. Influence of Body Condition on Serum Metabolic Indicators of Lipid Mobilization and Oxidative Stress in Dairy Cows During the Transition Period. Reprod. Domest. Anim. 2015, 50, 910–917. [Google Scholar] [CrossRef]
- Kozat, S.; Yüksek, N. Evaluation of Total Antioxidant, Total Calcium, Selenium, Insulin, Free Triiodothyronine and Free Thyroxine Levels in Cows with Ketosis. Iran. J. Appl. Anim. Sci. 2017, 7, 393–399. [Google Scholar]
- Ren, Z.-H.; Bai, L.-P.; Shen, L.-H.; Luo, Z.-Z.; Zhou, Z.-H.; Zuo, Z.-C.; Ma, X.-P.; Deng, J.-L.; Wang, Y.; Xu, S.-Y.; et al. Comparative iTRAQ Proteomics Reveals Multiple Effects of Selenium Yeast on Dairy Cows in Parturition. Biol. Trace Elem. Res. 2019, 197, 464–474. [Google Scholar] [CrossRef]
- He, P.P.; Jiang, T.; Ouyang, X.P.; Liang, Y.Q.; Zou, J.Q.; Wang, Y.; Shen, Q.Q.; Liao, L.; Zheng, X.L. Lipoprotein lipase: Biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin. Chim. Acta 2018, 480, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Andrieu, S. Is there a role for organic trace element supplements in transition cow health? Vet. J. 2008, 176, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J. Trace Elem. Med. Biol. 2004, 18, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sontag, T.J.; Parker, R.S. Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J. Lipid Res. 2007, 48, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Elsasser, T.H.; Kahl, S.; Lebold, K.M.; Traber, M.G.; Shaffer, J.; Li, C.-J.; Block, S. Short-term alpha- or gam-ma-delta-enriched tocopherol oil supplementation differentially affects the expression of proinflammatory mediators: Se-lective impacts on characteristics of protein tyrosine nitration in vivo. Vet. Sci. Dev. 2013, 3, 20–38. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Park, Y.H.; Lee, J.H.; Hong, J.H.; Kim, I.Y. SelenoproteinWenhances skeletal muscle differentiation by inhibiting TAZ binding to 14-3-3 protein. Biochim. Biophys. Acta 2014, 1843, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, Z.I.; Siddiq, M.; Lodhi, L.A.; Muhammad, G.; Jamil, H. Effect of vitamin E–selenium administration during late gestation on productive and reproductive performance in dairy buffaloes and on growth performance of their calves. Pak. Vet. J. 2010, 30, 83–86. [Google Scholar]
- Mardones, P.; Rigotti, A. Cellular mechanisms of vitamin e uptake: Relevance in alpha-tocopherolmetabolism and potential implications for disease. J. Nutr. Biochem. 2004, 15, 252–260. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef]
- Tucker, J.; Townsend, D. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef]
- Weiss, W.P. A 100-Year Review: From ascorbic acid to zinc—Mineral and vitamin nutrition of dairy cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef]
- Krueger, L.A.; Beitz, D.C.; Onda, K.; Osman, M.R.; O’Neil, M.; Lei, S.; Wattoo, F.H.; Stuart, R.L.; Tyler, H.D.; Nonnecke, B. Effects of d-α-tocopherol and dietary energy on growth and health of preruminant dairy calves. J. Dairy Sci. 2014, 97, 3715–3727. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Sordillo, L.M. Vitamin E analogs limit in vitro oxidant damage to bovine mammary endothelial cells. J. Dairy Sci. 2021, 104, 7154–7167. [Google Scholar] [CrossRef]
- Mokhber-Dezfouli, M.R.; Rahimikia, E.; Asadi, F.; Nadalian, M.G. The Role of Route of Vitamin E Administration on the Plasma Antioxidant Activity and Lipid Peroxidation in Newborn Calves. Basic Clin. Pharmacol. Toxicol. 2008, 103, 414–418. [Google Scholar] [CrossRef]
- Chatterjee, P.N.; Kaur, H.; Panda, N. Effect of Vitamin E Supplementation on Plasma Antioxidant Vitamins and Immunity Status of Crossbred Cows. Asian-Australas. J. Anim. Sci. 2003, 16, 1614–1618. [Google Scholar] [CrossRef]
- Smith, K.L.; Weiss, W.P.; Hogan, J.S. Role of vitamin E in optimizing mammary gland health and productivity of dairy cows: The next generation. In Proceedings of the Southwest Nutrition and Management Conference, Phoenix, AZ, USA; 2000; pp. 1–8. [Google Scholar]
- Erickson, K.L.; Medina, E.A.; Hubbard, N.E. Micronutrients and Innate Immunity. J. Infect. Dis. 2000, 182, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, S.; Von Soosten, D.; Meyer, U.; Drong, C.; Frahm, J.; Kluess, J.; Raschka, C.; Rehage, J.; Tröscher, A.; Pelletier, W.; et al. Influence of conjugated linoleic acid and vitamin E on performance, energy metabolism, and change of fat depot mass in transitional dairy cows. J. Dairy Sci. 2017, 100, 3193–3208. [Google Scholar] [CrossRef]
- Khatti, A.; Mehrotra, S.; Patel, P.K.; Singh, G.; Maurya, V.P.; Mahla, A.S.; Chaudhari, R.K.; Narayanan, K.; Das, G.K.; Singh, M.; et al. Supplementation of vitamin E, selenium and increased energy allowance mitigates transition stress and improves postpartum reproductive performance in crossbred cow. Theriogenology 2017, 104, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, Q.; Yuan, J.; Jiang, Z. Effects of supplementation with vitamin E on the performance and the tissue perox-idation of broiler chicks and the stability of thigh meat against oxidative deterioration. Anim. Feed Sci. Technol. 2001, 89, 165–173. [Google Scholar] [CrossRef]
- Gallardo, B.; Manca, M.G.; Mantecón, A.R.; Nudda, A.; Manso, T. Effects of linseed oil and natural or synthetic vitamin E supplementation in lactating ewes’ diets on meat fatty acid profile and lipid oxidation from their milk fed lambs. Meat Sci. 2015, 102, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.W.; Yoshimura, E.H.; Machado, E.; Matumoto-Pintro, P.T.; Montanher, P.F.; Visentainer, J.V.; Zeoula, L.M. Antioxidant effects of a propolis extract and vitamin E in blood and milk of dairy cows fed diet containing flaxseed oil. Livest. Sci. 2016, 191, 132–138. [Google Scholar] [CrossRef]
- Ripoll, G.; González-Calvo, L.; Molino, F.; Calvo, J.H.; Joy, M. Effects of finishing period length with vitamin e sup-plementation and alfalfa grazing on carcass color and the evolution of meat color and the lipid oxidation of light lambs. Meat Sci. 2013, 93, 906–913. [Google Scholar] [CrossRef]
- Maiorano, G.; Cavone, C.; McCormick, R.J.; Ciarlariello, A.; Gambacorta, M.; Manchisi, A. The effect of dietary energy and vitamin E administration on performance and intramuscular collagen properties of lambs. Meat Sci. 2007, 76, 182–188. [Google Scholar] [CrossRef]
- Politis, I. Reevaluation of vitamin E supplementation of dairy cows: Bioavailability, animal health and milk quality. Animal 2012, 6, 1427–1434. [Google Scholar] [CrossRef]
- Haga, S.; Miyaji, M.; Nakano, M.; Ishizaki, H.; Matsuyama, H.; Katoh, K.; Roh, S. Changes in the expression of α-tocopherol-related genes in liver and mammary gland biopsy specimens of peripartum dairy cows. J. Dairy Sci. 2018, 101, 5277–5293. [Google Scholar] [CrossRef] [PubMed]
- Persson, W.K.; Sandgren, H.C.; Emanuelson, U.; Jensen, S.K. Supplementation of RRR-alpha-tocopheryl acetate to per-iparturient dairy cows in commercial herds with high mastitis incidence. J. Dairy Sci. 2007, 90, 3640–3646. [Google Scholar] [CrossRef]
- Politis, I.; Bizelis, I.; Tsiaras, A.; Baldi, A. Effect of vitamin E supplementation on neutrophil function, milk composition and plasmin activity in dairy cows in a commercial herd. J. Dairy Res. 2004, 71, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, R.J.; Nielen, M.; Stegeman, J.A.; Dobbelaar, P.; Newbold, J.R.; Jansen, E.H.J.M.; Van Werven, T. Vitamin E supplementation during the dry period in dairy cattle. Part I: Adverse effect on incidence of mastitis postpartum in a double-blind randomized field trial. J. Dairy Sci. 2010, 93, 5684–5695. [Google Scholar] [CrossRef] [PubMed]
- Pritee, G.; Upadhyay, A.K.; Gangwar, N.K.; Rajput, M.K.S. Relationship of mineral and vitamin supplementation with mastitis. Vet. World 2008, 1, 103–104. [Google Scholar]
- Cengiz, M.; Bastan, A. Effectiveness of dry cow therapy antibiotic treatment, internal teat sealant, and a-tocopherol against new intramammary infections in cows. Bull. Vet. Inst. Pulawy. 2015, 59, 71–78. [Google Scholar] [CrossRef]
- Sharma, N.; Maiti, S.K. Effect of dietary supplementation of vitamin E and selenium in subclinical mastitis in dairy cows. Indian J. Vet. Med. 2005, 25, 76–79. [Google Scholar]
- Morgante, M.; Beghelli, D.; Pauselli, M.; Dall’Ara, P.; Capuccella, M.; Ranucci, S. Effect of Administration of Vitamin E and Selenium During the Dry Period on Mammary Health and Milk Cell Counts in Dairy Ewes. J. Dairy Sci. 1999, 82, 623–631. [Google Scholar] [CrossRef]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The effect of parenteral supplementation of vitamin E with selenium on the health and productivity of dairy cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bhakat, C.; Kumari, T.; Mandal, D.K.; Chatterjee, A.; Karunakaran, M.; Dutta, T.K. Influence of pre and postpartum alpha-tocopherol supplementation on milk yield, milk quality and udder health of Jersey crossbred cows at tropical lower Gangetic region. Vet. World 2020, 13, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Kolb, E. Seehawer, J. The role of selenium compounds, vitamin E and other factors in the prevention of retained placenta: A review. Tierarztliche Umschau 2002, 57, 666–672. [Google Scholar]
- Kamada, H.; Nonaka, I.; Takenouchi, N.; Amari, M. Effects of selenium supplementation on plasma progesterone con-centrations in pregnant heifers. Anim. Sci. J. 2014, 85, 241–246. [Google Scholar] [CrossRef]
- Nyman, A.-K.; Emanuelson, U.; Holtenius, K.; Ingvartsen, K.L.; Larsen, T.; Waller, K.P. Metabolites and Immune Variables Associated with Somatic Cell Counts of Primiparous Dairy Cows. J. Dairy Sci. 2008, 91, 2996–3009. [Google Scholar] [CrossRef]
- Jukola, E.; Hakkarainen, J.; Saloniemi, H.; Sankari, S. Blood selenium, vitamin E, vitamin A, and β-carotene concentrations and udder health, fertility treatments, and fertility. J. Dairy Sci. 1996, 79, 838–845. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.; Leslie, K.; Walton, J.; Leblanc, S. Risk factors for postpartum uterine diseases in dairy cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E., Jr.; Reinhardt, T.A. Decreased neutrophil function as a cause of retained placenta in dairy cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Pontes, G.; Monteiro, P.; Prata, A.; Guardieiro, M.; Pinto, D.; Fernandes, G.; Wiltbank, M.; Santos, J.; Sartori, R. Effect of injectable vitamin E on incidence of retained fetal membranes and reproductive performance of dairy cows. J. Dairy Sci. 2014, 98, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Laven, R.A. Effect of vitamin E supplementation on the health and fertility of dairy cows: A review. Vet. Rec. 2000, 147. [Google Scholar]
- Eger, S.; Drori, D.; Kadoori, I.; Miller, N.; Schindler, H. Effects of selenium and vitamin E on incidence of retained placenta. J. Dairy Sci. 1985, 68, 2119–2122. [Google Scholar] [CrossRef]
- Bourne, N.; Laven, R.; Wathes, D.C.; Martinez, T.; McGowan, M. A metaanalysis of the effects of vitamin E supplemen-tation on the incidence of retained foetal membranes in dairy cows. Theriogenology 2007, 67, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Tenhag, J.; Walton, J.S.; Johnson, W.H. The Effect of Prepartum Injection of Vitamin E on Health in Transition Dairy Cows. J. Dairy Sci. 2002, 85, 1416–1426. [Google Scholar] [CrossRef]
- Santos, J.E.P.; Bisinotto, E.S.; Ribeiro, F.S.; Lima, L.F.; Greco, C.R.; Thatcher, W.W. Applying nutrition and physiology to improve reproduction in dairy cattle. Reprod. Domest. Rumin. VII 2010, 67, 387–403. [Google Scholar] [CrossRef]

| Selenoproteins | Properties |
|---|---|
| Glutathione peroxidase 1 | Cellular reduction of H2O2 [67,68]. |
| Glutathione peroxidase 2 | Reduction of peroxide in the gut [69]. |
| Glutathione peroxidase 3 | Reduction of peroxide in the blood [70]. |
| Glutathione peroxidase 4 | Causes the Reduction of hydrogen peroxide radicals and facilitates lipid peroxides to water and lipid alcohols and the cellular ferroptosis induced by iron [71]. |
| Selenoprotein H | Responsible for Nuclear localization, which is associated with redox sensing and transcription [72,73]. |
| Selenoprotein O | Mitochondrial protein consisted of a cytosine-nucleotide-nucleotide-uridine motif suggestive of the redox role [62]. |
| Selenoprotein T | Deficiency leads to early embryonic lethality [74]. |
| Selenoprotein W | Have a role of putative antioxidant which is important for muscle growth [75]. |
| Selenophosphate synthetase 2 | Selenophosphate synthetase 2 has an essential role in the biogenesis of all selenoproteins together with itself [76]. |
| Vitamin E Treatment | Possible Outcomes | References |
|---|---|---|
| Vitamin E parenteral administration | Prevented suppression of TAS and GPx | [19] |
| Increased humoral immune response, | ||
| Enhanced daily growth in calves | ||
| 1 mg/kg of Vitamin E subcutaneous supplement | Enhance immunity and antioxidant system | [22] |
| Regulated tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), interferon gamma (IFNγ), SOD and GPx in calves | ||
| γ-tocopherol | Prevented cellular damage and loss of function of primary bovine mammary endothelial cells (BMECs) caused by oxidant challenge | [145] |
| Decreased cell cytotoxicity and enhanced cell viability | ||
| Reduced lipid peroxidation and apoptosis caused oxidative challenge | ||
| Vitamin E intramuscular injection (40 IU/kg body weight) | Enhanced antioxidant activity | [146] |
| Suppressed lipid peroxidation | ||
| Decreased MDA values in plasma | ||
| Increased α-tocopherol in plasma of calves | ||
| Vitamin E supplementation | Prevented oxidative stress caused by aluminum in rats | [133] |
| Enhanced antioxidative status in rats | ||
| Decreased lipid peroxidation | ||
| Suppressed MDA concentration in plasma of rats | ||
| Decreased Plasma thiobarbituric acid-reacting substances (TBARS) | ||
| Vitamin E supplementation | Enhanced antioxidative status and suppressed oxidative stress in perinatal cattle Enhanced GSH-Px concentration Decreased the SOD level | [43] |
| Vitamin E supplementation | Decreased the SOD, MDA and catalase (CAT) level Enhanced the activity of TAOC, phagocytic activity (PA) of granulocytes and lymphocyte proliferation assay (LPA) in transition dairy cows | [151] |
| Vitamin E supplementation (A review) | Enhanced the antioxidant capacity and immunity in transition dairy cattle | [112] |
| Vitamin E supplementation | Reduced tissue peroxidation in chicken | [152] |
| Vitamin E supplementation | Reduced lipid peroxidation in meat and enhance antioxidative status | [153] |
| Vitamin E supplementation | Enhanced the antioxidative status in dairy cattle | [154] |
| α-tocopherol supplementation | Enhanced antioxidant status Suppressed lipid peroxidation | [155] |
| Vitamin E injection | Decreased Plasma thiobarbituric acid-reacting substances (TBARS) in muscle | [156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. https://doi.org/10.3390/antiox10101555
Xiao J, Khan MZ, Ma Y, Alugongo GM, Ma J, Chen T, Khan A, Cao Z. The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants. 2021; 10(10):1555. https://doi.org/10.3390/antiox10101555
Chicago/Turabian StyleXiao, Jianxin, Muhammad Zahoor Khan, Yulin Ma, Gibson Maswayi Alugongo, Jiaying Ma, Tianyu Chen, Adnan Khan, and Zhijun Cao. 2021. "The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation" Antioxidants 10, no. 10: 1555. https://doi.org/10.3390/antiox10101555
APA StyleXiao, J., Khan, M. Z., Ma, Y., Alugongo, G. M., Ma, J., Chen, T., Khan, A., & Cao, Z. (2021). The Antioxidant Properties of Selenium and Vitamin E; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants, 10(10), 1555. https://doi.org/10.3390/antiox10101555








