Dimeric Histidine as a Novel Free Radical Scavenger Alleviates Non-Alcoholic Liver Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of H-Bihistidine
2.2. Detection of H2O2 Content
2.3. Animals
2.4. Cell Culture
2.5. Preparation of Damaged Cells by Free Radicals and Biochemical Assay
2.6. NAFL Model Preparation and H-Bihistidine Administration
2.7. Preparation of CCl4-Induced Liver Injury Mice
2.8. Preparation of Mouse Model of Liver Fibrosis
2.9. Statistical Analysis
3. Results
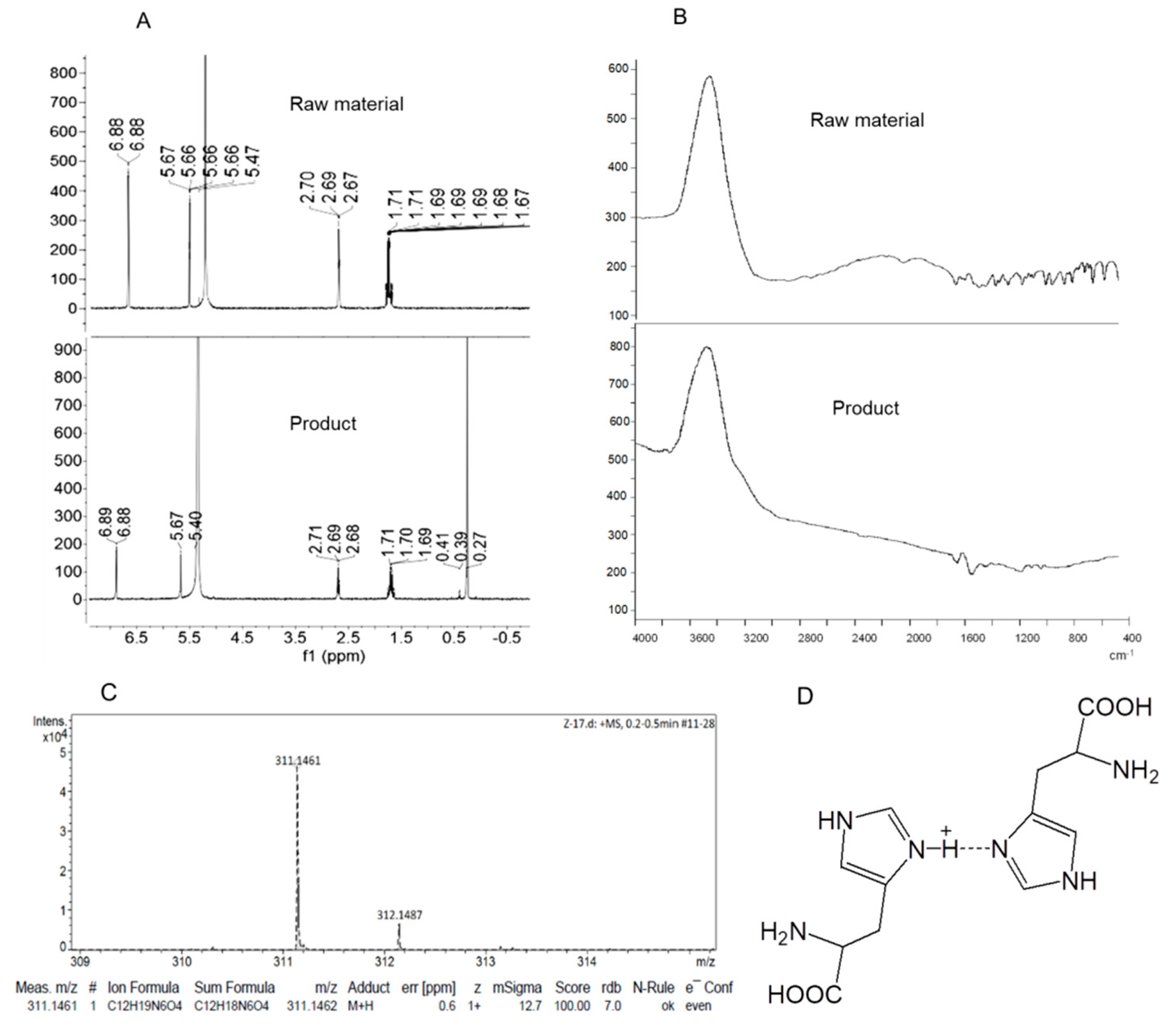
3.1. Synthesis of the Dimeric Histidine (H-Bihistidine)
3.2. H-Bihistidine Scavenged H2O2 and Reduced Cell Injury Induced by H2O2
3.3. H-Bihistidine Prevented Palmitate-Induced Cell Injury
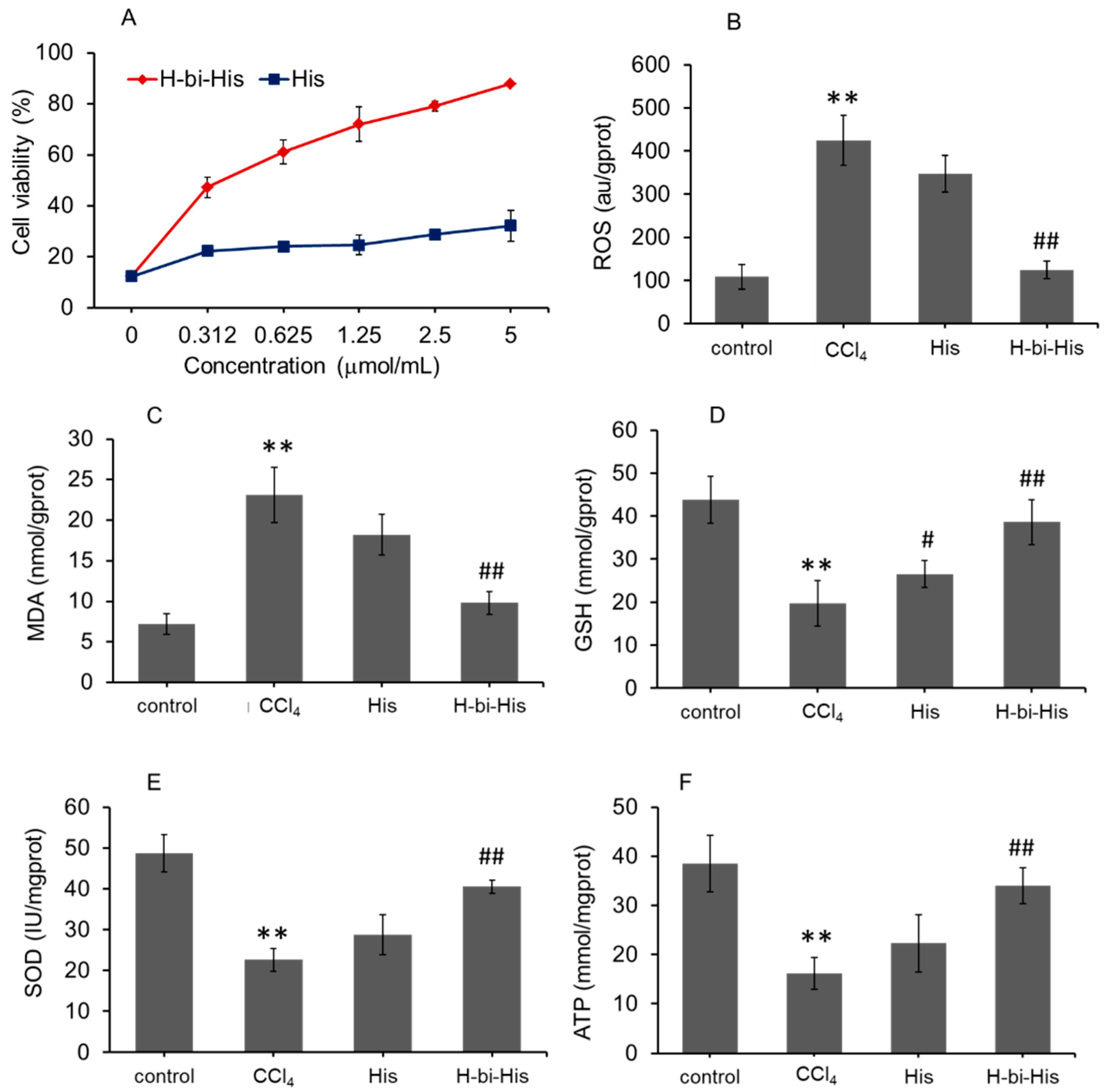
3.4. H-Bihistidine Restored Cell Viability in CCl4-Induced Cell Injury
3.5. H-Bihistidine Reduced Serum Transaminase and Lipid In Vivo
3.6. Treatment of Mouse Fatty Liver with H-Bihistidine
3.7. H-Bihistidine Prevented CCl4-Induced Liver Injury
3.8. H-Bihistidine Prevented Liver Fibrosis Induced by Both High-Fat Diet and CCl4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264. [Google Scholar] [CrossRef] [Green Version]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, S.D.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell Longev. 2020, 2020, 9829176. [Google Scholar] [PubMed]
- Félix, F.; Oliveira, C.C.V.; Elsa Cabrita, E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants 2021, 10, 36. [Google Scholar] [CrossRef]
- Lat Vera-Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Katunde-Dada, G.O. Protective Role of Histidine Supplementation Against Oxidative Stress Damage in the Management of Anemia of Chronic Kidney Disease. Pharmaceuticals 2018, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.C.; Azzout-Marniche, D. Histidine: A Systematic Review on Metabolism and Physiological Effects in Human and Different Animal Species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Hejna, A.; Olszewski, A.; Zedler, Ł.; Kosmela, P.; Formela, K. The Impact of Ground Tire Rubber Oxidation with H2O2 and KMnO4 on the Structure and Performance of Flexible Polyurethane/Ground Tire Rubber Composite Foams. Materials 2021, 14, 499. [Google Scholar] [CrossRef]
- Seglen, P.O. Preparation of rat liver cells. I. Effect of Ca2+ on enzymatic dispersion of isolated, perfused liver. Exp. Cell Res. 1972, 74, 450–454. [Google Scholar] [CrossRef]
- Klingmüller, U.; Bauer, A.; Bohl, S.; Nickel, P.J.; Hengstler, J.G. Primary mouse hepatocytes for systems biology approaches: A standardized in vitro system for modelling of signal transduction pathways. IEE Proc. Syst. Biol. 2006, 153, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Xie, K.; Jin, B.; Zhu, H.; Zhou, P.; Du, L.; Jin, X. Ferulic acid (FA) protects human retinal pigment epithelial cells from H2O2-induced oxidative injuries. J. Cell Mol. Med. 2020, 24, 13454–13462. [Google Scholar] [CrossRef]
- Shen, C.; Dou, X.; Ma, Y.; Ma, W.; Songtao, L.; Song, Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr. Res. 2017, 40, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Hou, Y.X.; Zhou, Z.; Keerthiga, R.; Fu, A. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng. Transl. Med. 2021, 6, e10209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Chen, C. Effects of exogenous thymosin β4 on carbon tetrachloride-induced liver injury and fibrosis. Sci. Rep. 2017, 7, 5872. [Google Scholar] [CrossRef]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W. The Effects of RKI-1447 in a Mouse Model of Nonalcoholic Fatty Liver Disease Induced by a High-Fat Diet and in HepG2 Human Hepatocellular Carcinoma Cells Treated with Oleic Acid. Med. Sci. Monit. 2020, 26, e919220-1–e919220-17. [Google Scholar] [CrossRef]
- Zhao, J.; Posa, D.K.; Kumar, V.; Hoetker, D.; Kumar, A.; Ganesan, S.; Riggs, D.W.; Bhatnagar, A.; Wempe, M.F.; Baba, S.P. Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 2019, 51, 123–138. [Google Scholar] [CrossRef]
- Nair, N.G.; Perry, G.; Smith, M.A.; Reddy, V.P. NMR studies of zinc, copper, and iron binding to histidine, the principal metal ion complexing site of amyloid- peptide. J. Alzheimer’s Dis. 2010, 20, 57–66. [Google Scholar] [CrossRef]
- Vistoli, G.; Aldini, G.; Fumagalli, L.; Dallanoce, C.; Angeli, A.; Supuran, C.T. Activation Effects of Carnosine- and Histidine- Containing Dipeptides on Human Carbonic Anhydrases: A Comprehensive Study. Int. J. Mol. Sci. 2020, 21, 1761. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of alpha-tocopherol, gamma-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The Structural Integrity of the Model Lipid Membrane during Induced Lipid Peroxidation: The Role of Flavonols in the Inhibition of Lipid Peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef]
- Fu, A.; Shi, X.S.; Zhang, H.Q.; Fu, B. Mitotherapy for fatty liver by intravenous administration of exogenous mitochondria in male mice. Front. Pharmacol. 2017, 8, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther. 2020, 20, 115. [Google Scholar] [CrossRef] [Green Version]
- Bala, S.; Calenda, C.D.; Catalano, D.; Babuta, M.; Kodys, K.; Nasser, I.A.; Vidal, B.; Szabo, G. Deficiency of miR-208a Exacerbates CCl4-Induced Acute Liver Injury in Mice by Activating Cell Death Pathways. Hepatol. Commun. 2020, 4, 1487–1501. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Fu, C.; Zhang, Y.; Fu, A. Dimeric Histidine as a Novel Free Radical Scavenger Alleviates Non-Alcoholic Liver Injury. Antioxidants 2021, 10, 1529. https://doi.org/10.3390/antiox10101529
Zhao Z, Fu C, Zhang Y, Fu A. Dimeric Histidine as a Novel Free Radical Scavenger Alleviates Non-Alcoholic Liver Injury. Antioxidants. 2021; 10(10):1529. https://doi.org/10.3390/antiox10101529
Chicago/Turabian StyleZhao, Zizhen, Chen Fu, Yuping Zhang, and Ailing Fu. 2021. "Dimeric Histidine as a Novel Free Radical Scavenger Alleviates Non-Alcoholic Liver Injury" Antioxidants 10, no. 10: 1529. https://doi.org/10.3390/antiox10101529
APA StyleZhao, Z., Fu, C., Zhang, Y., & Fu, A. (2021). Dimeric Histidine as a Novel Free Radical Scavenger Alleviates Non-Alcoholic Liver Injury. Antioxidants, 10(10), 1529. https://doi.org/10.3390/antiox10101529





