Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Herb Sample Collection and Preparation
2.3. Taguchi Experimental Design for Ultrasonic Extraction
2.4. Determination of Antioxidant Activity
2.5. Determination of TPC
2.6. Determination of TFC
2.7. Determination of SPC Content
2.8. Statistical Analysis
3. Results
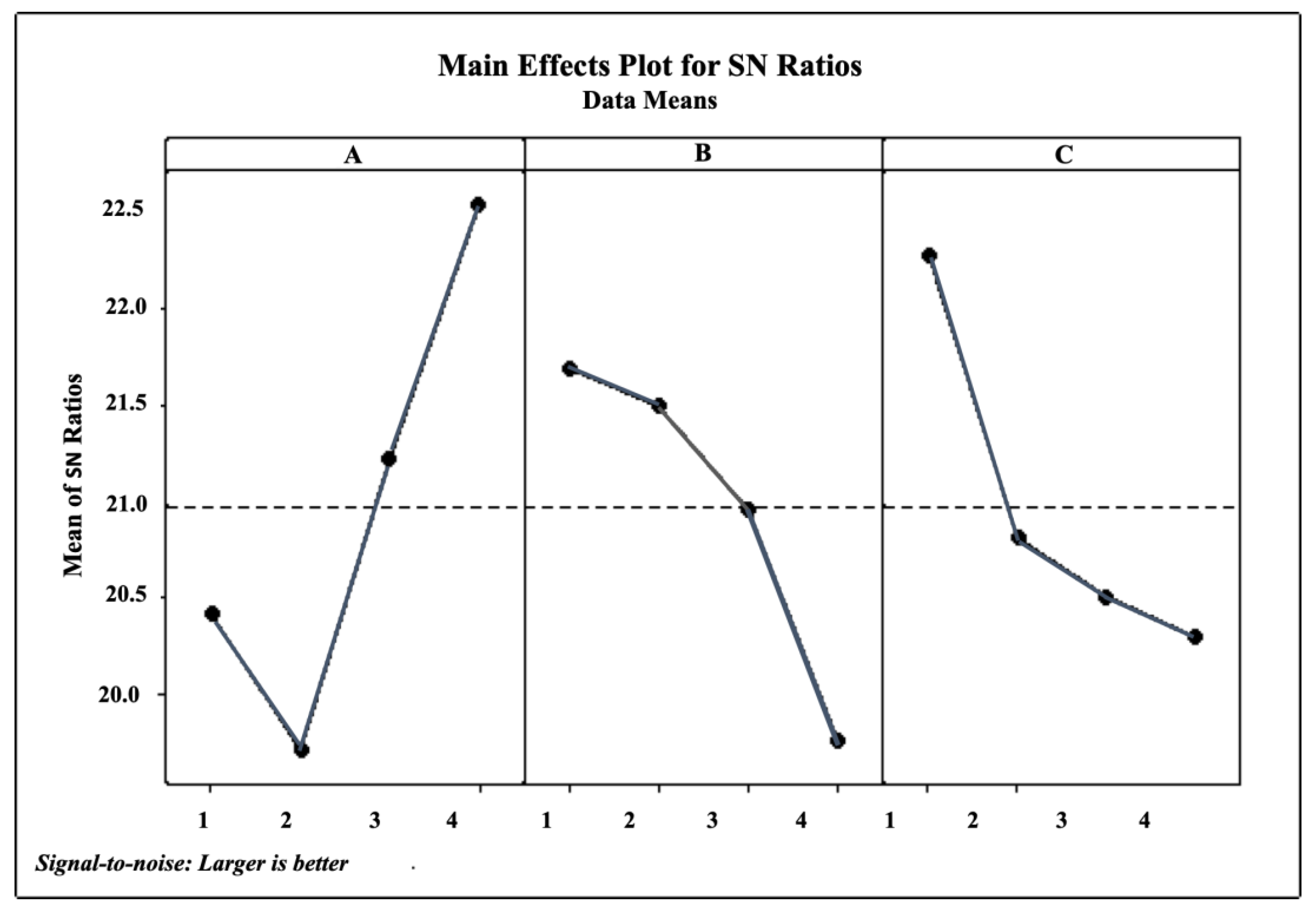
3.1. Optimum Conditions for Ultrasonic Extraction
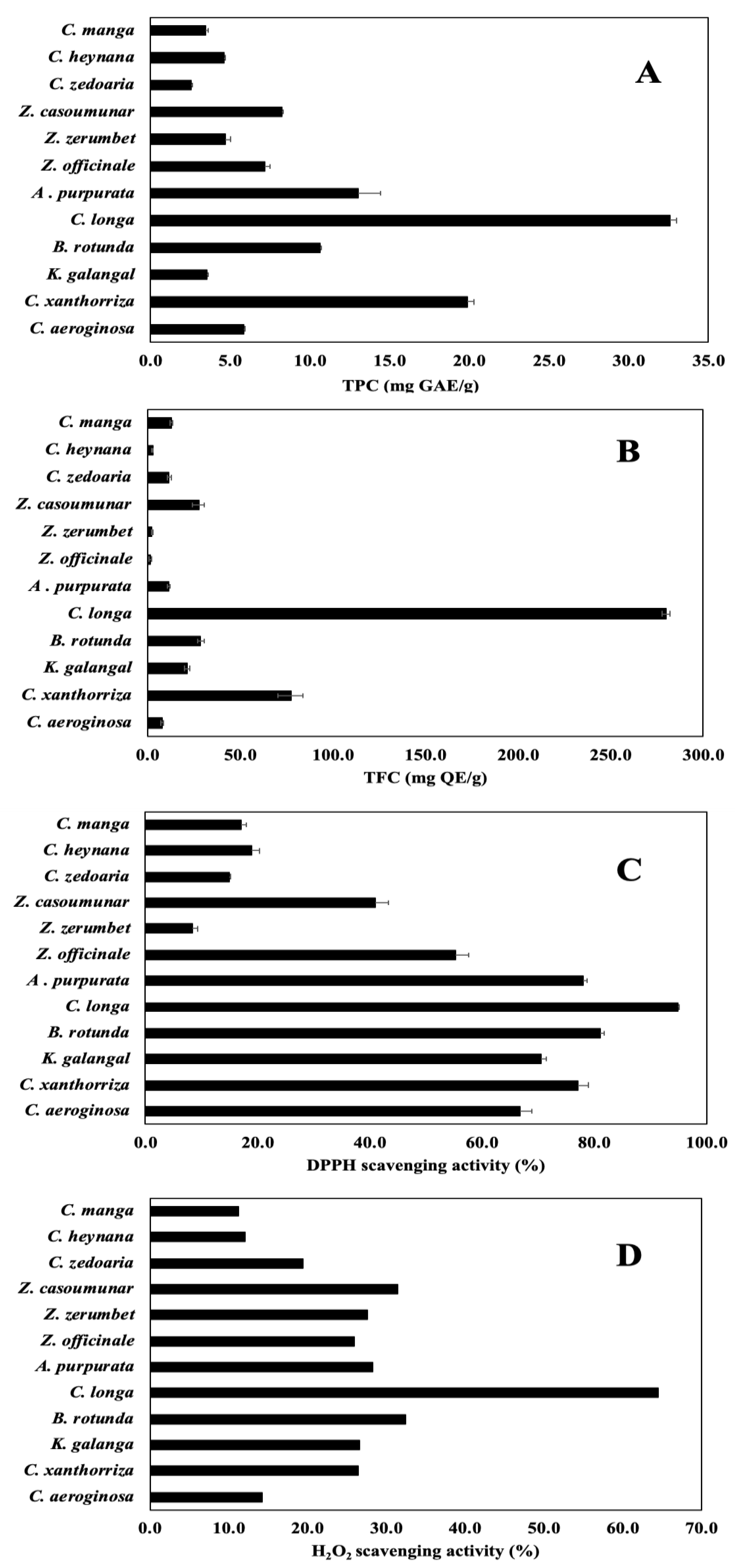
3.2. TPC, TFC, and Antioxidant Activity
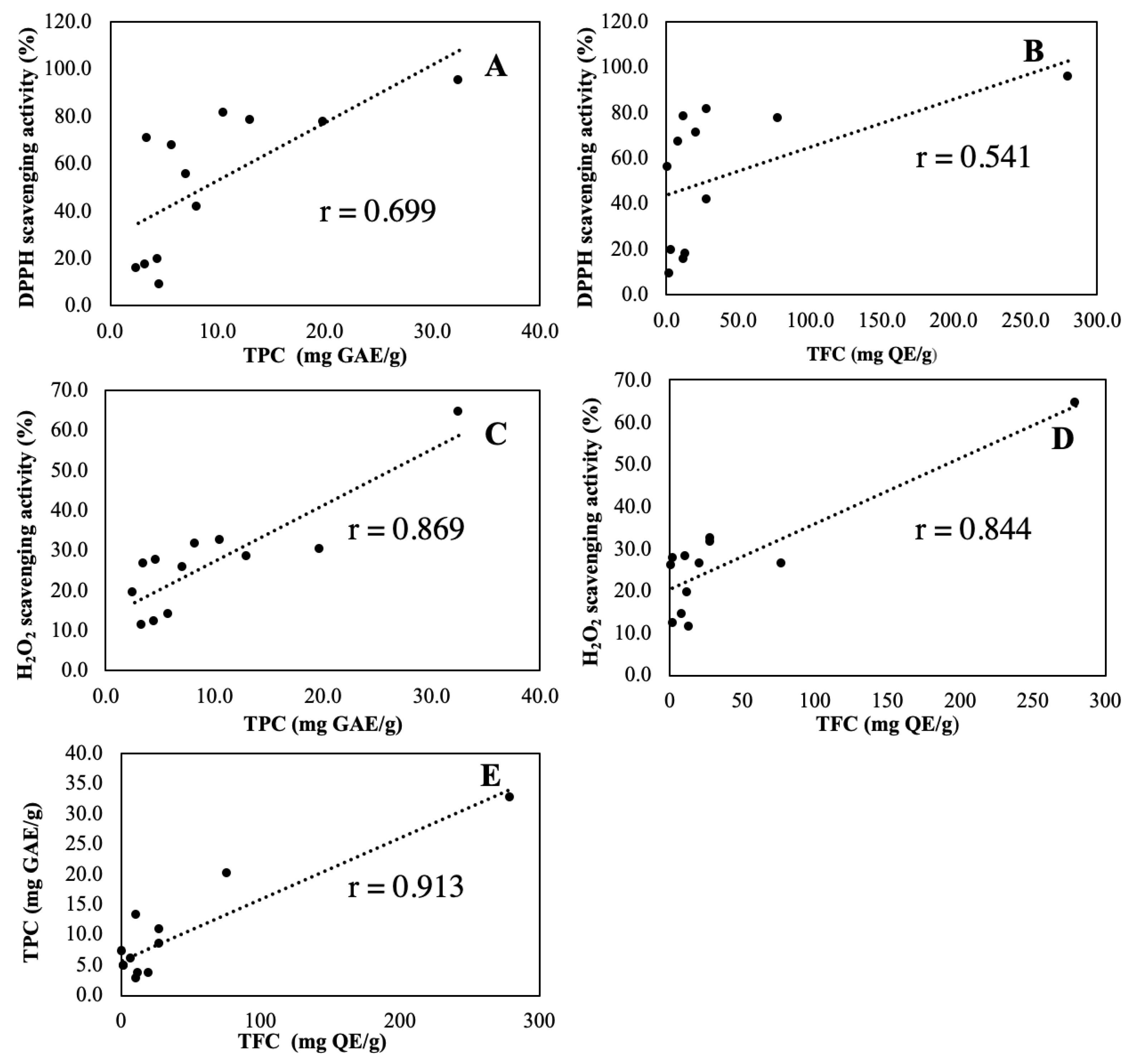
3.3. Correlation Analysis of AA with TPC and TFC
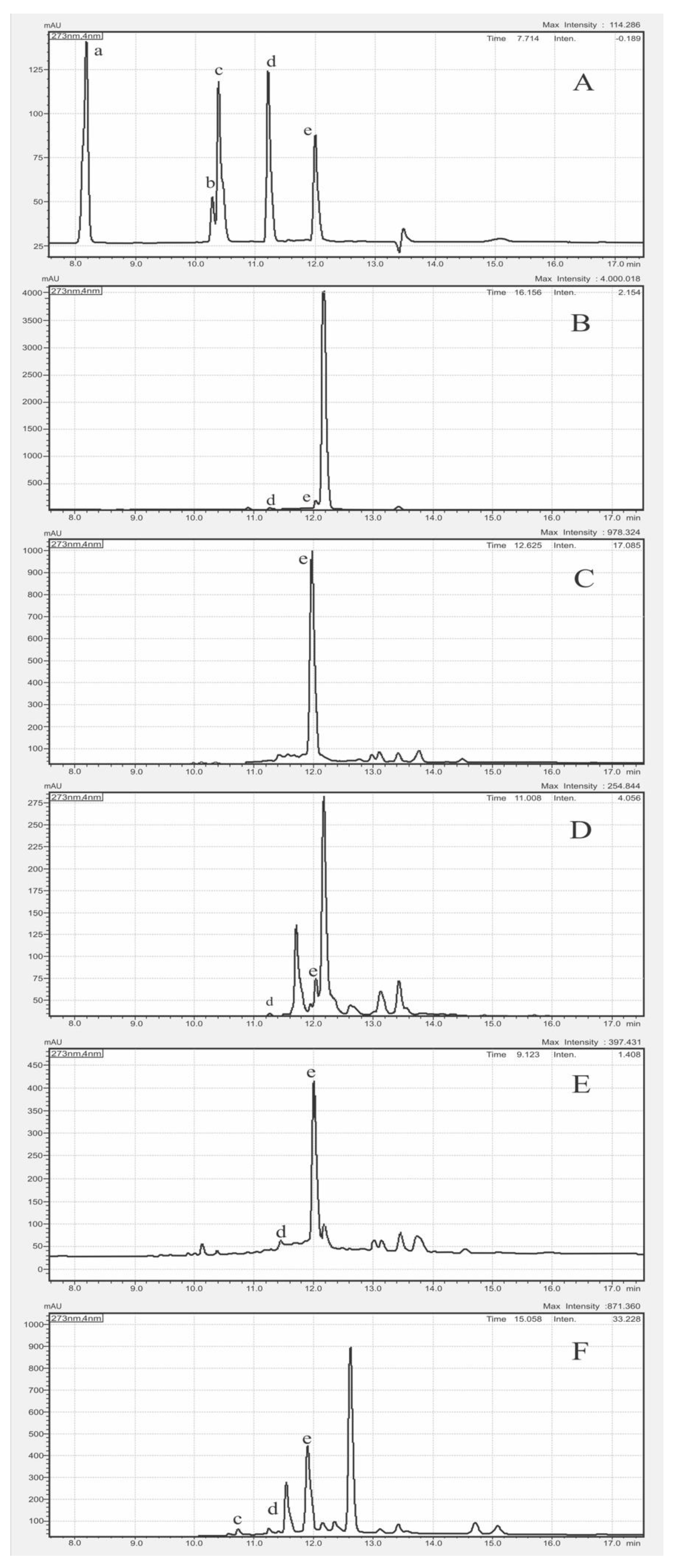
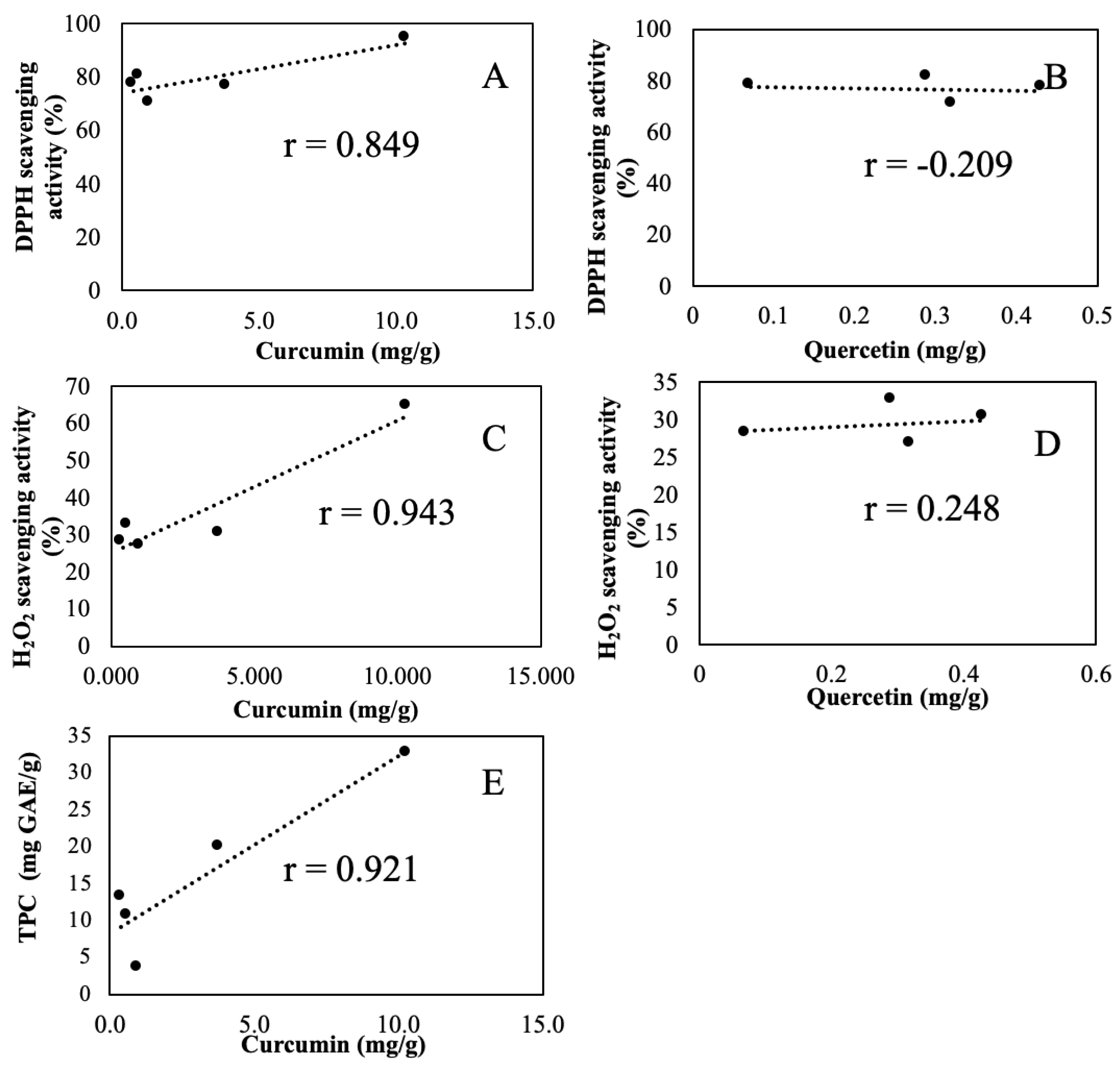
3.4. Correlation Analysis of AA with SPC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Li, M.; Paré, P.W.; Zhang, J.; Kang, T.; Zhang, Z.; Yang, D.; Wang, K.; Xing, H. Antioxidant Capacity Connection with Phenolic and Flavonoid Content in Chinese Medicinal Herbs. Rec. Nat. Prod. 2018, 12, 239–250. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Szmigiel-Merena, B.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Natural antioxidants–properties and possible applications. J. Appl. Biotechnol. Bioeng. 2018, 5, 1. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.D.; Rawat, S.; Rawal, R.S. Antioxidants in Medicinal Plants. In Biotechnology for Medicinal Plants; Chandra, S., Lata, H., Varma, A., Eds.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; pp. 295–326. [Google Scholar]
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhães, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.S.; Almeida, I.F. Evolution of the use of antioxidants in anti-ageing cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Cahyaningsih, R.; Brehm, J.M.; Maxted, N. Gap analysis of Indonesian priority medicinal plant species as part of their conservation planning. Glob. Ecol. Conserv. 2021, 26, e01459. [Google Scholar] [CrossRef]
- Hamzah, B.; Zubair, M.S. Traditional Usages and Phytochemical Screenings of Selected Zingiberaceae from Central Sulawesi, Indonesia. Pharmacogn. J. 2019, 11, 505–510. [Google Scholar] [CrossRef]
- Widyowati, R.; Agil, M. Chemical Constituents and Bioactivities of Several Indonesian Plants Typically Used in Jamu. Chem. Pharm. Bull. 2018, 66, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Krishna, S.R.R.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Yordi, E.G.; Perez, E.M.; Matos, M.J.; Villares, U.V. Antioxidant and pro-oxidant effects of polyphenolic compounds and struc-ture-activity relationship evidence. In Nutrition, Well-Being and Health. Bouyaed; Intechopen: London, UK, 2012; pp. 24–41. Available online: https://www.intechopen.com/chapters/29974 (accessed on 18 July 2021).
- Şahin, S. Optimization of ultrasonic-assisted extraction parameters for antioxidants from Curcuma longa L. Trak. Univ. J. Nat. Sci. 2018, 19, 121–128. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Ling, Y.-C. An overview of supercritical fluid extraction in Chinese herbal medicine: From preparation to analysis. J. Food Drug Anal. 2000, 8, 2. [Google Scholar] [CrossRef]
- Song, J.; Li, D.; Liu, C.; Zhang, Y. Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Lou, S.-N.; Hsu, Y.-S.; Ho, C.-T. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food Drug Anal. 2014, 22, 290–295. [Google Scholar] [CrossRef]
- Wisdom, J.; Creswell, J.W. Mixed Methods: Integrating Quantitative and Qualitative Data Collection and Analysis While Studying Patient-Centered Medical Home Models; AHRQ Publication No. 13-0028-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013. Available online: https://pcmh.ahrq.gov/page/mixed-methods-integrating-quantitative-and-qualitative-data-collec-tion-and-analysis-while (accessed on 26 September 2021).
- Hernández-Rodríguez, G.; Espinosa-Solares, T.; Hernandez-Eugenio, G.; Villa-García, M.; ReyesTrejo, B.; Guerra-Ramírez, D. Influence of Polar Solutions on the Extraction of Phenolic Compounds from Capulín Fruits (Prunus serotina). J. Mex. Chem. Soc. 2017, 60, 73–78. [Google Scholar] [CrossRef]
- Duan, S.-C.; Kwon, S.-J.; Eom, S.-H. Effect of Thermal Processing on Color, Phenolic Compounds, and Antioxidant Activity of Faba Bean (Vicia faba L.) Leaves and Seeds. Antioxidants 2021, 10, 1207. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; E Babiker, E.; Özcan, M.M.; Jamiu, F.G. The Effect of Heating Temperature on Total Phenolic Content, Antioxidant Activity, and Phenolic Compounds of Plum and Mahaleb Fruits. Int. J. Food Eng. 2019, 15, 11–12. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia lasoar PAM. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Akinola, A.; Ahmad, S.; Maziah, M. Total Anti-Oxidant Capacity, Flavonoid, Phenolic Acid And Polyphenol Content In Ten Selected Species of Zingiberaceae rhizomes. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Akarchariya, N.; Sirilun, S.; Julsrigival, J.; Chansakaowa, S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017, 7, 881–885. [Google Scholar] [CrossRef]
- Victorio, C.P.; Kuster, R.M.; Lage, C.L.S. Detection of flavonoids in Alpinia purpurata (Vieill.) K. Schum. leaves using high-performance liquid chromatography. Rev. Bras. Plantas Med. 2009, 11, 147–153. [Google Scholar] [CrossRef][Green Version]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2010, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Ovando-Domínguez, M.Y.; Luján-Hidalgo, M.C.; González-Mendoza, D.; Vargas-Díaz, A.A.; Ruiz-Lau, N.; Gutiérrez-Miceli, F.A.; Lecona-Guzman, C.A. Total phenols, flavonoids and antioxidant activity in Annona muricata and Annona purpurea callus culture. Phyton 2019, 88, 139–147. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Siriwardhana, S.S.K.W.; Shahidi, F. Antiradical activity of extracts of almond and its by-products. J. Am. Oil Chem. Soc. 2002, 79, 903–908. [Google Scholar] [CrossRef]
- Yusuf, N.A.; Suffian, M.; Annuar, M.; Khalid, N. Existence of bioactive flavonoids in rhizomes and plant cell cultures of Boesenbergia rotunda (L.). Mansf. Kulturpfl. Aust. J. Crop. Sci. 2013, 7, 730–734. [Google Scholar]
- Raj, C.A. Leaf extract of Alpinia purpurata (Vieill.) K. Schum screened for its phytochemical constituents and antibacterial and anticancer activities. J. Chin. Integr. Med. 2012, 10, 1460–1464. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between Chemical Composition of Curcuma domestica and Curcuma xanthorrhiza and Their Antioxidant Effect on Human Low-Density Lipoprotein Oxidation. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Parida, R.; Singh, S.; Padhy, R.N.; Nayak, S. Evaluation of yield, quality and antioxidant activity of essential oil of in vitro propagated Kaempferia galanga Linn. J. Acute Dis. 2014, 3, 124–130. [Google Scholar] [CrossRef]
- Desai, S.; Tatke, P.; Gabhe, S. Enhanced HPLC-DAD Method for Fast Determination of Quercetin-3-O-β-d-Glucoside in Extracts and Polyherbal Formulations Containing Azadirachta indica—Optimization and Validation. J. Chromatogr. Sci. 2017, 55, 706–711. [Google Scholar] [CrossRef]

| Exp. No | Parameters | TPC (mg GAE/g) | TFC (mg QE/g) | AA (%) | ||
|---|---|---|---|---|---|---|
| Solvent (%) | Time (min) | S-L (g/mL) | ||||
| 1 | 50 | 50 | 0.02 | 4.81 | 8.96 | 12.85 |
| 2 | 50 | 60 | 0.04 | 3.87 | 9.27 | 11.35 |
| 3 | 50 | 70 | 0.06 | 2.02 | 3.45 | 9.36 |
| 4 | 50 | 80 | 0.08 | 1.51 | 2.59 | 8.86 |
| 5 | 60 | 50 | 0.04 | 3.55 | 7.82 | 11.06 |
| 6 | 60 | 60 | 0.02 | 8.25 | 15.83 | 11.06 |
| 7 | 60 | 70 | 0.08 | 1.80 | 4.02 | 7.97 |
| 8 | 60 | 80 | 0.06 | 2.30 | 4.60 | 8.96 |
| 9 | 70 | 50 | 0.06 | 2.81 | 14.48 | 10.86 |
| 10 | 70 | 60 | 0.08 | 2.79 | 15.25 | 11.45 |
| 11 | 70 | 70 | 0.02 | 7.70 | 25.27 | 15.94 |
| 12 | 70 | 80 | 0.04 | 1.95 | 3.63 | 8.86 |
| 13 | 80 | 50 | 0.08 | 3.55 | 14.48 | 14.14 |
| 14 | 80 | 60 | 0.06 | 5.14 | 21.75 | 13.84 |
| 15 | 80 | 70 | 0.04 | 4.42 | 21.35 | 13.05 |
| 16 | 80 | 80 | 0.02 | 4.45 | 12.76 | 12.65 |
| Level | Solvent (%) | Time (min) | S–L (mg/mL) |
|---|---|---|---|
| 1 | 20.42 | 21.69 | 22.89 |
| 2 | 19.71 | 21.50 | 20.81 |
| 3 | 21.22 | 20.95 | 20.50 |
| 4 | 22.55 | 19.75 | 20.29 |
| Delta | 2.84 | 1.94 | 1.99 |
| Rank | 1 | 3 | 2 |
| Compound | Retention Time (min) | LOD (µg/mL) | LOQ (µg/mL) | Repeatability (RSD%, n = 3) | Recovery (%) |
|---|---|---|---|---|---|
| Gallic acids | 8.20 ± 0.014 | 0.20 | 0.52 | <2% | 98 ± 1 |
| Naringin | 10.30 ± 0.004 | 0.29 | 0.44 | <5% | 97 ± 4 |
| Ferulic acids | 10.41 ± 0.003 | 0.02 | 0.02 | <4% | 100 ± 3 |
| Quercetin | 11.24 ± 0.003 | 0.02 | 0.02 | <4% | 100 ± 4 |
| Curcumin | 12.02 ± 0.002 | 0.18 | 0.33 | <6% | 98 ± 5 |
| SPC | Gallic Acid | Naringin | Ferulic Acid | Quercetin | Curcumin |
|---|---|---|---|---|---|
| C. longa | ND | ND | ND | ND | 10.34 ± 0.02 |
| B. rotunda | ND | ND | ND | 0.29 ± 0.007 | 0.61 ± 0.06 |
| A. purpurata | ND | ND | ND | 0.07 ± 0.002 | 0.38 ± 0.022 |
| C. xanthorrhiza | ND | ND | 0.04 ± 0.005 | 0.43 ± 0.026 | 3.78 ± 0.553 |
| K. galangal | ND | ND | ND | 0.32 ± 0.02 | 0.99 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. https://doi.org/10.3390/antiox10101530
Muflihah YM, Gollavelli G, Ling Y-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants. 2021; 10(10):1530. https://doi.org/10.3390/antiox10101530
Chicago/Turabian StyleMuflihah, Yeni Maulidah, Ganesh Gollavelli, and Yong-Chien Ling. 2021. "Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs" Antioxidants 10, no. 10: 1530. https://doi.org/10.3390/antiox10101530
APA StyleMuflihah, Y. M., Gollavelli, G., & Ling, Y.-C. (2021). Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants, 10(10), 1530. https://doi.org/10.3390/antiox10101530








